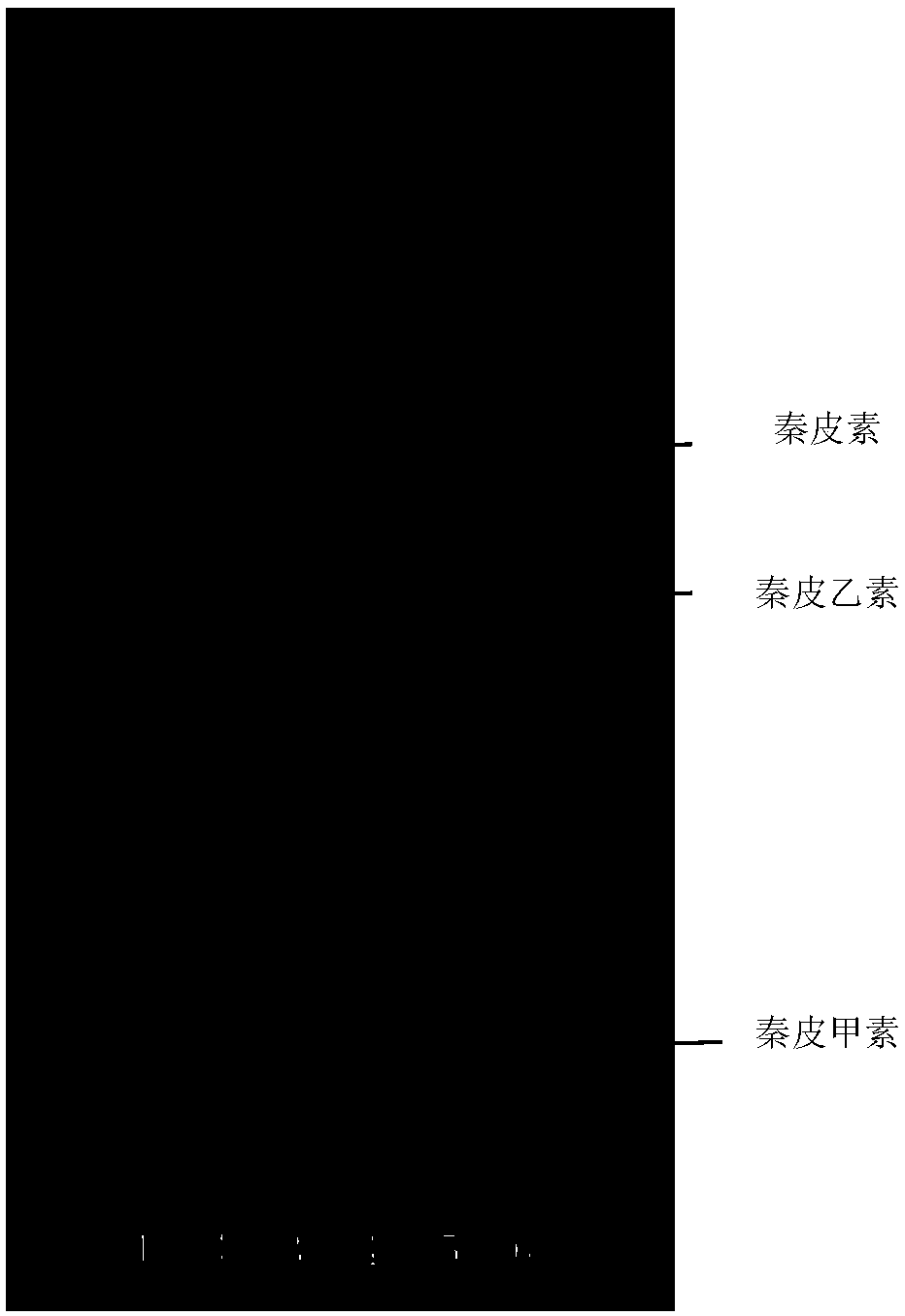
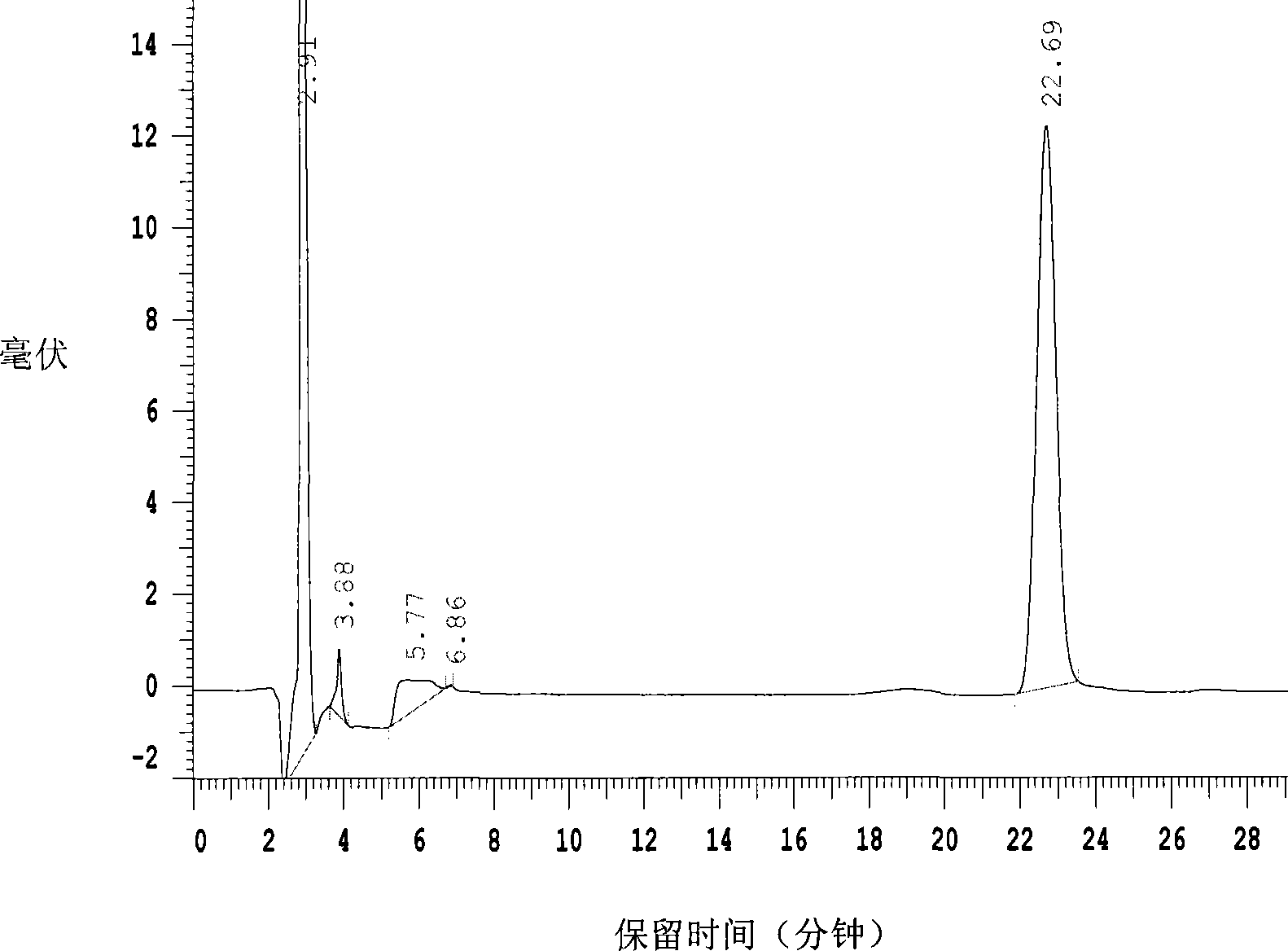
Patents
Literature
72results about How to "Increase assay" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
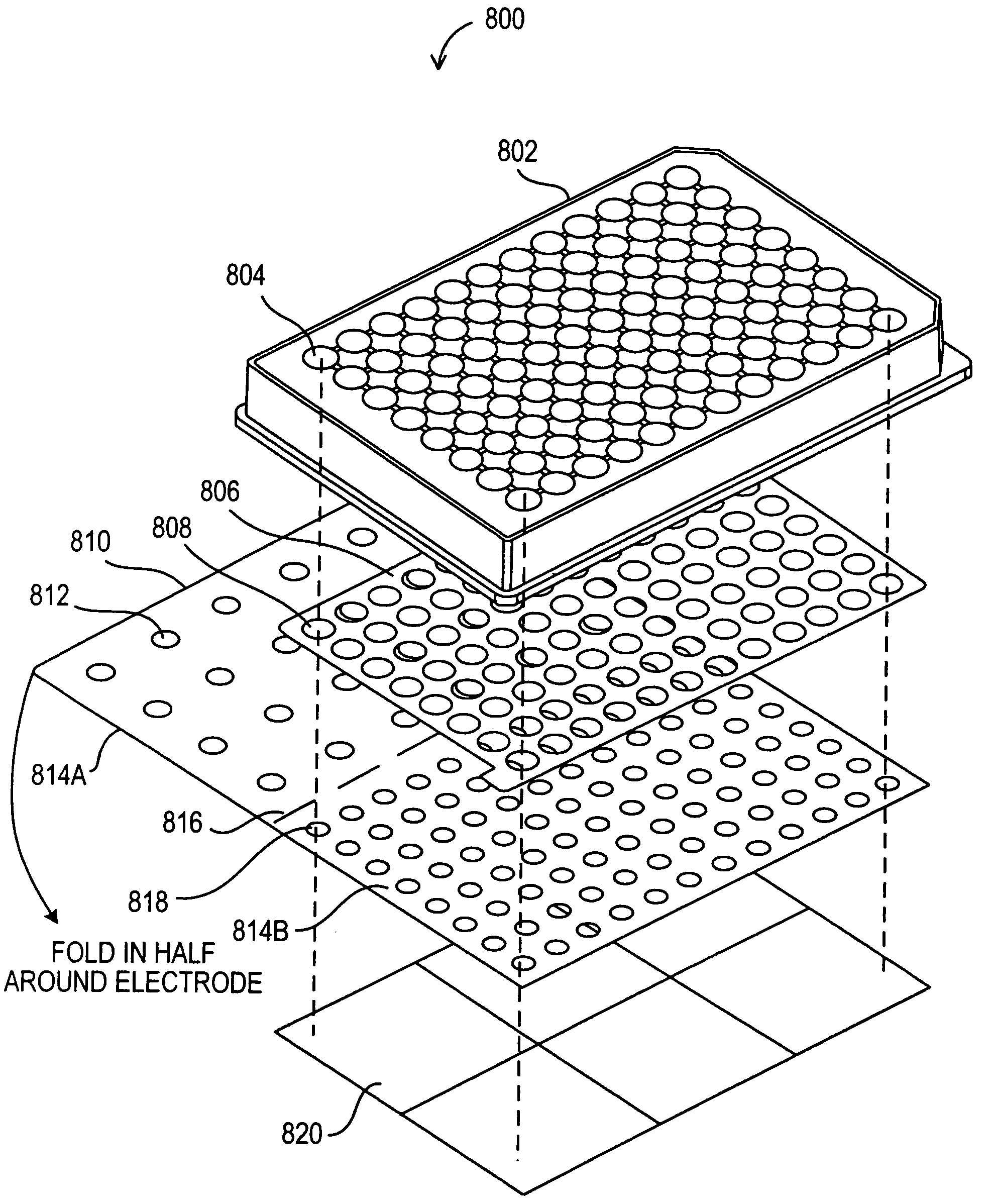
Modular assay plates, reader systems and methods for test measurements
ActiveUS20050142033A1Improve collection efficiencyIncrease assayAnalysis using chemical indicatorsMicrobiological testing/measurementTest measurementBiology
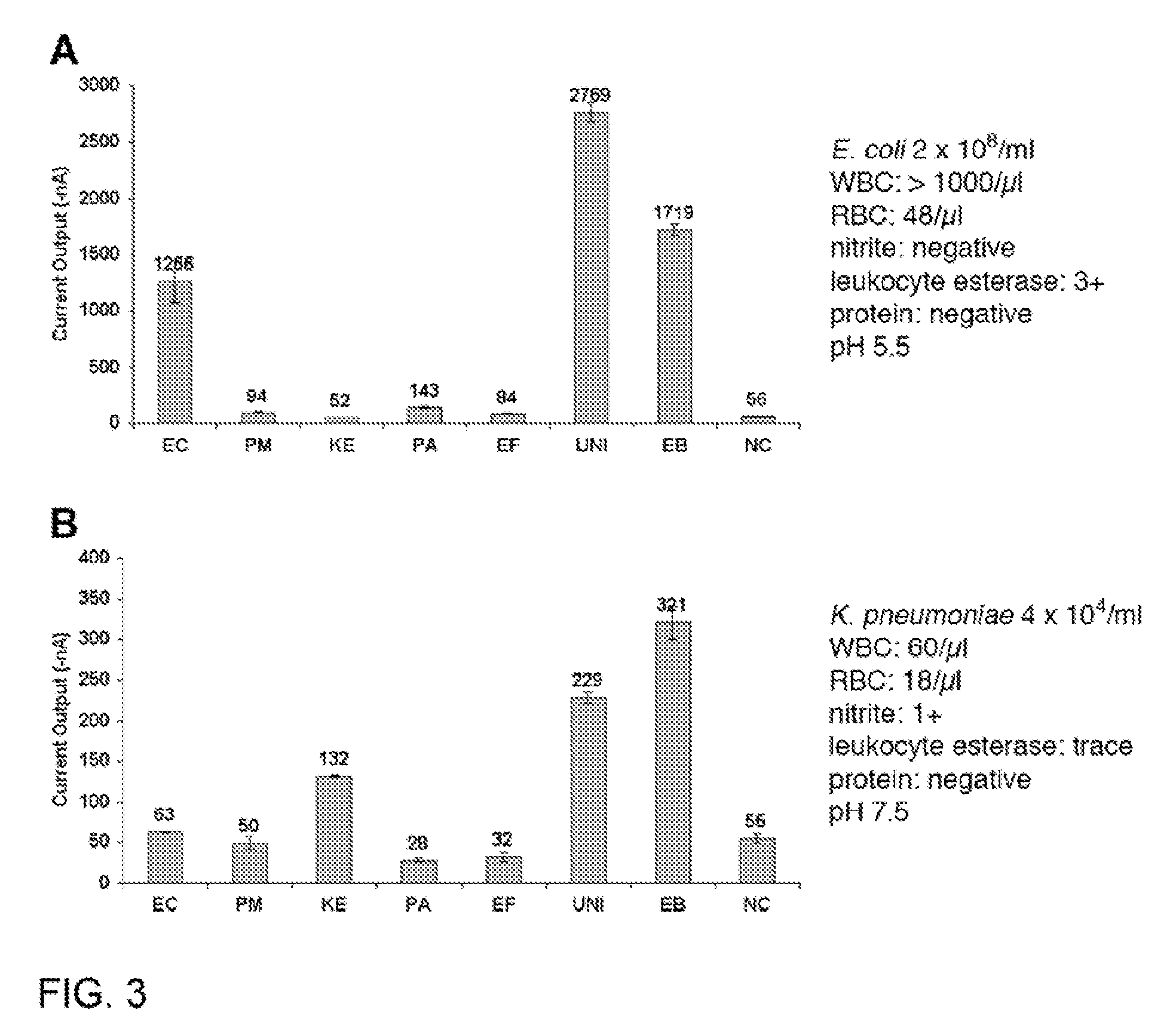
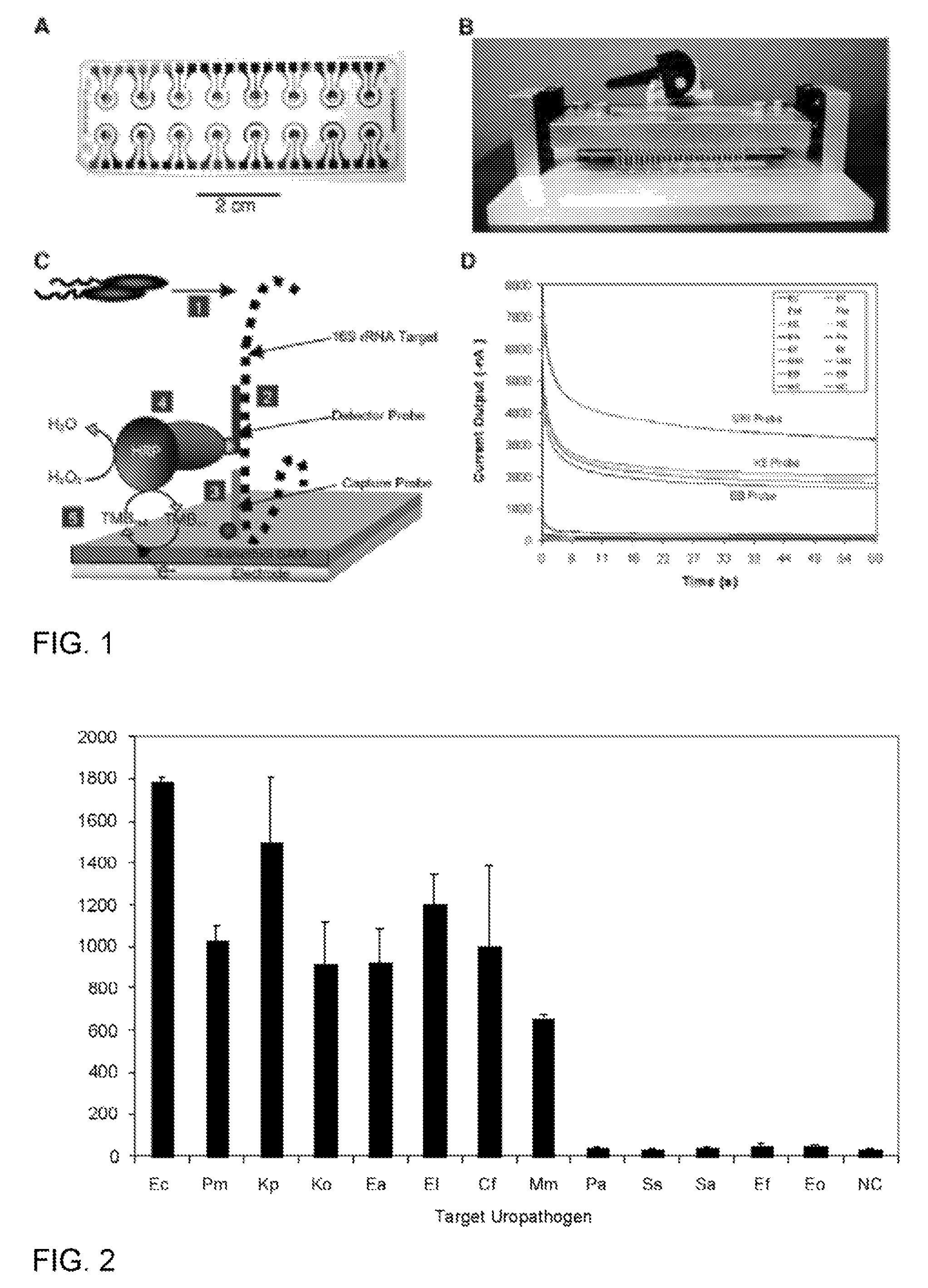
