Pharmaceutical composition for treating cold in children and preparation method thereof
A composition and drug technology, applied in the direction of drug combination, anti-infective drug, pharmaceutical formula, etc., can solve the problems of poor water solubility of dextromethorphan, unresolved mutual antagonism, and inaccurate curative effect, etc., to improve the taste and water solubility , good compliance and exact efficacy of the preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
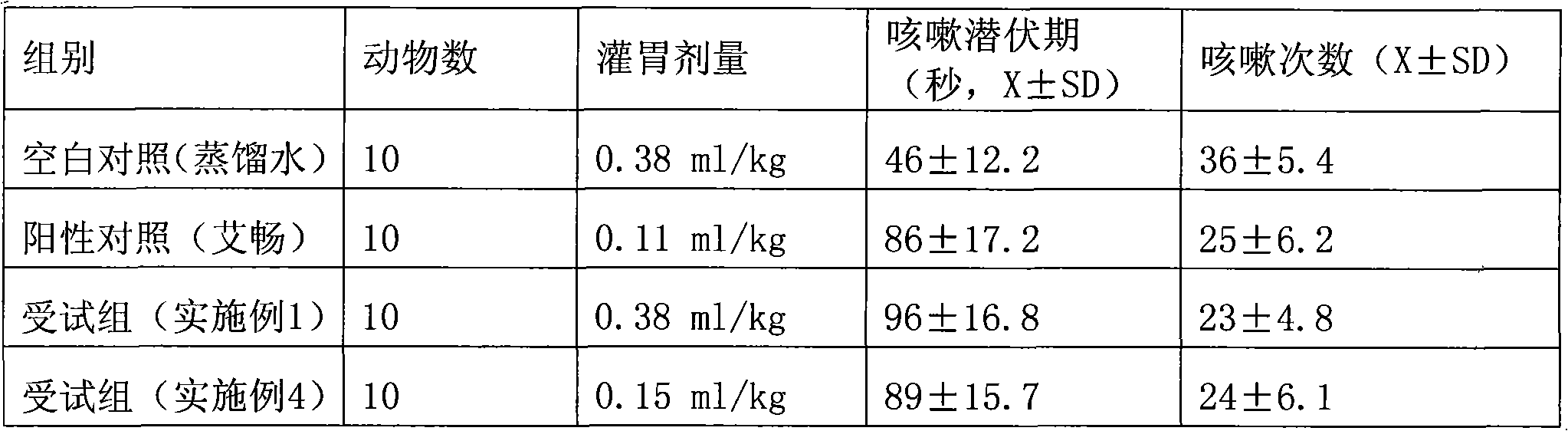
Examples
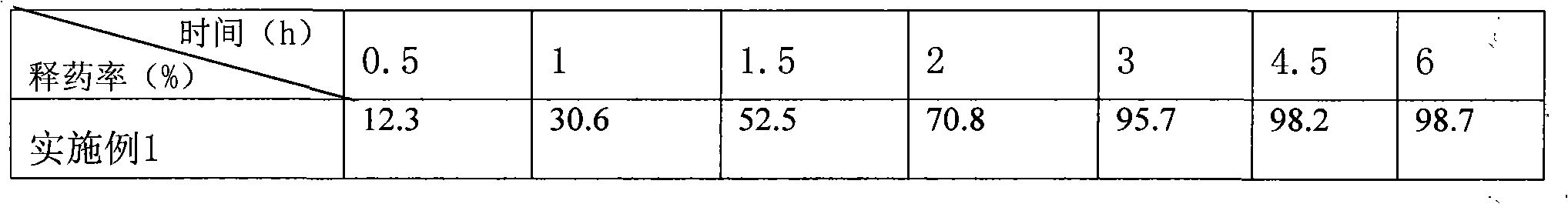
Embodiment 1
[0045] Embodiment 1 (preparation of syrup)
[0046] Recipe ratio:
[0047]
[0048] Preparation Process:
[0049] 1. Weigh beta-cyclodextrin and dissolve it in 200ml of water, dissolve 0.9g of dextromethorphan hydrobromide in 10ml of ethanol, add it to the beta-cyclodextrin solution, stir at room temperature for 2 hours, it can be observed that the initial solution is a band The opalescent suspension solution turned into a clear and transparent solution, heated to 60°C, and continued to stir for 30 minutes. After all ethanol was removed, the solution was still clear and transparent, tasteless and odorless. At this time, dextromethorphan had been absorbed by betacycline. Dextrin contains.
