Compound methoxyphenamine quick-release and sustained-release preparation
A technology for compound methoxyphenamine and sustained-release preparations, which is applied to medical preparations containing active ingredients, pill delivery, and pharmaceutical formulations, and can solve the problem that compound methoxyphenamine products do not have sustained release, large dosage, and Problems such as taking too many times
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
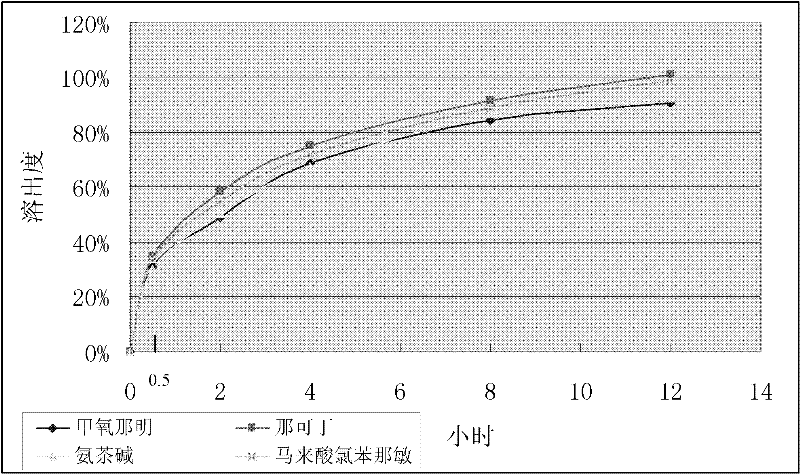
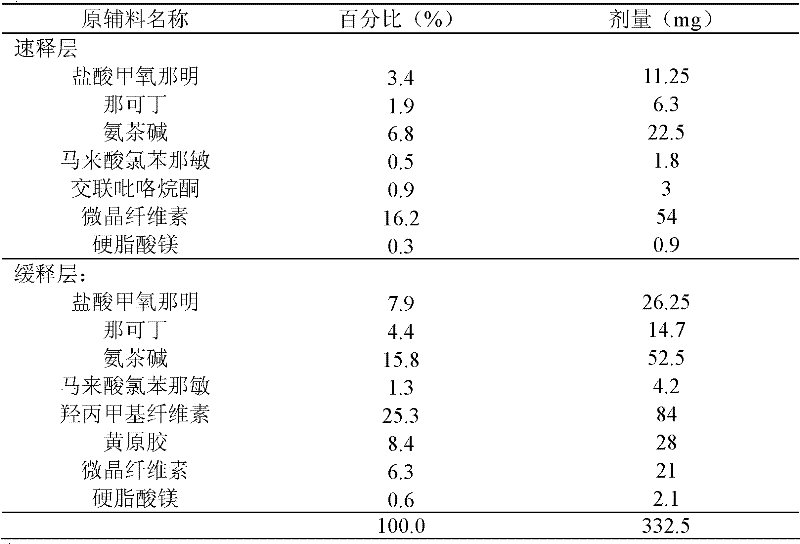
[0062] Embodiment 1: The quick-release-sustained-release compound tablet whose sustained release can reach 12 hours
[0063]
[0064] Preparation Process:
[0065] According to the proportion of the above formula, use dry blending, or dry granulation, or wet granulation process to make the immediate release layer mixture:
[0066] Dry-mixing process preparation method: add active ingredients (methoxyphenamine hydrochloride, narcotine, aminophylline, chlorpheniramine maleate) into cross-linked pyrrolidone and microcrystalline cellulose, and stir evenly; add hard Magnesium fatty acid, stir until uniform to make an immediate-release layer mixture.
[0067] Dry granulation process preparation method: add active ingredients (methoxyphenamine hydrochloride, narcotine, aminophylline, chlorpheniramine maleate) into cross-linked pyrrolidone and microcrystalline cellulose, and stir to form a uniform Mixture, the above-mentioned mixture is extruded into flakes or lumps; then the fla...
Embodiment 2
[0074] Embodiment 2: The quick-release-sustained-release compound tablet whose sustained release can reach 12 hours
[0075] The immediate-release layer of the above-mentioned example 1 was divided into 2 equal parts, and the above-mentioned materials were pressed into three-layer tablets according to the feeding sequence of the immediate-release layer-sustained-release layer-immediate-release layer by using a specific three-layer tablet press;
Embodiment 3
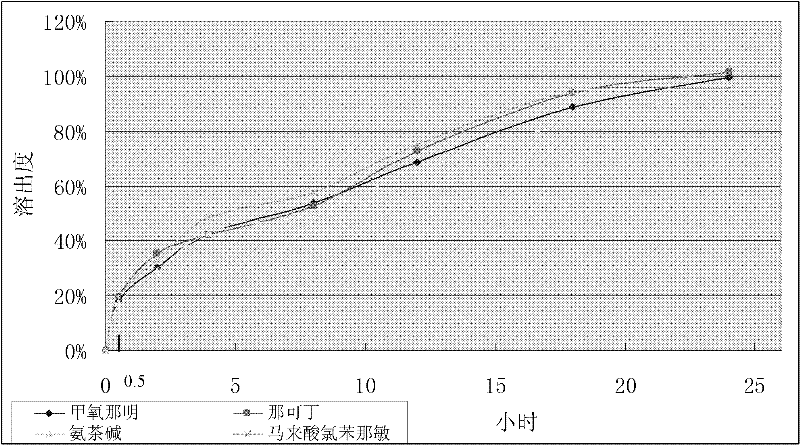
[0076] Embodiment 3: The rapid-release-sustained-release compound tablet whose sustained release can reach 24 hours
[0077]
[0078] Preparation Process:
[0079] According to the proportion of the above formula, use dry blending, or dry granulation, or wet granulation process to make the immediate release layer mixture:
[0080] Dry-mixing process preparation method: add active ingredients (methoxyphenamine hydrochloride, narcotine, aminophylline, chlorpheniramine maleate) into cross-linked pyrrolidone and microcrystalline cellulose, and stir evenly; add hard Magnesium fatty acid, stir until uniform to make an immediate-release layer mixture.
[0081] Dry granulation process preparation method: add active ingredients (methoxyphenamine hydrochloride, narcotine, aminophylline, chlorpheniramine maleate) into cross-linked pyrrolidone and microcrystalline cellulose, and stir to form a uniform Mixture, the above-mentioned mixture is extruded into flakes or lumps; then the fla...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com