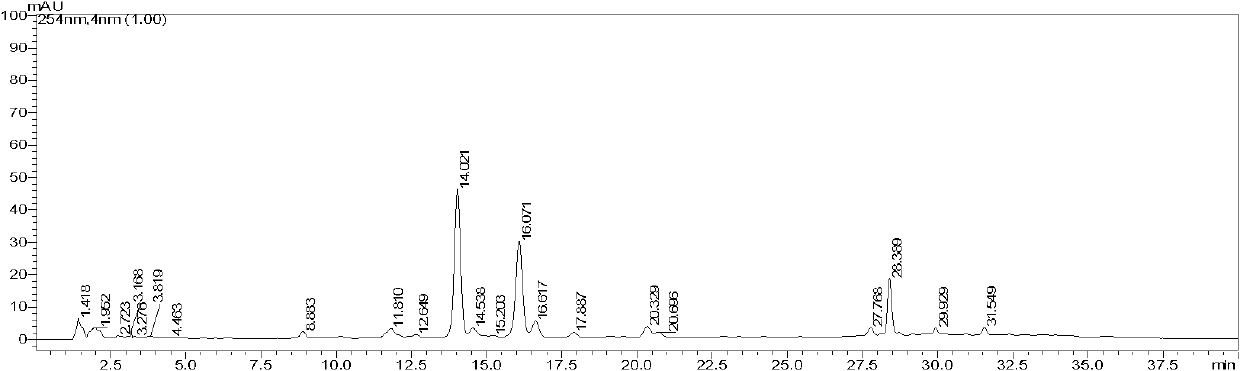
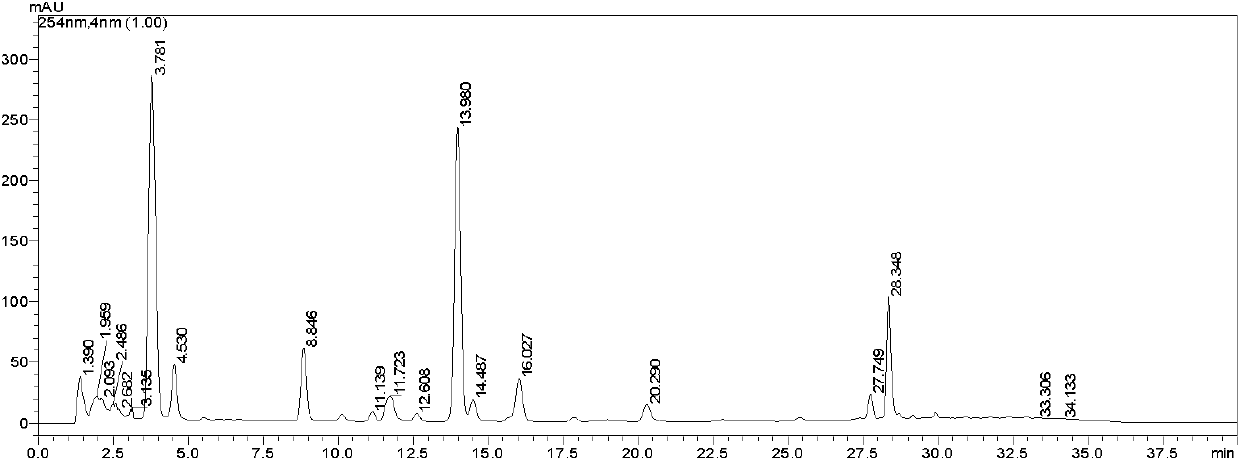
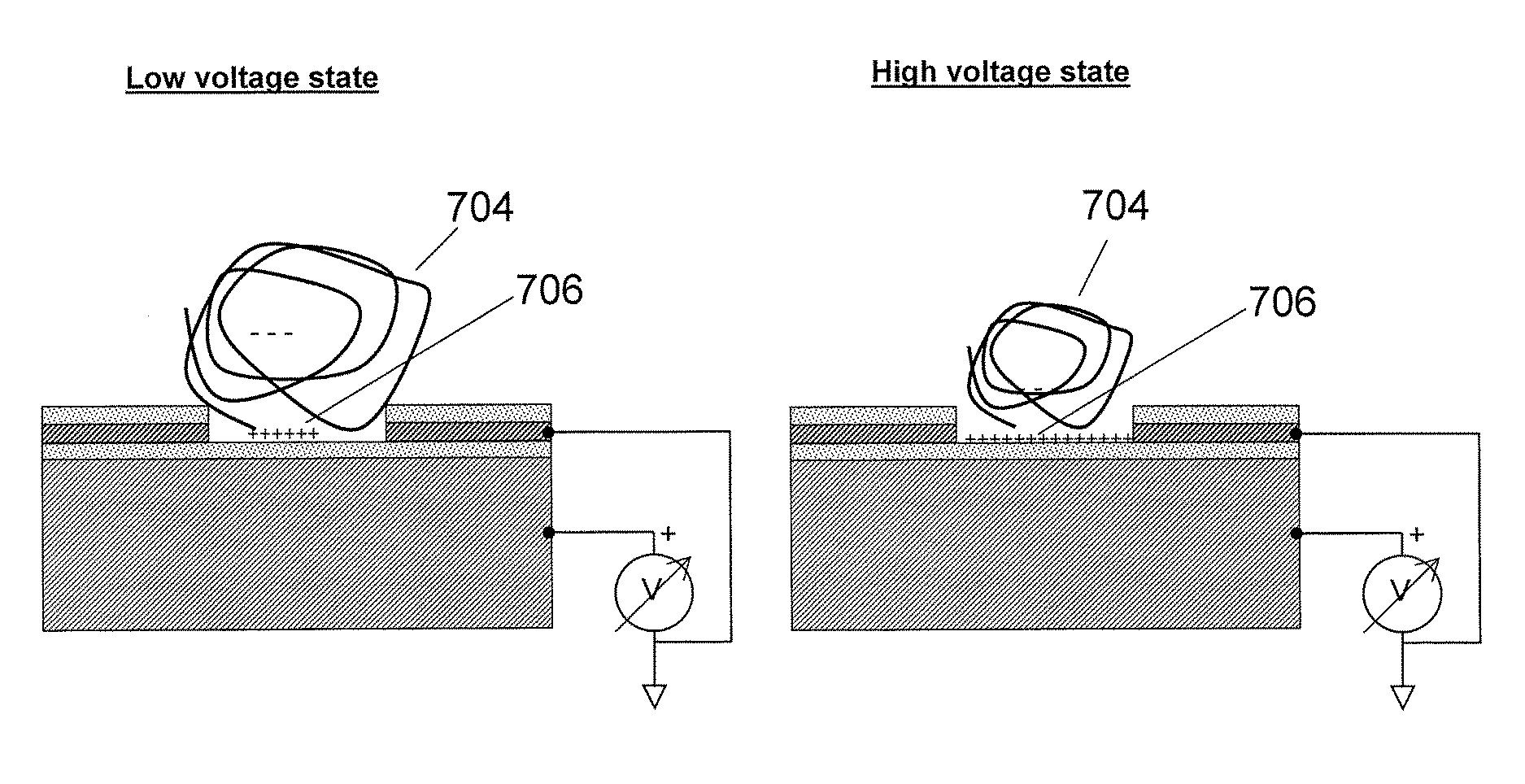
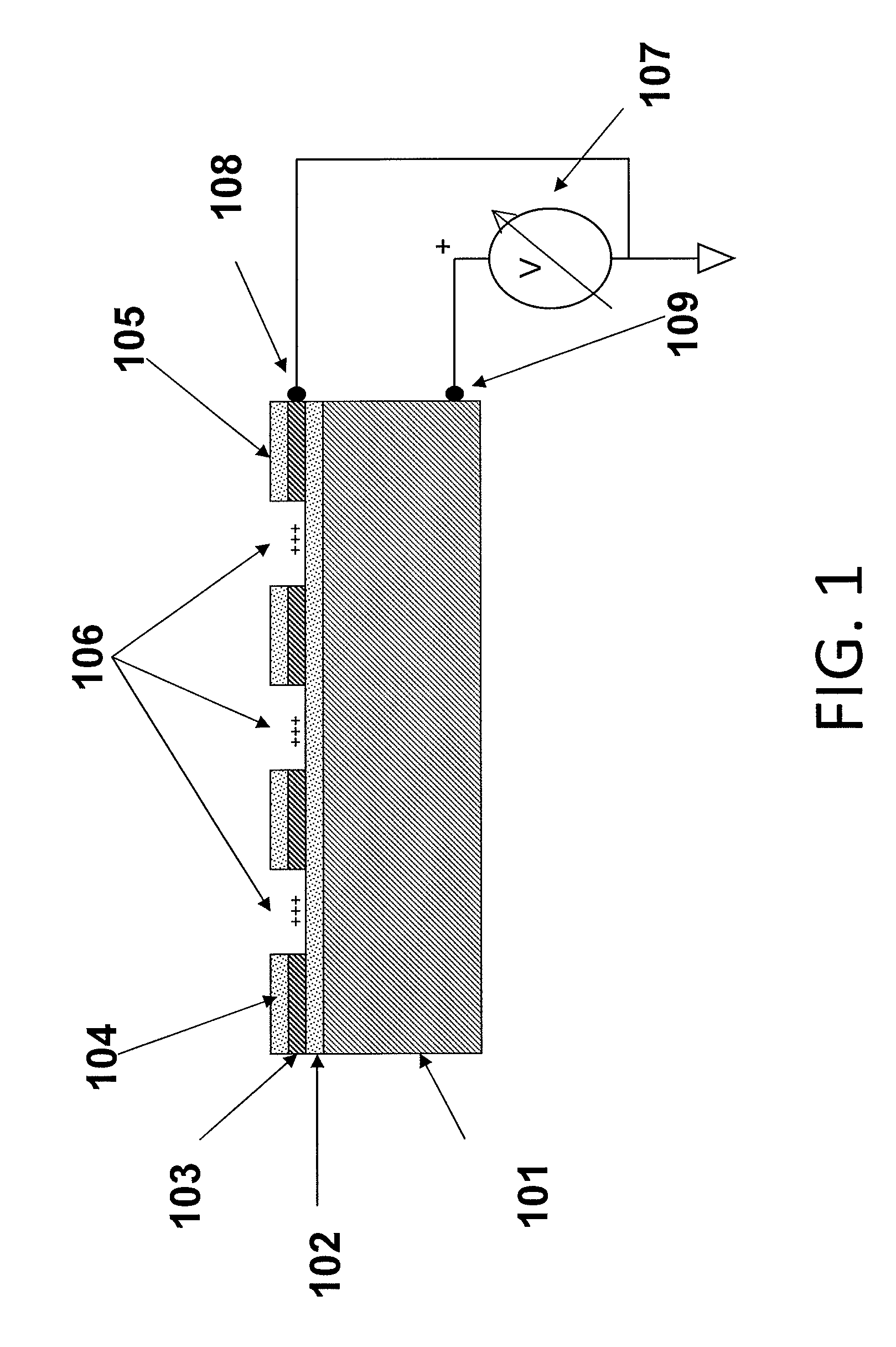
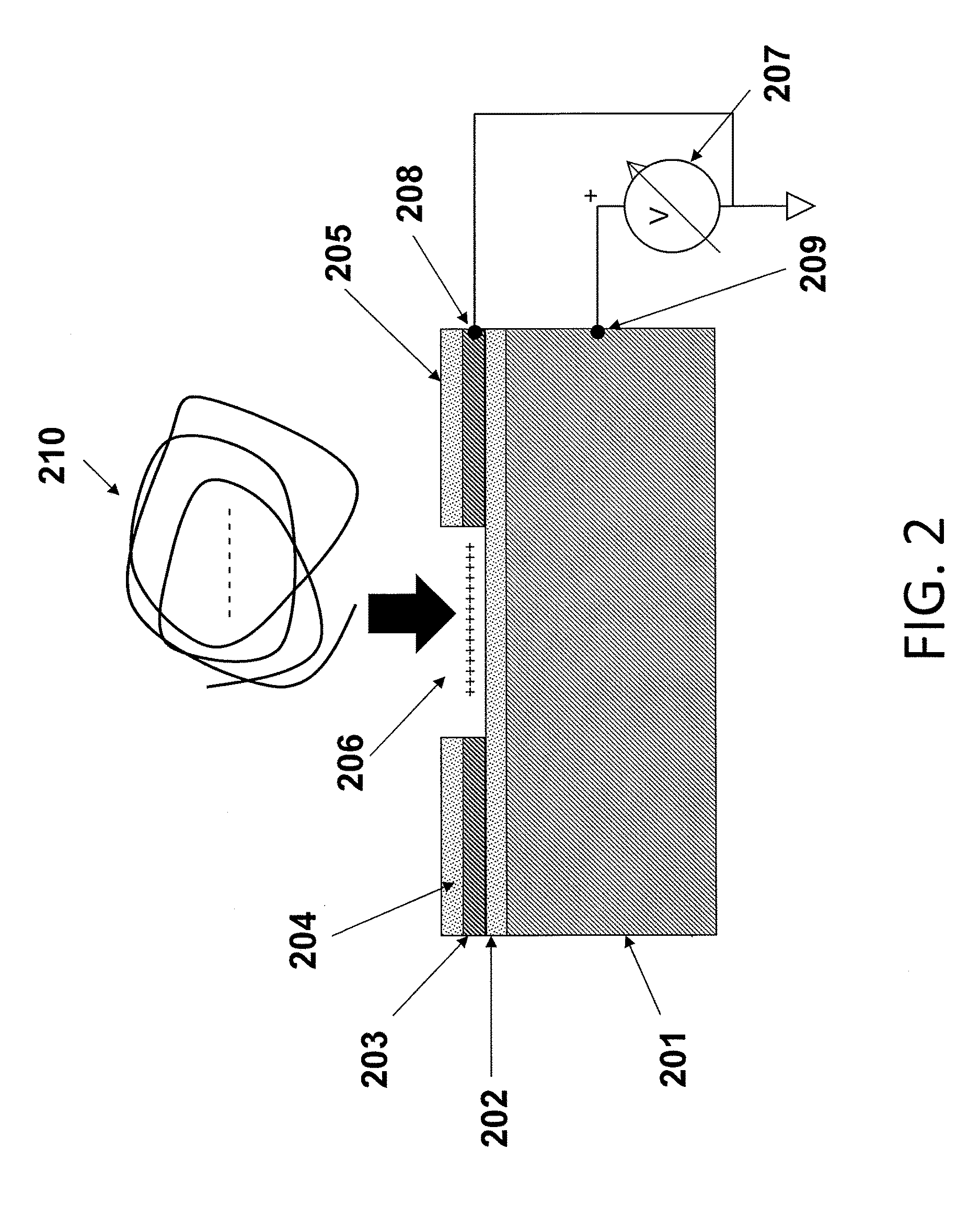
Luminescence test measurements are conducted using an assay module having integrated electrodes with a reader apparatus adapted to receive assay modules, induce luminescence, preferably electrode induced luminescence, in the wells or assay regions of the assay modules and measure the induced luminescence.