[0050] 2. Weigh sucrose, add appropriate amount of boiling water, heat to 100°C, stir to dissolve, heat and keep warm (95±5°C) for 45 minutes while stirring, and filter while hot to obtain simple syrup.
[0051] 3. Weigh chlorpheniramine maleate, ammonium chloride, sodium citrate, citr...
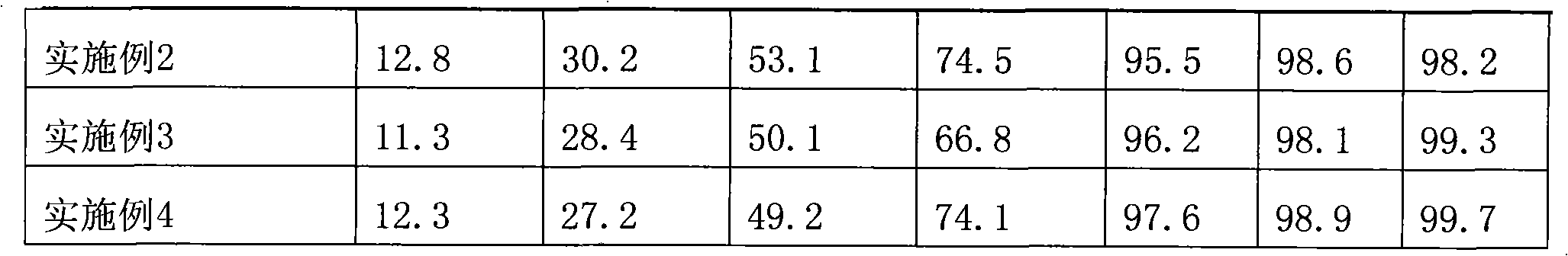
Embodiment 2
[0053] Embodiment 2 (preparation of oral solution)
[0054] Recipe ratio:
[0055]
[0056] Preparation Process:
[0057] 1. Weigh beta-cyclodextrin and dissolve it in 200ml of water, dissolve 0.9g of dextromethorphan hydrobromide in 10ml of ethanol, add it to the beta-cyclodextrin solution, stir at room temperature for 2 hours, it can be observed that the initial solution is a band The opalescent suspension solution turned into a clear and transparent solution, heated to 60°C, and continued to stir for 30 minutes. After all ethanol was removed, the solution was still clear and transparent, tasteless and odorless. At this time, dextromethorphan had been absorbed by betacycline. Dextrin inclusion.
[0058] 2. Weigh chlorpheniramine maleate, ammonium chloride, sodium citrate, citric acid, benzoic acid, glycerin, sorbitol, and sodium saccharin according to the prescription ratio, dissolve in freshly boiled water, and add dextromethorphan Cyclodextrin inclusion compound, ora...
Embodiment 3
[0060] Embodiment 3 (preparation of oral solution)
[0061] Recipe ratio:
[0062]
[0063] Preparation Process:
[0064] 1. Weigh beta-cyclodextrin and dissolve it in 200ml of water, dissolve 0.9g of dextromethorphan hydrobromide in 10ml of ethanol, add it to the beta-cyclodextrin solution, stir at room temperature for 2 hours, it can be observed that the initial solution is a band The opalescent suspension solution turned into a clear and transparent solution, heated to 60°C, and continued to stir for 30 minutes. After all ethanol was removed, the solution was still clear and transparent, tasteless and odorless. At this time, dextromethorphan had been absorbed by betacycline. Dextrin contains.
[0065] 2. Weigh chlorpheniramine maleate, ammonium chloride, sodium citrate, citric acid, benzoic acid, sucrose, sorbitol, stevioside according to the prescription ratio, dissolve in freshly boiled water, add dextromethorphan Cyclodextrin inclusion compound, orange essence, alm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com