Owner:MESO SCALE TECH LLC
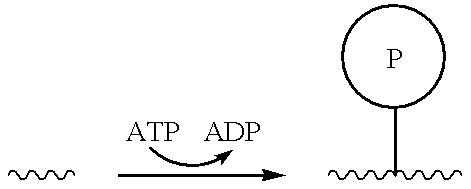
Molecular modification assays
InactiveUS7070921B2Increase brightnessLow mobilityBioreactor/fermenter combinationsBiological substance pretreatmentsPhosphateEnzyme
Assays for detecting molecular modifications such as phosphate modifications and the presence and / or activity of enzymes and other agents involved in facilitating or otherwise regulating such modifications.
Owner:MOLECULAR DEVICES
Probes and methods for detection of pathogens and antibiotic resistance
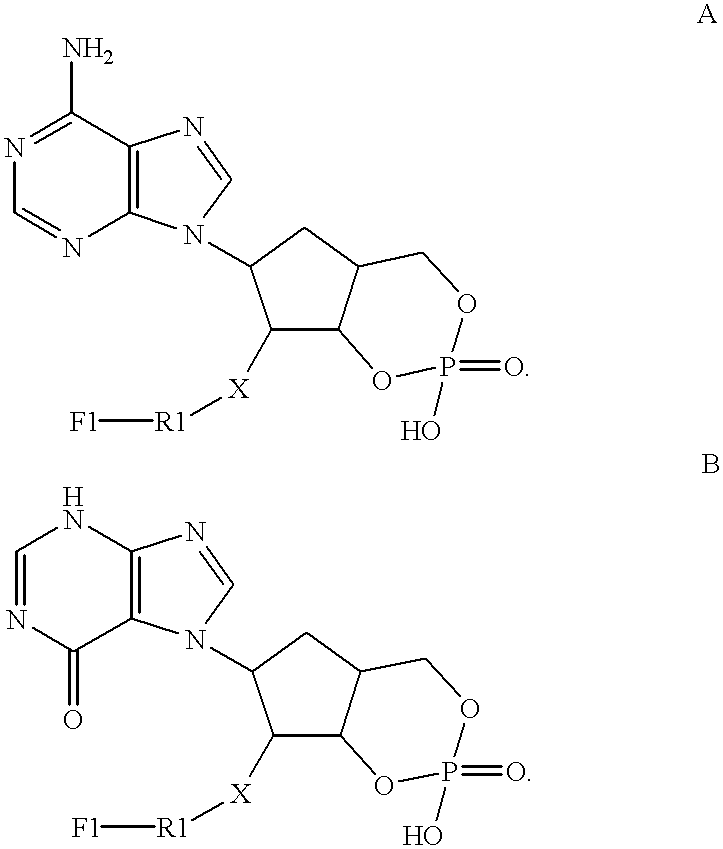
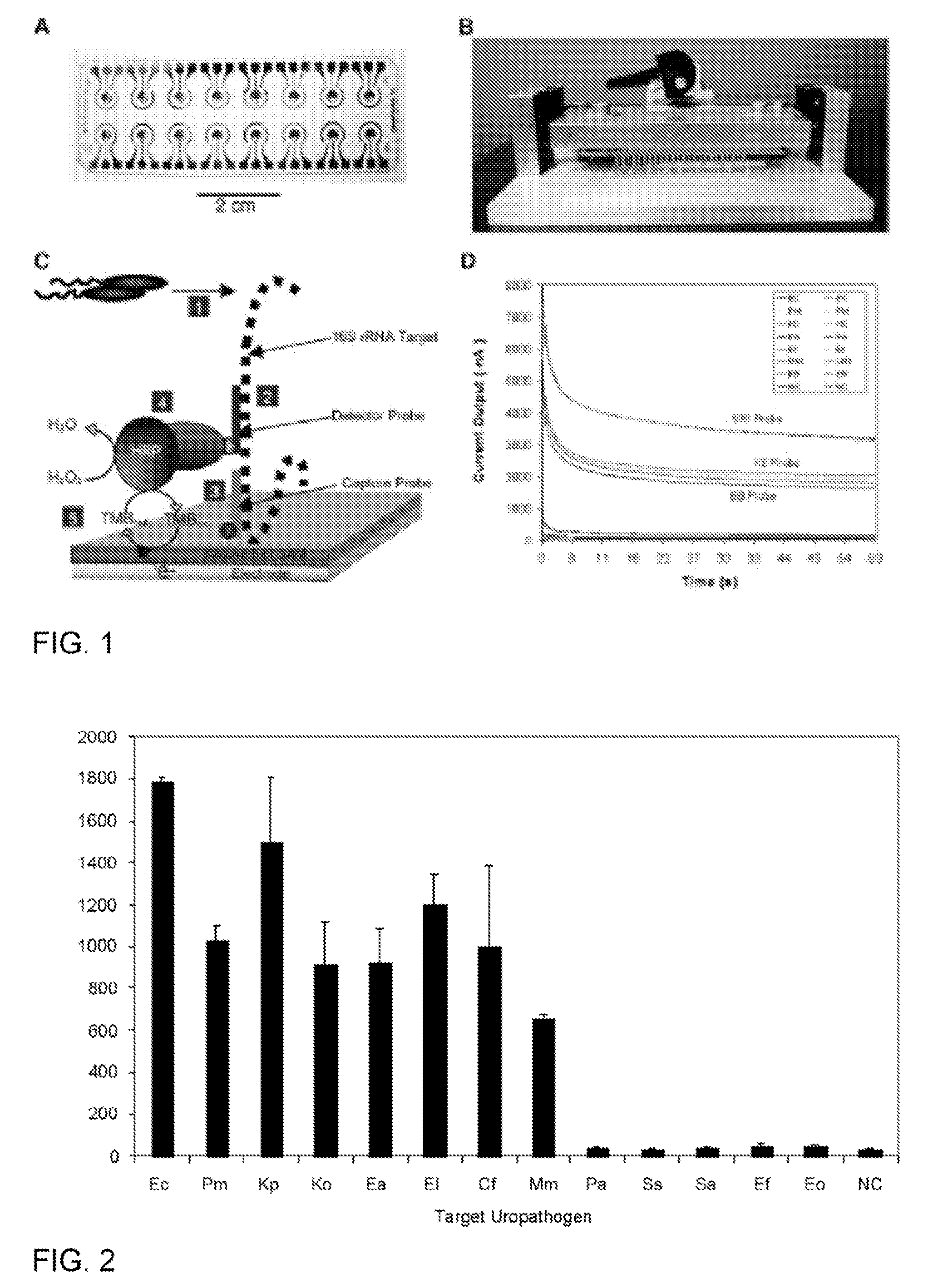
ActiveUS20080199863A1Increase assayRapid detection of antimicrobialSugar derivativesMicrobiological testing/measurementAntibiotic resistanceNucleic acid sequencing
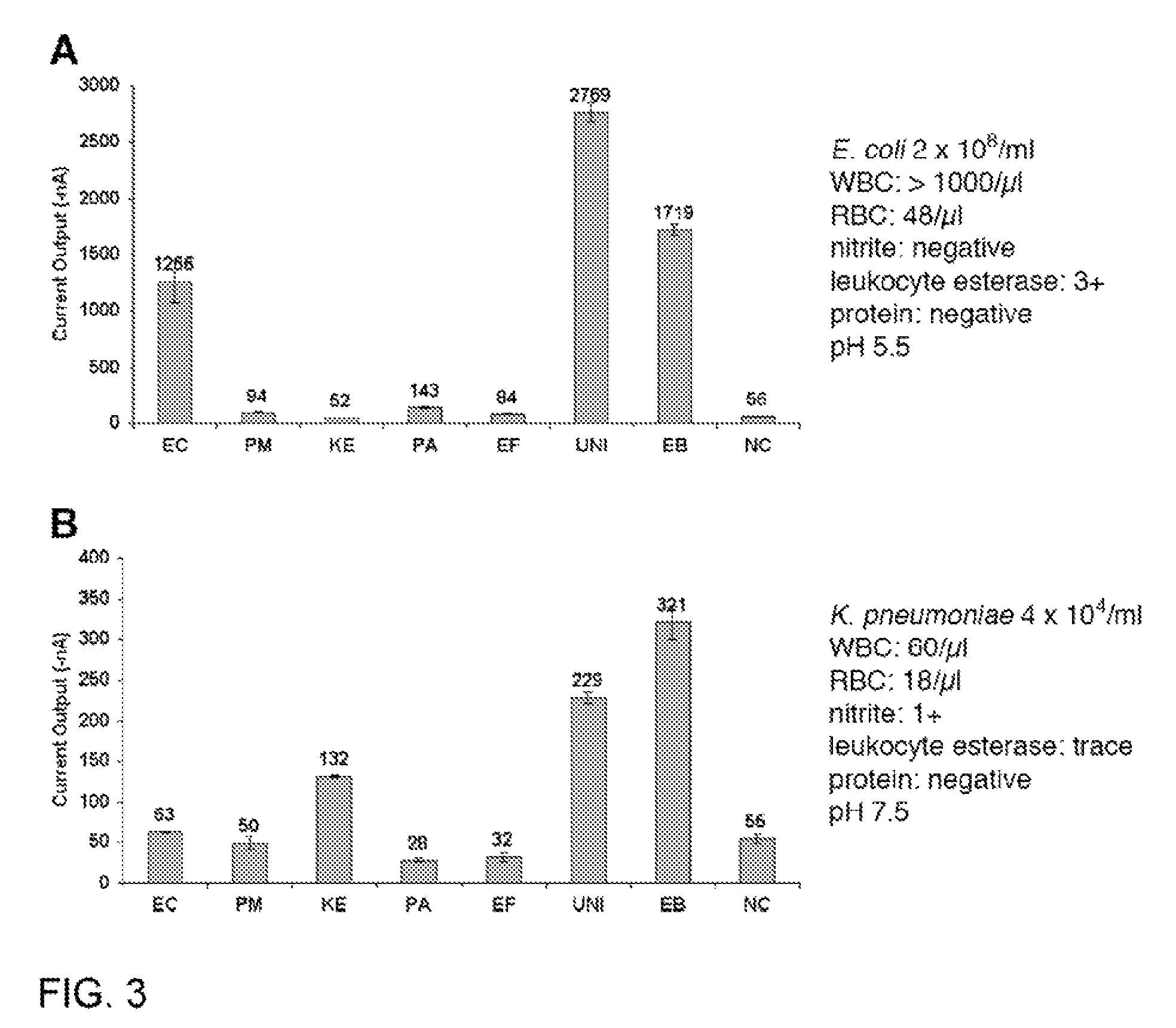
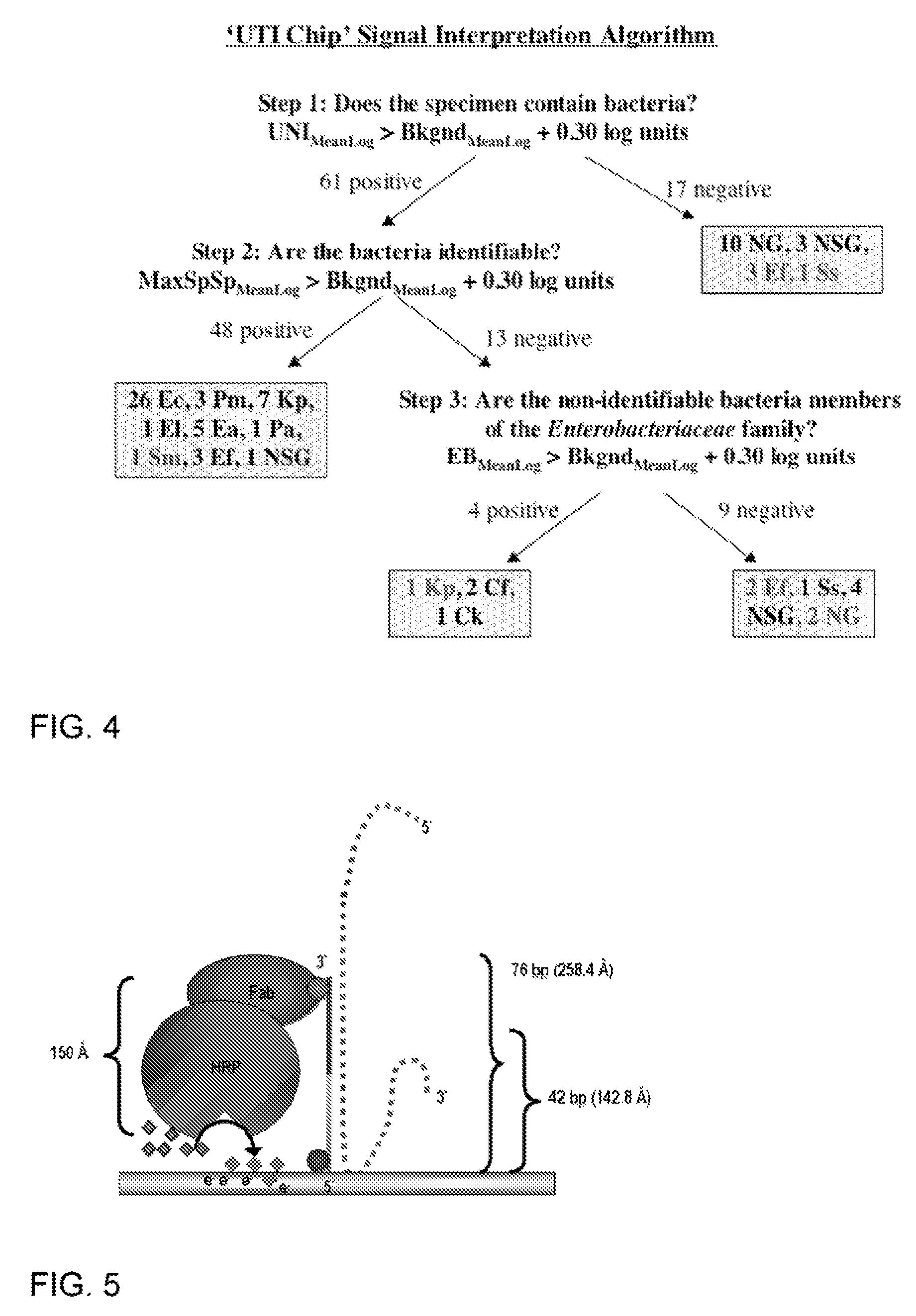
Described are probes and methods for detecting pathogens and antibiotic resistance of a specimen. The method comprises contacting the specimen with a growth medium; and lysing the specimen to release nucleic acid molecules from the specimen. The lysate of the specimen is contacted with a capture probe immobilized on a substrate, wherein the capture probe comprises an oligonucleotide that specifically hybridizes with a first target nucleic acid sequence region of ribosomal RNA. The lysate is in contact with a detector probe that comprises a detectably labeled oligonucleotide that specifically hybridizes with a second target nucleic acid sequence region of ribosomal RNA. The presence or absence of labeled oligonucleotide complexed with the substrate is determined. Detection of labeled oligonucleotide complexed with the substrate is indicative of the presence of pathogen. Performing the method in the presence and absence of an antibiotic permits detection of antibiotic resistance.
Owner:THE GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPT OF VETERANS AFFAIRS +1
Traditional Chinese medicinal composition for treating nephropathy as well as preparation method and detection method thereof
InactiveCN104758515AGood curative effectImprove the effect of the effectComponent separationUrinary disorderAchyranthesSalvia miltiorrhiza
The invention relates to a traditional Chinese medicinal composition for treating nephropathy as well as a preparation method and a detection method thereof. The traditional Chinese medicinal composition is prepared from the following raw materials: rheum officinale, radix pseudostellariae, coptis chinensis, rhizoma pinellinae praeparata, dried orange peel, poria cocos, salvia miltiorrhiza, radix achyranthis bidentatae, safflower and liquorice root by combining water extraction and alcohol extraction, and stability and mass content detection is carried out. According to the invention, the classic formula of existing Shenshuaining is researched and improved, so that the full extraction and effect development of active ingredients in the raw materials are achieved, the relative dosage of patients is reduced, and the preparation method is simple, convenient and easy in operation; in the detection method, the content determination of the rheum officinale which serves as a monarch drug and the salvia miltiorrhiza which functions as a ministerial drug is added, high performance liquid chromatography for the simultaneous determination of the contents of active ingredients of the rheum officinale and the salvia miltiorrhiza is established, and the detection method can be used for the convenient and efficient qualitative and quantitative determination of the contents of active ingredients in the traditional Chinese medicinal composition for treating nephropathy, so as to objectively, comprehensively and accurately evaluate the mass of Shenshuaining-like drugs; and the detection method has significance in controlling the quality of the Shenshuaining-like drugs and guaranteeing the curative effect.
Owner:云南雷允上理想药业有限公司
Probes and methods for detection of Escherichia coli and antibiotic resistance
ActiveUS7763426B2Rapid and species-specific detectionRapid detection of antimicrobialSugar derivativesMicrobiological testing/measurementEscherichia coliAntibiotic resistance
Described are probes and methods for detecting pathogens and antibiotic resistance of a specimen. The method comprises contacting the specimen with a growth medium; and lysing the specimen to release nucleic acid molecules from the specimen. The lysate of the specimen is contacted with a capture probe immobilized on a substrate, wherein the capture probe comprises an oligonucleotide that specifically hybridizes with a first target nucleic acid sequence region of ribosomal RNA. The lysate is in contact with a detector probe that comprises a detectably labeled oligonucleotide that specifically hybridizes with a second target nucleic acid sequence region of ribosomal RNA. The presence or absence of labeled oligonucleotide complexed with the substrate is determined. Detection of labeled oligonucleotide complexed with the substrate is indicative of the presence of pathogen. Performing the method in the presence and absence of an antibiotic permits detection of antibiotic resistance.
Owner:U S GOVERNMENT REPRESENTED BY THE DEPT OF VETERANS AFFAIRS +1
Traditional Chinese medicine for treating acute and chronic gastroenteritis and bacterial dysentery and its processing technology
InactiveCN101007071AWeight increaseReduce weightAntibacterial agentsDigestive systemGastritisChinese knotweed
The invention relates to a process for preparing Chinese medicinal composition for the treatment of chronic enteritis, gastritis and bacillary dysentery, which is prepared from Chinese herbal materials including hairy euphorbia, Chinese knotweed and iron holly bark through steps of grinding, grilling, merging grilling liquid, concentrating into electuary, drying and granulating.
Owner:刘晓
Detection method of Guwei collaterals-activating tincture
ActiveCN101623469AImprove monitoring levelRaise the level of monitoringAnthropod material medical ingredientsHydroxy compound active ingredientsDrugMedical department
The invention discloses a detection method of Guwei collaterals-activating tincture. A method for effectively detecting main drugs of the Guwei collaterals-activating tincture is added in the prior production process so that the main drug components of the finished production of the Guwei collaterals-activating tincture can be ensured relatively, thereby the monitoring level of the product quality is greatly improved. The invention is not only convenient for the product monitoring of manufacturers and management departments, but also can provide better guarantees for medical departments and the treatment of patients.
Owner:GUIZHOU SHENGSHI LONGFANG PHARMA
Mass detection method of Kesuting syrup
ActiveCN101664509AIncrease the limit of ephedra contentImproved quality controlComponent separationPharmaceutical delivery mechanismQuality standardEphedra herb
The invention discloses a mass detection method of Kesuting syrup, which improves TLC identification to white mulberry root-bark and pink reineckea herb on the basis of the existing quality standard,increases the content limitation of morphine in poppy shell, and enhances the content limitation of ephedra herb, thus leading the level of monitoring the quality of Kesuting syrup to be greatly improved. The application of the mass detection method not only is more beneficial to the monitoring on products by manufacturers and regulatory authorities, but also provides better guarantee for treatment of medical authorities and patients.
Owner:GUIZHOU BAILING GRP PARMACEUTIAL CO LTD
Technology for processing coastal glehnia root medicinal materials in planting and producing area and quality standard
InactiveCN102027855AIncrease assayImprove efficacyAntipyreticComponent separationChemical synthesisBud
The invention discloses technology for processing coastal glehnia root medicinal materials in a planting and producing area and a quality standard. The technology comprises the following steps of: natural environment and planting field selection, seed treatment, sowing and seedling, field management, pest prevention and control, harvesting, processing and quality control standard. The producing area with an atmosphere environment and a soil environment both reaching a good agricultural practice (GAP) standard is selected in the sowing step; in the production management process, any harmful synthetic chemical substance is not used or a limited amount of appointed synthetic chemical substance is allowed; the production and processing are performed according to a production operating procedure worked out by GAP requirements; check and detection are performed; sandy or semi-sandy soil with loose and fertile soil texture is selected; if seeds are sowed in spring, the seeds are required to be stored in sand at low temperature; in the field management step, the water content of soil is controlled and reasonable relative humidity is kept; buds are picked timely; the pest prevention and control work is well performed; after harvesting, the coastal glehnia root medicinal material which is peeled or not peeled is processed timely, wherein the medicinal effect is better if the coastal glehnia root medicinal material is not peeled; and fingerprint and content measurement is added in the quality control, so the quality control standard is raised.
Owner:INNER MONGOLIA TIANQI HAN&MONGOLIA PHARMA CO
Method for nucleic acid detection using voltage enhancement
ActiveUS20120122721A1Easy to analyzeAid removalMicrobiological testing/measurementLibrary screeningNucleic acid detectionAttachment site
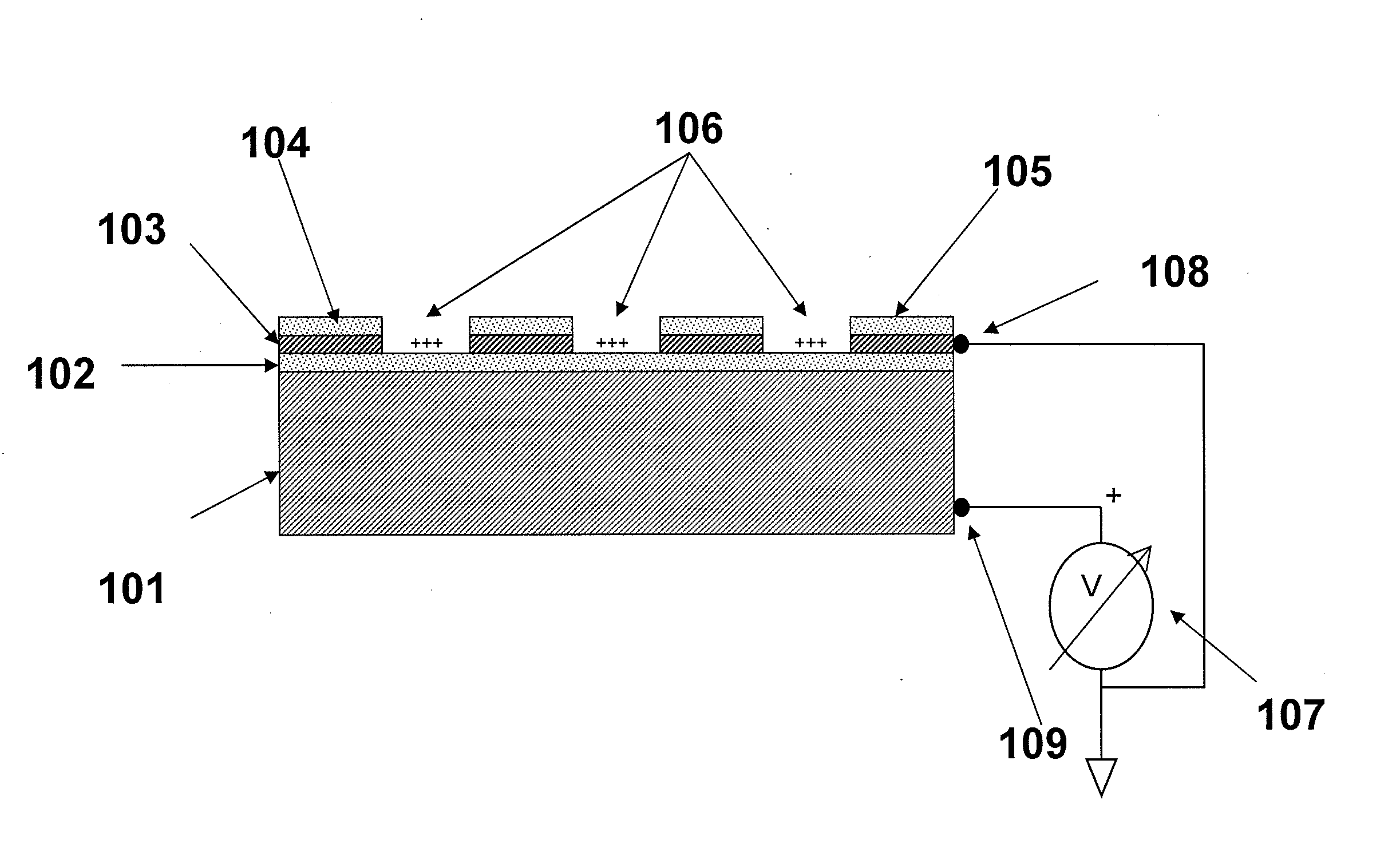
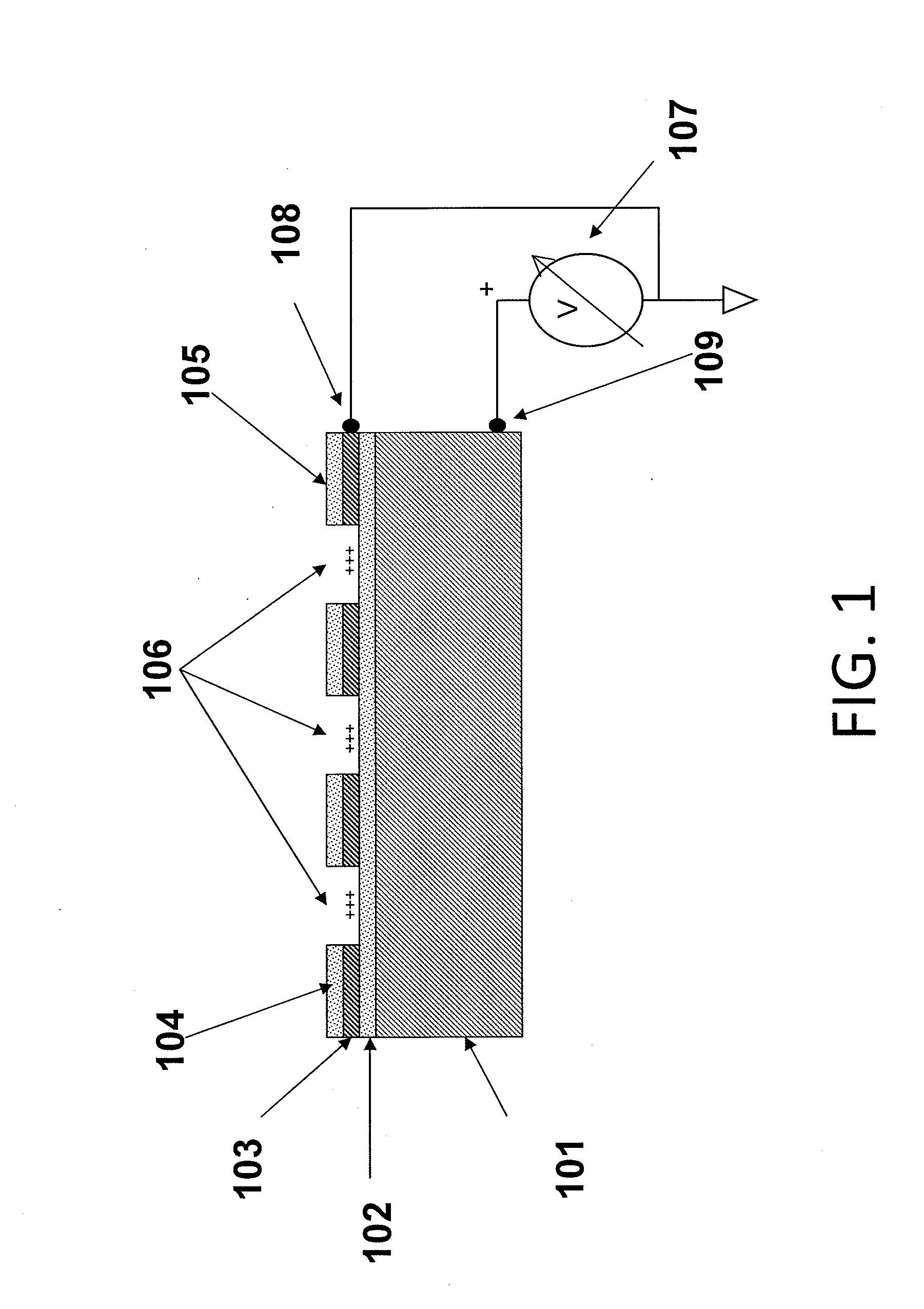
Methods are provided for carrying out nucleic acid analysis, including sequence identification, employing voltage and / or controlled electric charge to enhance operation. A device comprises substrates for nucleic acid analysis, a first electrically conductive layer, a first electrically insulative layer of dielectric material on the first conductive layer, a second electrically conductive layer disposed upon the first insulative layer in a pattern to define discrete attachment sites for macromolecules on the first insulative layer, the second conductive layer provided with means for resisting affinity for the macromolecules to impede their attachment to sites on the second conductive layer, and terminals for the first and second conductive layers for applying a voltage pattern between the first and the second conductive layers to control affinity between the macromolecules and the discrete attachment sites.
Owner:COMPLETE GENOMICS INC
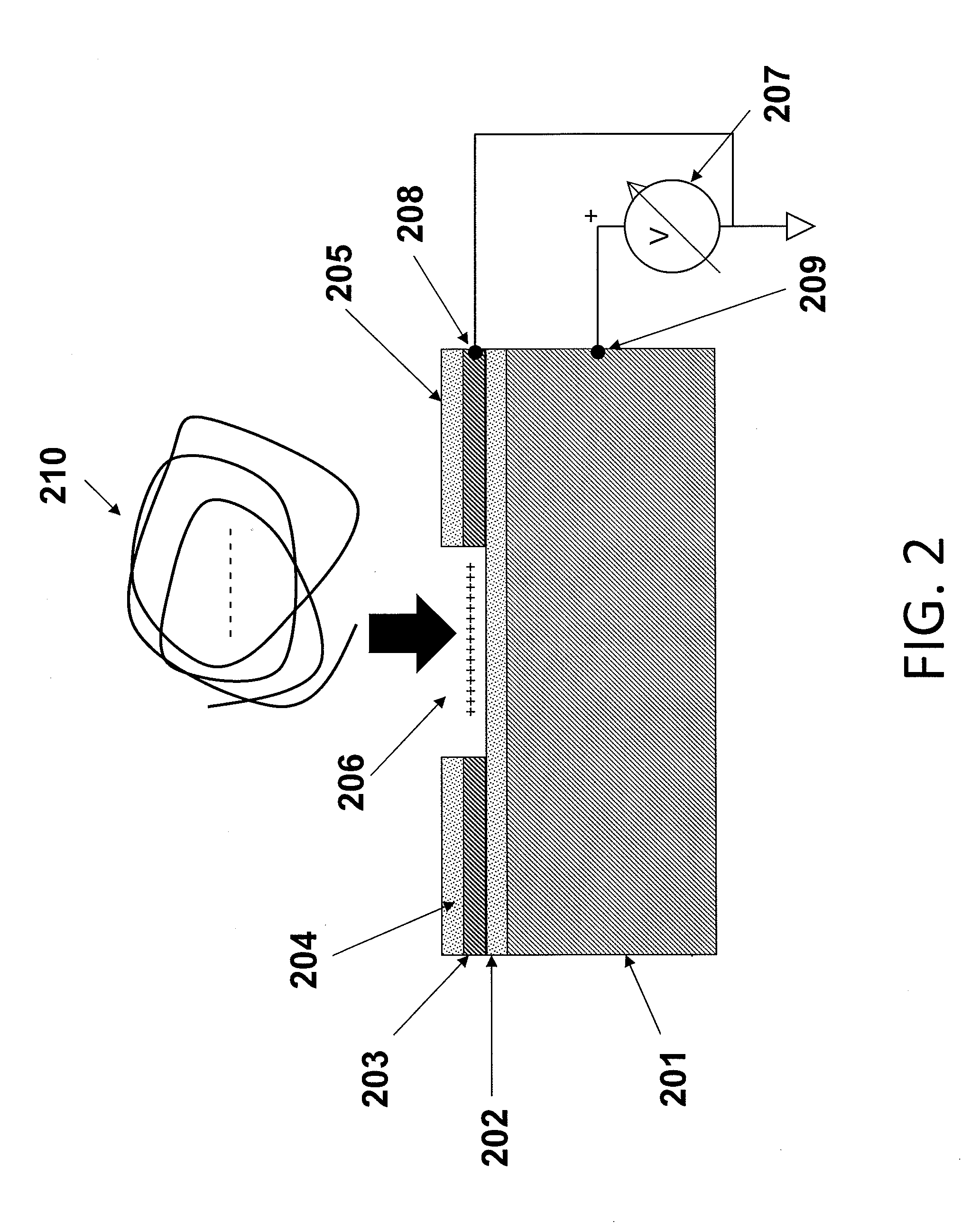
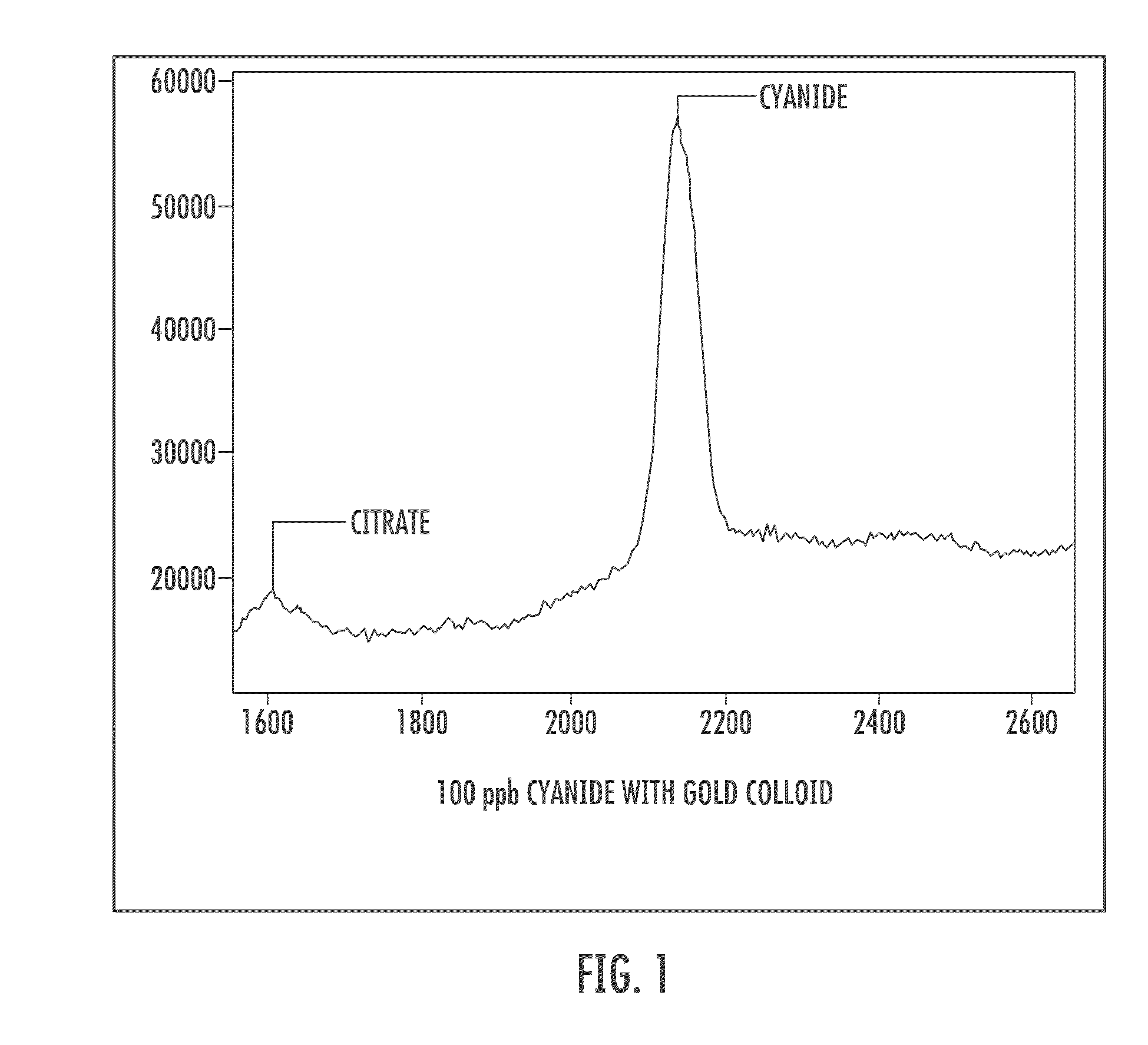
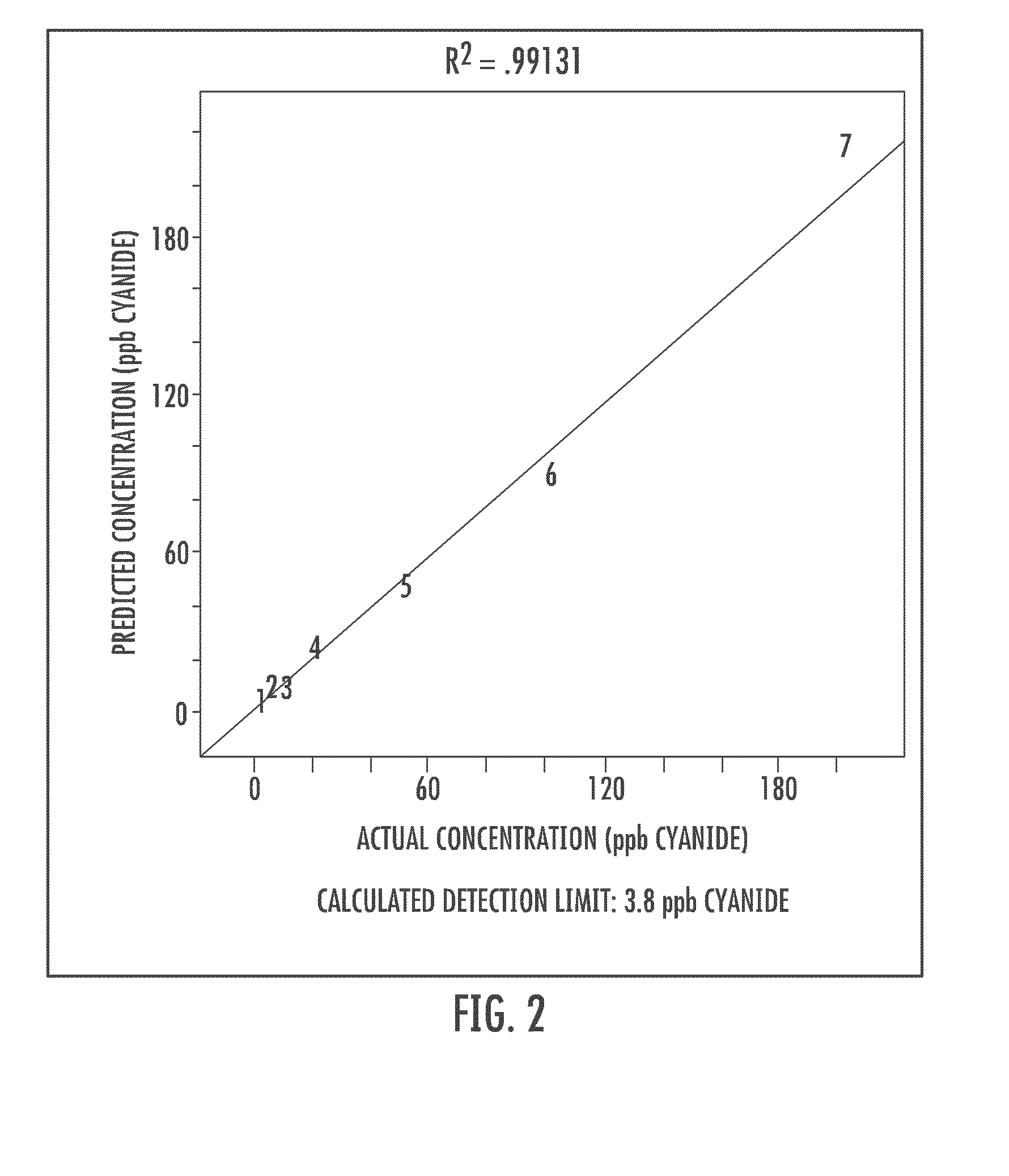
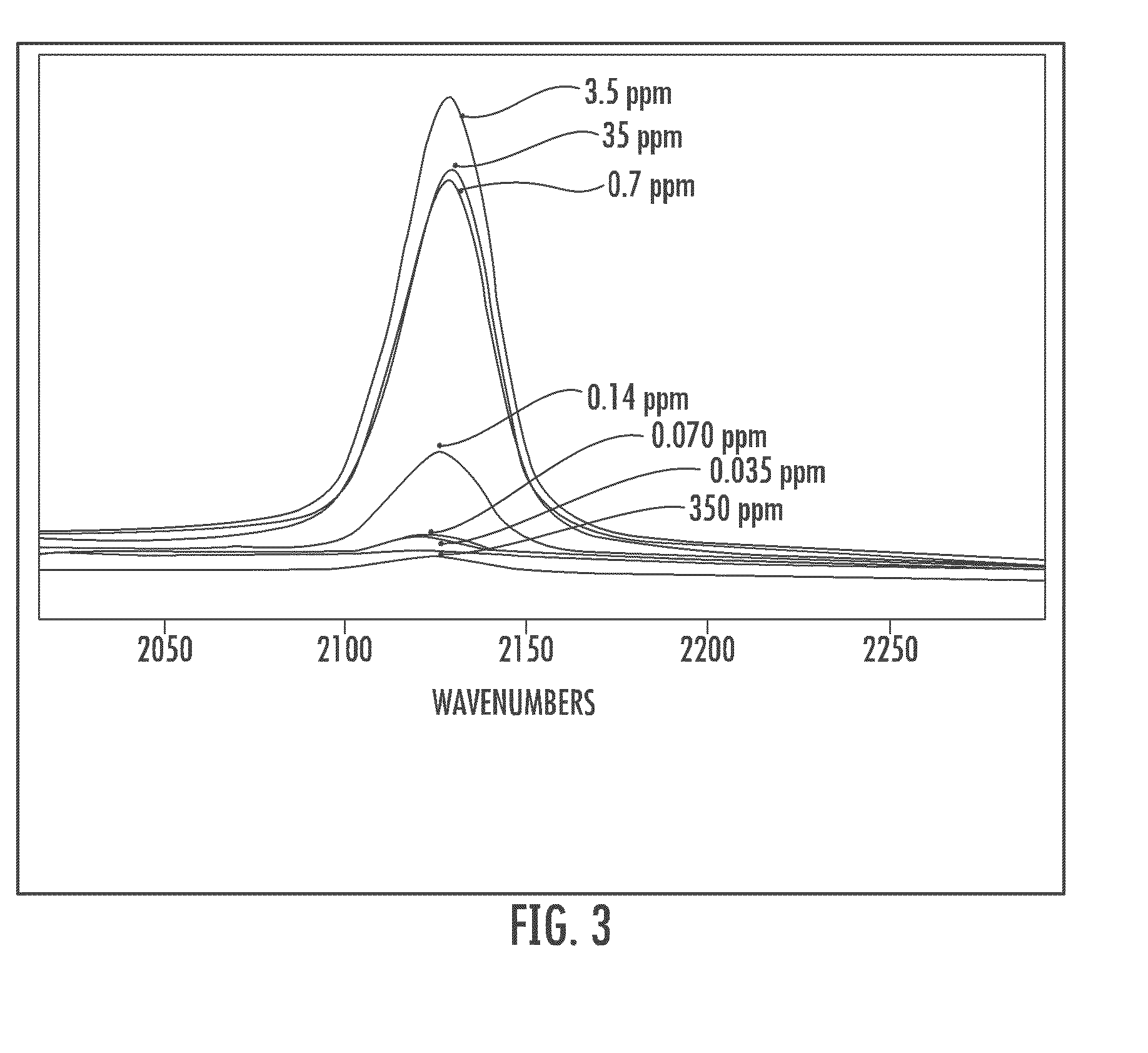
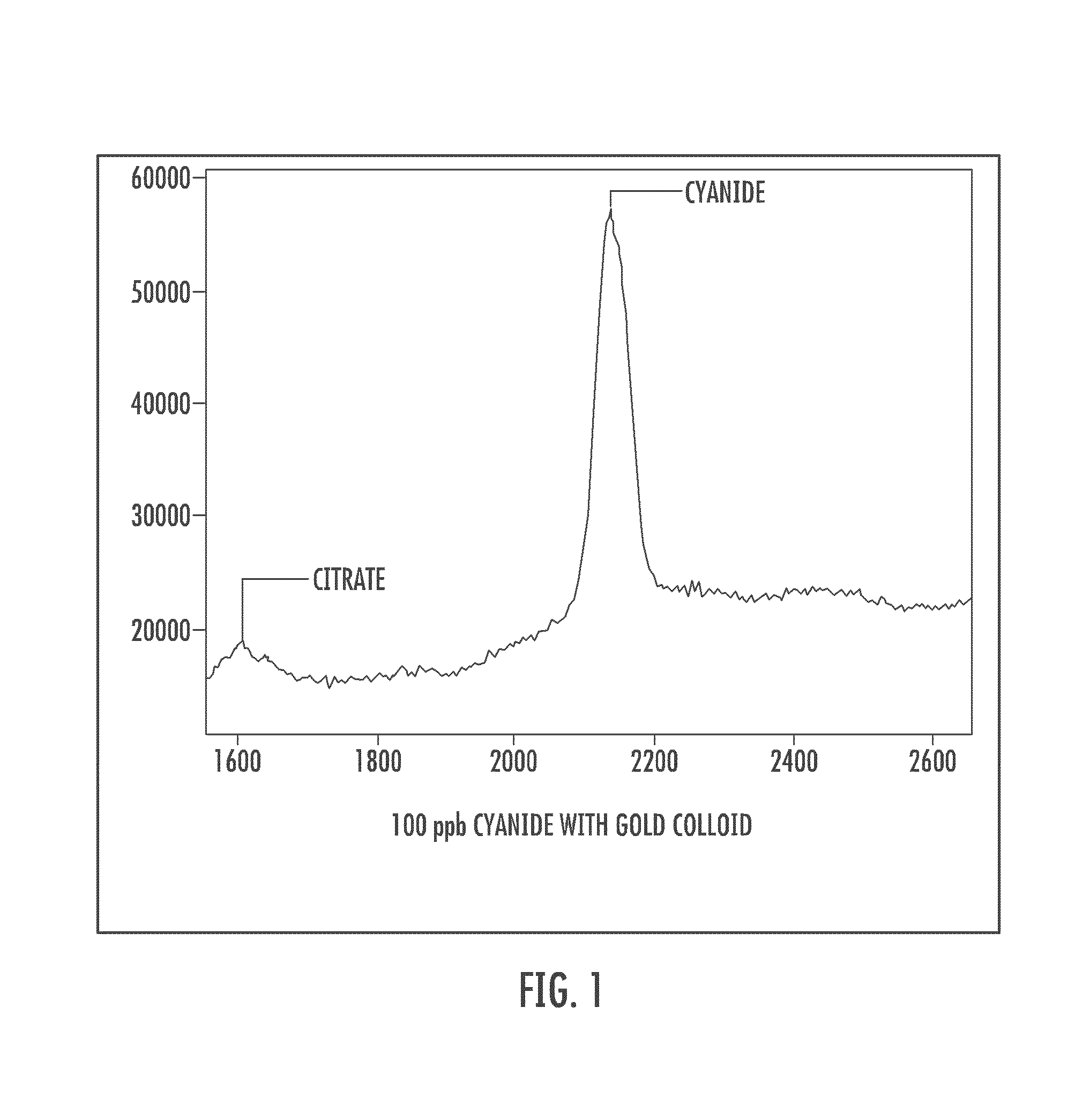
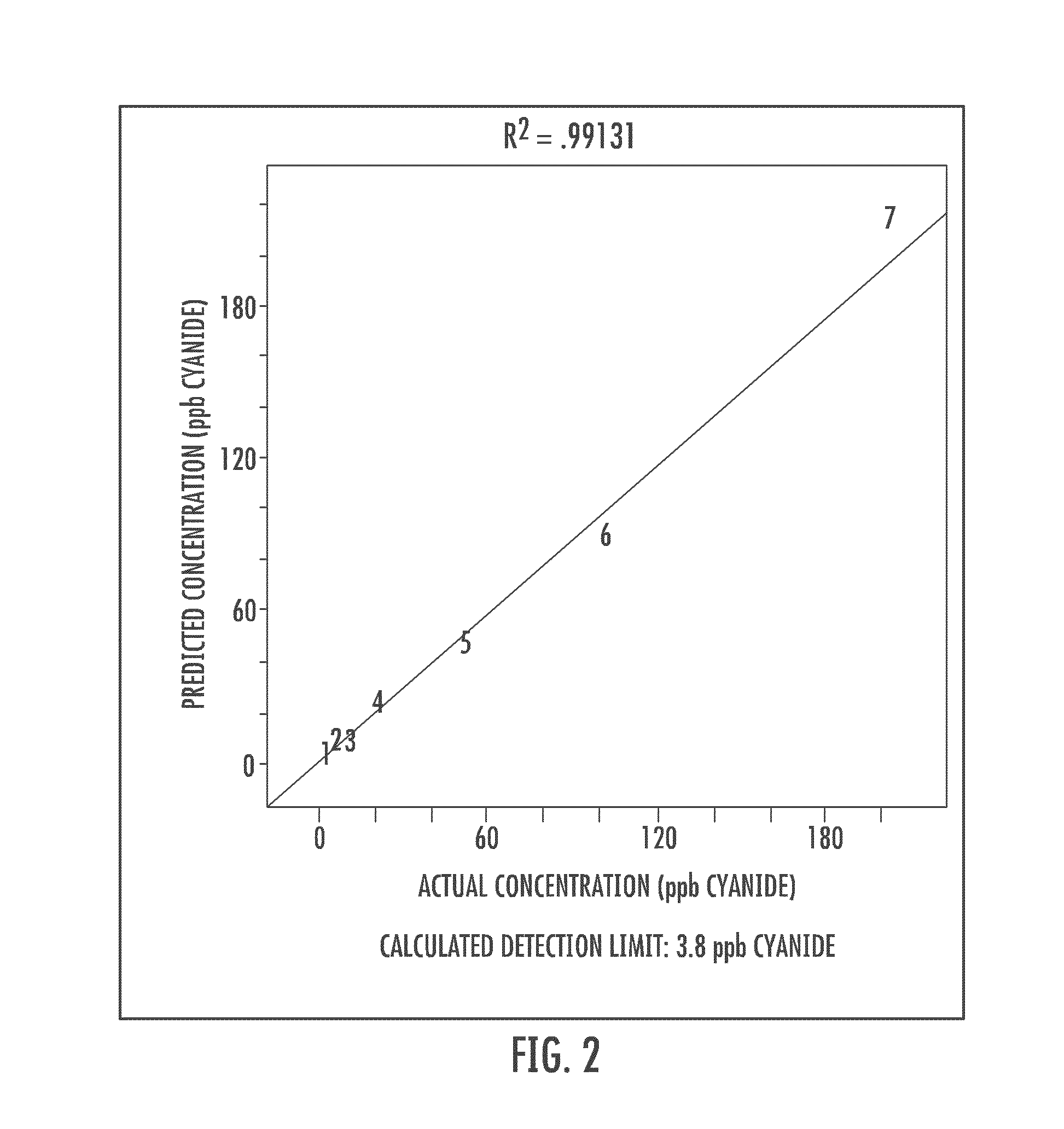
Cyanide and related species detection with metal surfaces
InactiveUS20100291701A1Enhanced signalStrong Raman signalAnalysis using chemical indicatorsMicrobiological testing/measurementCyanideNanoparticle
An assay method and kit for detecting a chemical. The method and kit utilize a metal surface capable of surface enhanced Raman Scattering. The metal surface may be provided in the form of one or more nanoparticles, to increase the surface enhanced Raman Scattering capability of the metal surface. The nanoparticles may be treated with one or more additives to further enhance or maintain the surface enhanced Raman Scattering capability of the nanoparticles.
Owner:UNIVERSITY OF WYOMING
Preparation of Chinese medicine oral preparation
ActiveCN101264154AReasonable workmanshipImprove formulation qualitySkeletal disorderPharmaceutical non-active ingredientsDiseaseSilica gel
The invention relates to a preparation method for a traditional Chinese medicine oral preparation with traditional Chinese medicines as main materials. One, two or three raw medicines of the medicine composites are shattered and sifted out; the other non-shattering raw medicines are grouped or not grouped, decocted and extracted with water, concentrated and refined; the shattering and sifting rawmedicine powder is used as basic charge by added in one or two components of proper starch, dextrine and micro silica gel, and water extract is injected for a one-step granulation for troche, capsule, pill, granular formulation and other formulations. The invention has a feasible technology, decreases the impurity in the extract and the loss of the active ingredient in the extraction process, thegranulation is more convenient, the grain is more uniform, the subsequent working procedures of tabletting and enpsulation are easier; the prepared traditional Chinese medicine compound preparation for curing the gout disease has the advantages of high preparation quality, low clinical dose and other advantages; each index of the product is fully qualified, and the quality of the product is improved greatly.
Owner:SICHUAN SUNNYHOPE PHARM CO LTD
Extraction method for hermetia illucens antibacterial peptide and application of hermetia illucens antibacterial peptide in preparation of fertilizer used for resisting tobacco diseases
ActiveCN111334547AImprove agronomic traitsImprove economyBacteriaAlkali orthophosphate fertiliserBiotechnologyNicotiana tabacum
The invention belongs to the field of fertilizer preparation, and specifically relates to an extraction method for hermetia illucens antibacterial peptide and an application of the hermetia illucens antibacterial peptide in preparation of a fertilizer used for resisting tobacco diseases. In order to prepare the fertilizer used for resisting the tobacco diseases from the hermetia illucens antibacterial peptide, a composite bacterial liquid composed of a tobacco ralstonia solanacearum bacterial liquid, a tobacco wildfire bacterial liquid and a tobacco angular leaf spot bacterial liquid is used as an induction liquid; hermetia illucens is induced to generate a large amount of antibacterial peptide under acupuncture soaking treatment; the hermetia illucens antibacterial peptide is prepared through an enzymolysis method; and the obtained hermetia illucens antibacterial peptide is high in content and high in activity and has good inhibiting effect on tobacco ralstonia solanacearum, tobacco wildfire pathogenic bacteria and tobacco angular leaf spot pathogenic bacteria. Meanwhile, a foliar fertilizer used for resisting the tobacco diseases is prepared from the hermetia illucens antibacterial peptide, and shows good prevention and treatment effects on tobacco bacterial angular leaf spot, tobacco bacterial wilt and tobacco wild fire. The agronomic traits and quality of the tobacco can beimproved, and the economic value of the tobacco is improved.
Owner:松颉环保科技(深圳)有限公司
Quality control method of pharmaceutical composition for preventing and treating coronary heart disease
InactiveCN101411735APrevention and treatment of anginaPrevention and treatment of chest tightnessHeavy metal active ingredientsHydroxy compound active ingredientsSalvia miltiorrhizaCoronary artery disease
The invention discloses a method for controlling the quality of a medicine composition for preventing coronary heart disease. The medicine composition comprises a composition prepared from bezoar, muskiness, pearl, toad venom, red ginseng, notoginseng, borneol, hyocholalic cream, ochre, concentrated powder of cornu bubali, a salvia miltiorrhiza extract and and so on, and a preparation thereof; the quality control method at least comprises character identification, microscopical identification and physicochemical identification; in addition, the quality control method also comprises the identification of thin-layer chromatography; the optimal quality control method also comprises content measuration, wherein the identification of thin-layer chromatography comprises the identification of thin layer chromatography carried out on any one or several of the borneol, the toad venom and salvia miltiorrhiza; the content measuration is carried out by measuring the content of index compositions of any one or several of the toad venom, the salvia miltiorrhiza, the red ginseng and the notoginseng; and the measuration method is a high-efficient liquid phase chromatography. The quality control method has wide range of quality control, simple and reliable method, easy operation and good sensitivity and repeatability, and can effectively ensure the curative effect and safe medication.
Owner:大连美罗中药厂有限公司
Detection method of capsule for reinforcing kidney and warming yang
InactiveCN103217502AIncrease assayThe identification method is simpleComponent separationValineKidney
The invention discloses a detection method of a capsule for reinforcing a kidney and warming yang. The capsule is prepared by hydrolyzing a sheep placenta by enzyme and adding starch. According to the detection method disclosed by the invention, the determination of the content of lysine is added on the basis of existing detection, so as to improve an identification method of glycine and valine; and main medical components of the capsule are effectively controlled so that the quality monitoring level of the capsule is greatly improved. The detection method is good for the monitoring of product quality by manufacturers and supervision and administration departments, and can also provide good guarantees to the treatment of medical treatment departments and patients.
Owner:ZHEJIANG DADE PHARMACEUTICAL GROUP CO LTD
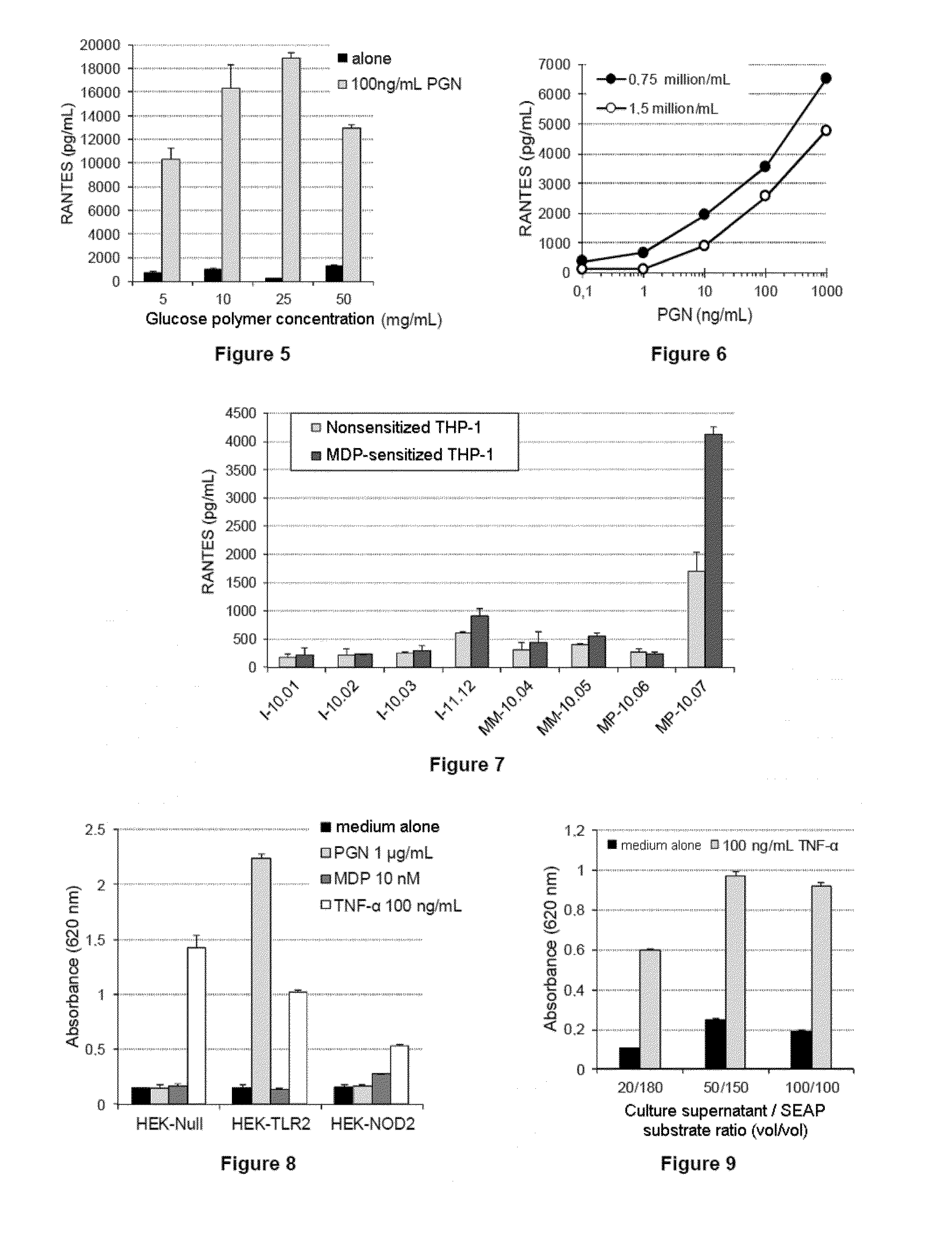
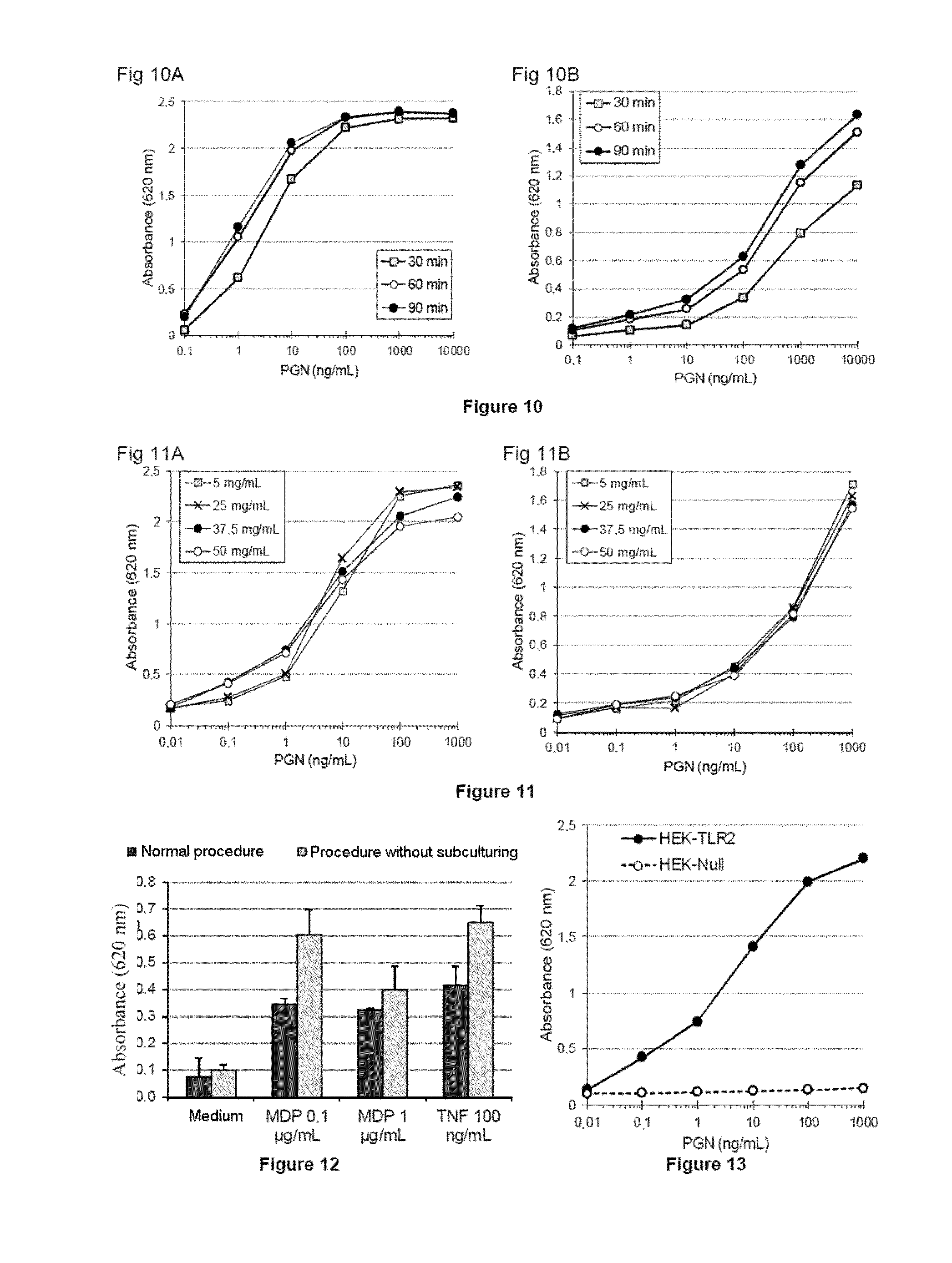
Methods for detecting contaminants in solutions containing glucose polymers
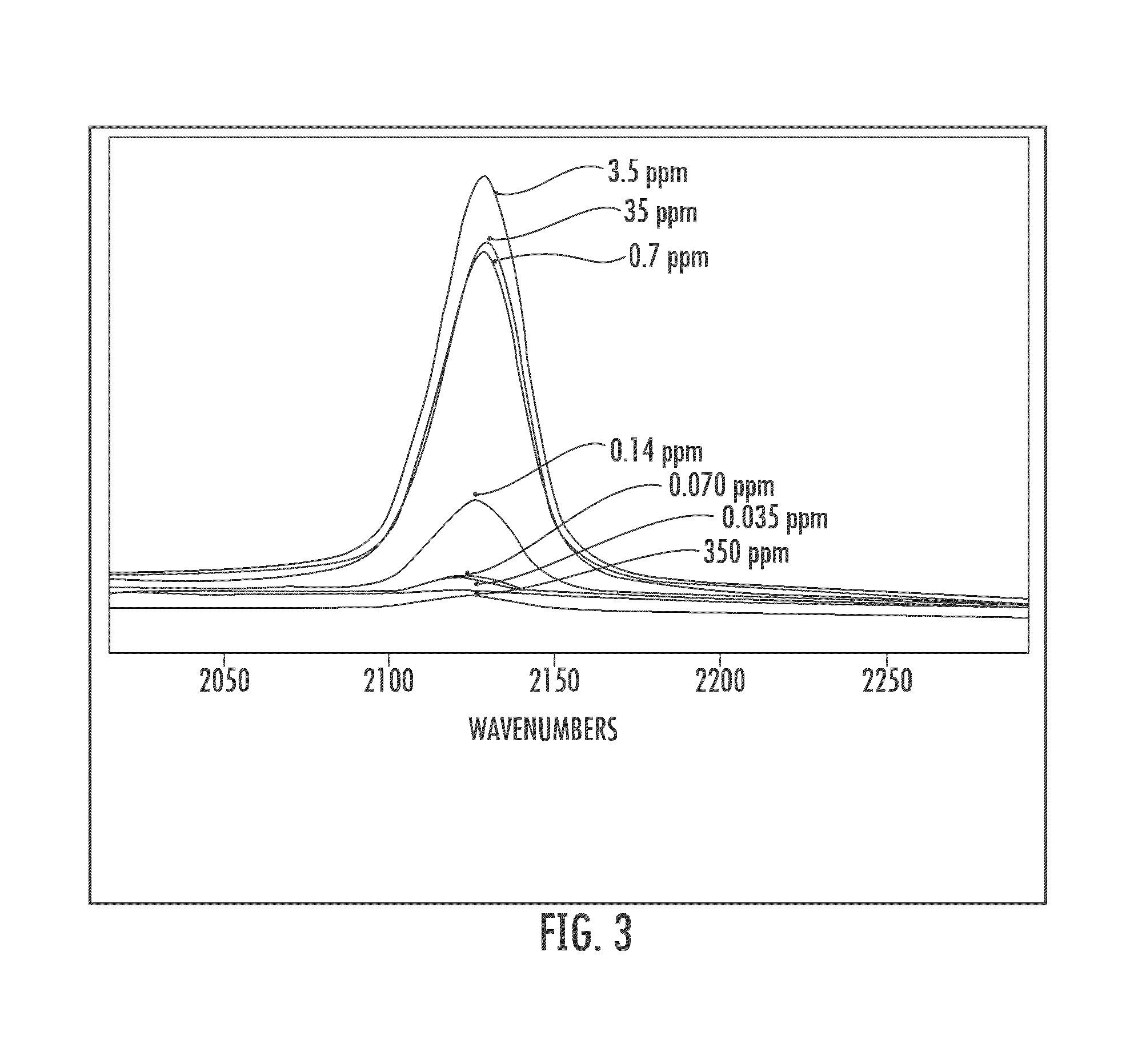
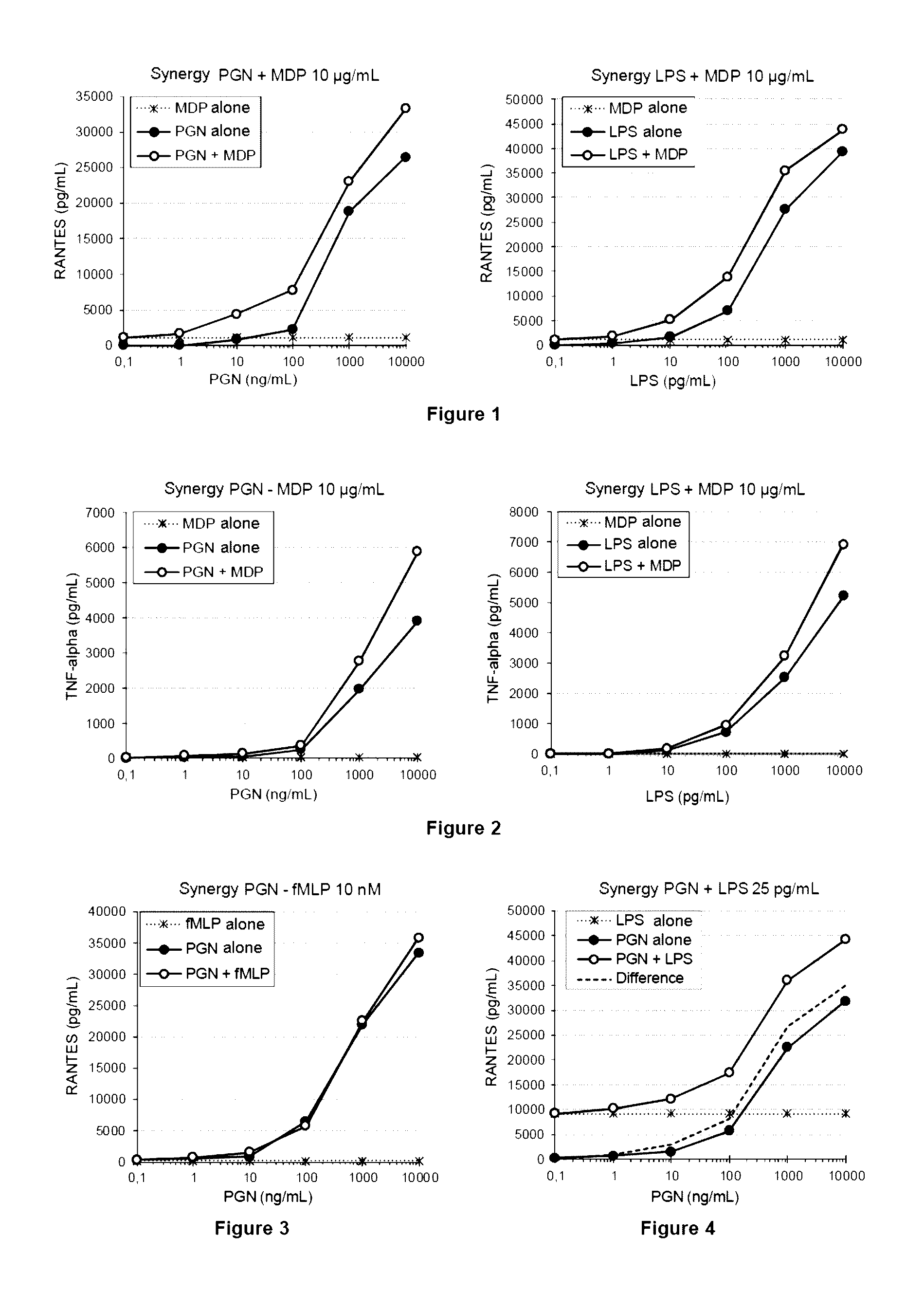
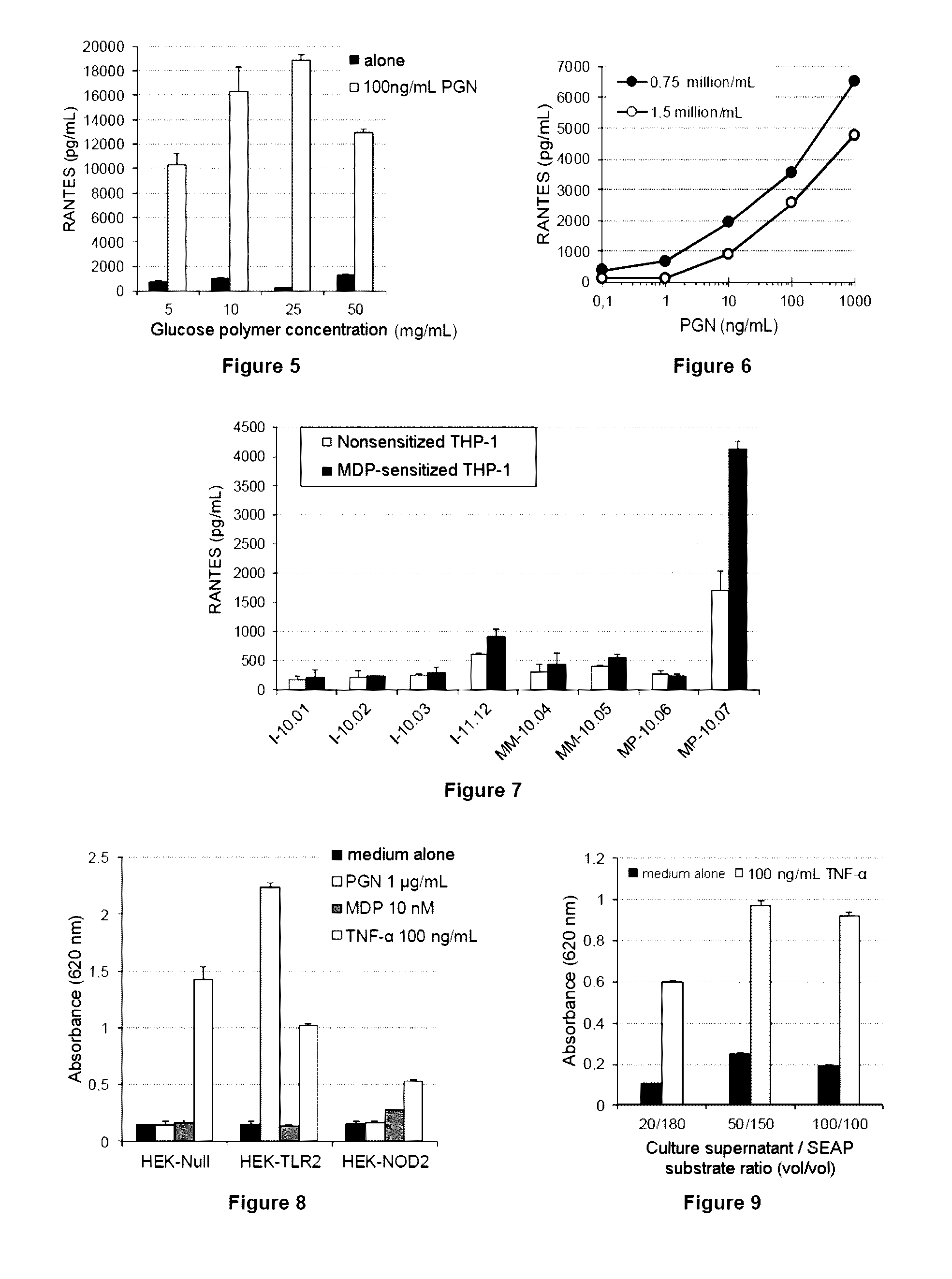
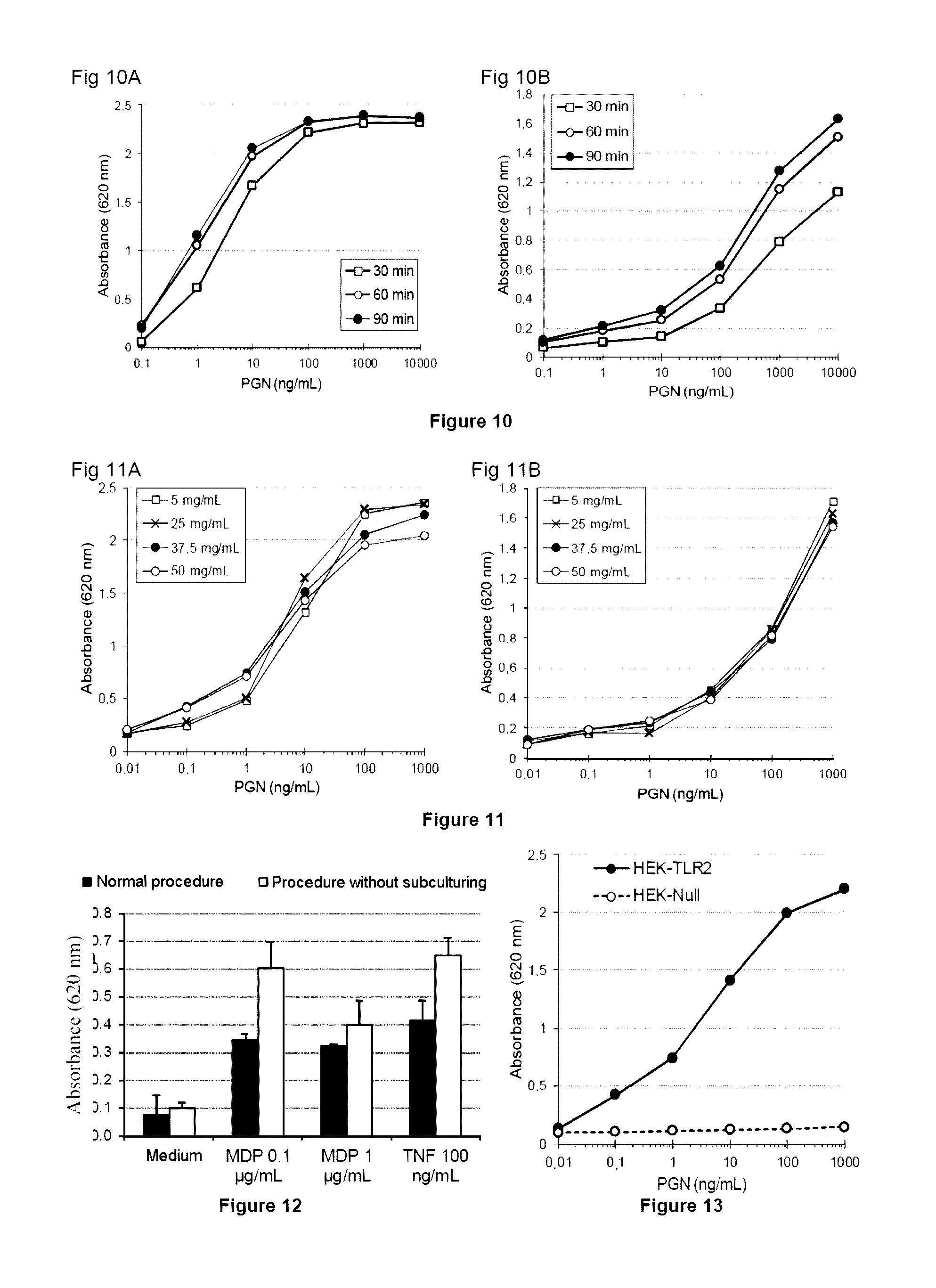
ActiveUS20140051097A1Increase productionIncrease assayMetabolism disorderMicrobiological testing/measurementGlucose polymersGlycopolymer
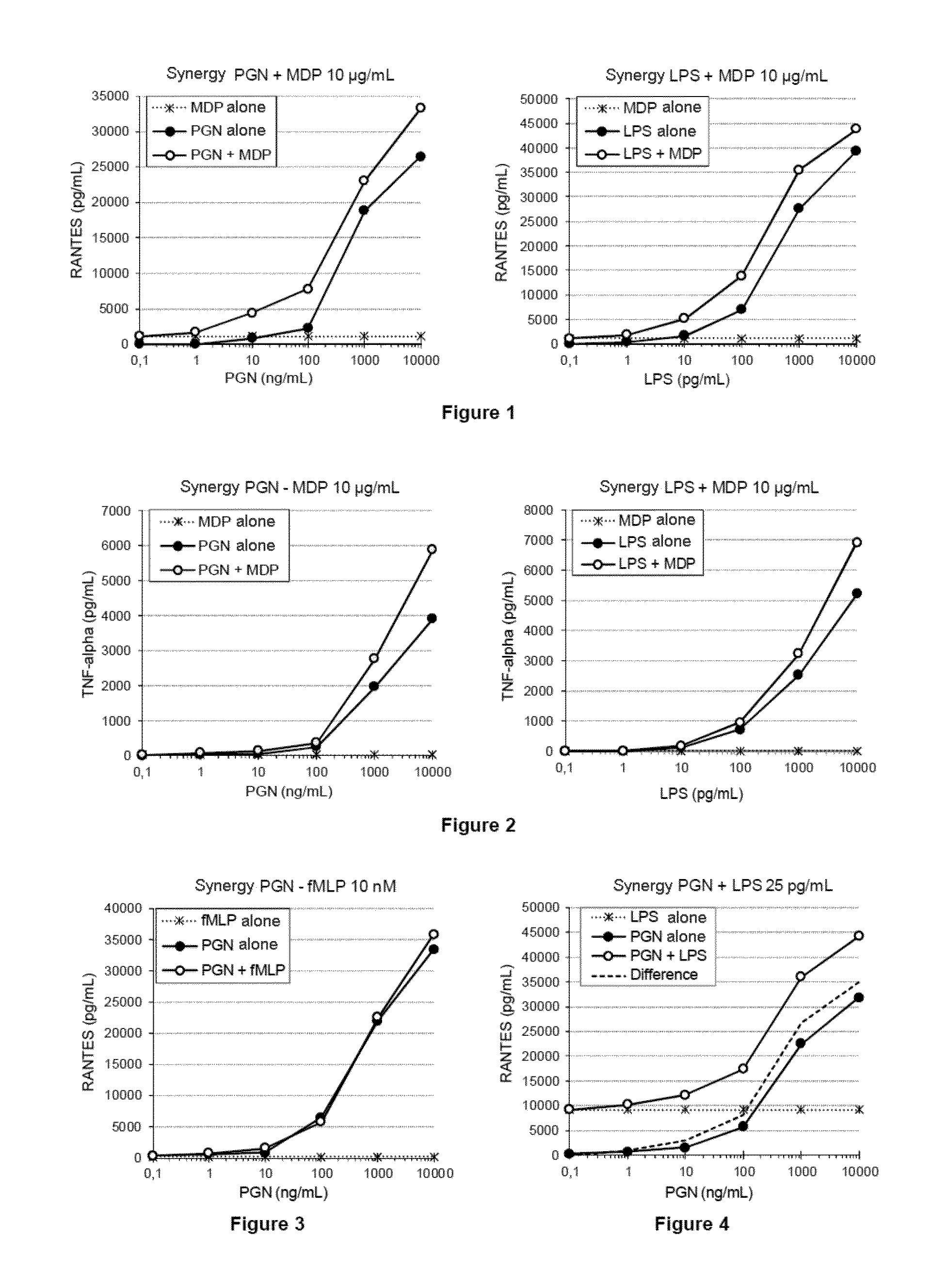
The invention relates to a method for detecting contaminants of glucose polymers, said contaminants being capable of acting in synergy with one another so as to trigger an inflammatory reaction, characterized in that it comprises an in vitro inflammatory response test using modified cell lines.
Owner:ROQUETTE FRERES SA
Quality control method of Shangke bone-knitting medicine
InactiveCN101278976AIncrease assayGuaranteed curative effectHeavy metal active ingredientsAnthropod material medical ingredientsElectrophoresisQuality control
The invention discloses a quality control method for orthopedics-traumatology coaptation. The method adopts TLC to carry out qualitative identification for notoginseng, borneol, safflower and Nux vomica powder and uses electrophoresis for carrying out qualitative identification for starfish. HPLC and GC are respectively adopted to carry out quantitative identification for strychnine and the borneol. Chemical titration is adopted to carry out quantitative identification for mercury sulfide. Therefore, the quality control scope of the orthopedics-traumatology coaptation is enlarged. The quality control method for orthopedics-traumatology coaptation is easy to be practiced and has strong specialization. In addition, the quantitative identification has high precision and good repeatability, thus ensuring that the quality of compound medicine is uniform, stable, effective and controllable.
Owner:大连美罗中药厂有限公司
Detection method for compound Dan Yin paste
ActiveCN107102093AThe detection method is scientific and reasonableThe detection method is feasibleComponent separationClinical efficacySalvianolic acid B
The invention provides a detection method for compound Dan Yin paste. The method includes identification on characters and components, inspection, and content measurement. The invention aims to incomplete detection standards of the compound Dan Yin paste in the prior art, in which there are no any quality detection measures besides detections on common indices according to preparation general rules, so that the detection methods, in the prior art, has defects of monitoring medicine quality. In the method, the following items are added: identification between capillary artemisia and artemisia apiacea, identification between salvia miltiorrhiza and gentiana macrophylla, identification on radix bupleuri and licorice roots, content measurement of tanshinone IIA, and content measurement of salvianolic acid B. The method can effectively detect whether the corresponding medicine raw materials are less than standard contents or are not added due to lawless enterprises or not, and also can detect adulteration of the artemisia apiacea and gentiana macrophylla, which are liable to be confused, thereby preventing mis-use. The method can effectively guarantee the quality of the compound Dan Yin paste, ensures that the compound Dan Yin paste to be safe and effective, can guarantees clinical curative effects of the medicine and protects patient benefits.
Owner:SICHUAN FENGCHUN PHARMA
Multi-component comprehensively quantificational method for assessing and controlling quality of traditional Chinese medicine and application
ActiveCN104897839AEmbody strengthIn line with the development directionComponent separationTesting medicinal preparationsMedicineControl quality
The application of the invention provides a multi-component comprehensively quantificational method for assessing and controlling the quality of traditional Chinese medicine and application. The method for assessing and controlling the quality of traditional Chinese medicine combines measurement of chemical content and measurement of biological potency, focuses on comprehensively quantificational assessment on the quality of the rheum palmatum herbs or medicinal slices through biological potency, and establishes the equivalent computation method for components causing the rheum palmatum purgative effect; the method can be applied to preliminary assessment on the quality of rheum palmatum herbs or medicinal slices with different producing areas, different batches and different growth years.
Owner:302 MILITARY HOSPITAL OF CHINA +1
Method for nucleic acid detection using voltage enhancement
ActiveUS9551026B2Easy to controlIncrease assayMicrobiological testing/measurementLibrary screeningNucleic acid detectionDNA fragmentation
Methods are provided for carrying out DNA sequencing on a device having upper and lower conductive layers separated by an insulative layer. Holes in the upper conductive layer create discrete attachment sites for DNA fragments. Voltage is applied to the surface to control affinity between the attachment sites and the DNA fragments, and to compact the DNA fragments for discrete optical detection.
Owner:COMPLETE GENOMICS INC
Quality detection method for spleen-invigorating, cold-dispersing and antidiarrheal medicine composition
ActiveCN102621268ASimple manufacturing methodReduce dosageComponent separationQuality controlContent determination
The invention discloses a quality detection method for a spleen-invigorating, cold-dispersing and antidiarrheal medicine composition, which includes identification and content determination. The existing quality standard is raised, on one hand, a preparation method of thin-layer sample solution identification is simplified, solvent usage, pollution, labor and materials are reduced, and cost is saved; and on the other hand, content tests of cinnamon and fructus piperis longi are added, and by a series of inspection on specificity, linear relation, stability, repeatability and the like, the method conforms to Chinese Pharmacopoeia 2010, quality control in industrial production and quality stability of products are benefited.
Owner:YABAO PHARMA GRP CO LTD
Cyanide and related species detection with metal surfaces
ActiveUS7776610B2Enhanced signalStrong Raman signalAnalysis using chemical indicatorsMicrobiological testing/measurementCyanide compoundAssay
Owner:UNIVERSITY OF WYOMING
Methods for detecting contaminants in solutions containing glucose polymers
ActiveUS9494576B2Increase productionIncrease assayMetabolism disorderMicrobiological testing/measurementGlucose polymersPolymer
The invention relates to a method for detecting contaminants of glucose polymers, said contaminants being capable of acting in synergy with one another so as to trigger an inflammatory reaction, characterized in that it comprises an in vitro inflammatory response test using modified cell lines.
Owner:ROQUETTE FRERES SA
Extended release pharmaceutical composition comprising metoprolol succinate
InactiveUS20100255105A1Easy to manufactureSuitable pharmacotechnical parameterBiocideOrganic active ingredientsDiluentExcipient
An extended release pharmaceutical composition comprising metoprolol succinate and at least two pharmaceutically acceptable excipients, wherein the first pharmaceutically acceptable excipient is an extended release agent; the second pharmaceutically acceptable excipient is selected from a binder, a diluent and mixtures thereof; and metoprolol succinate is in a crystalline form having a D50 ranging from 5 to 16 microns and a D90 below 50 microns.
Owner:ZAKLADY FARMACEUTYCZNE POLPHARMA SA
Preparation method and testing method of tablets for treating gouty arthritis
InactiveCN107753724ASolve the defect of identification lack of specificityIncrease assayComponent separationSkeletal disorderDrugThin layer
The invention relates to the field of drugs, in particular to a preparation method and testing method of tablets for treating gouty arthritis. The testing method is obtained through screening with creative experiments, and in the testing method, through the screening of sample treatment methods and the selection of a developing solvent system, distinguishing specialization is good, the testing method is economical and applicable, a result can be quickly obtained, and the defects that existing thin-layer identification of cassia twig and cortex acanthopanacis has no specialization are overcome.In a content testing method, the test of the astragaloside content is added through the optimization of the test sample treatment methods, so that the quality of the product can be effectively controlled by means of the content testing method, and the practical production requirements can be met.
Owner:JIANGSU KANION PHARMA CO LTD
Quality control method of Shangke bone-knitting medicine
InactiveCN101278976BIncrease assayGuaranteed curative effectHeavy metal active ingredientsAnthropod material medical ingredientsElectrophoresisQuality control
The invention discloses a quality control method for orthopedics-traumatology coaptation. The method adopts TLC to carry out qualitative identification for notoginseng, borneol, safflower and Nux vomica powder and uses electrophoresis for carrying out qualitative identification for starfish. HPLC and GC are respectively adopted to carry out quantitative identification for strychnine and the borneol. Chemical titration is adopted to carry out quantitative identification for mercury sulfide. Therefore, the quality control scope of the orthopedics-traumatology coaptation is enlarged. The qualitycontrol method for orthopedics-traumatology coaptation is easy to be practiced and has strong specialization. In addition, the quantitative identification has high precision and good repeatability, thus ensuring that the quality of compound medicine is uniform, stable, effective and controllable.
Owner:大连美罗中药厂有限公司
Detection method of traditional Chinese medicine preparation Jiuhua hemorrhoid suppository
InactiveCN110927323AIncrease assayPlay a curative effectComponent separationMedicinal herbsGas liquid chromatographic
The invention relates to a quality detection method of a Jiuhua hemorrhoid suppository. The detection method disclosed by the invention comprises a method for identifying and determining rheum officinale, borneol, mangnolia officinalis and lithospermum, a method for determining the high performance liquid chromatography content of rheum officinale and a method for determining the gas chromatography content of borneol and isoborneol. The thin-layer identification method for rheum officinale, borneol, magnolia officinalis and lithospermum erythrorhizon comprises the following steps: taking jiuhua hemorrhoid suppository, grinding and adding methanol, dipping, filtering, evaporating filtrate to dryness, adding water into residues to dissolve, adding hydrochloric acid, heating, refluxing, immediately cooling, adding diethyl ether, shaking and extracting, merging diethyl ether liquid, recovering a solvent to dryness, adding methanol into residues to dissolve, and then taking the solution asa testing sample solution; taking a rhubarb reference medicinal material, preparing a reference medicinal material solution by the same method, taking a chrysophanol reference substance, and adding methanol to prepare a reference substance solution; and identifying according to the general rule 0502 in Chinese Pharmacopoeia 2015 edition by thin-layer chromatography.
Owner:JIANGXI JIUHUA PHARMA
Quality control method of compound Chinese lobelia oral preparation and use thereof
InactiveCN101480440APerfect quality control methodQuality improvementComponent separationRespiratory disorderCoumaric acidP-Coumaric acid
The invention relates to a mass control method of a traditional Chinese medicine compound Chinese Lobelia oral formulation and the application thereof. Based on the mass control standard of compound Chinese Lobelia injecta, the content of coumaric acid in Chinese Lobelia, herba scutellariae barbatae and herba oldenlandiae is additionally measured to synthetically control the prescription. The invention improves the mass control method of compound Chinese Lobelia oral formulation, more effectively controls the quality of the compound Chinese Lobelia oral formulation, and ensures the safety of people taking the compound Chinese Lobelia oral formulation.
Owner:FUREN PHARMA GROUP
Quality detection method of pharmaceutical composition for relieving cough and asthma
ActiveCN112710797AShort detection cycleReduce testing costsPreparing sample for investigationTesting medicinal preparationsUrsolic acidPharmaceutical drug
The invention relates to a quality detection method of a pharmaceutical composition for relieving cough and asthma, wherein the pharmaceutical composition is composed of pinecones, cotton roots and loquat leaves; the detection method comprises identification of the active components pinecones, cotton roots and loquat leaves, and determination of oleanolic acid and ursolic acid content in loquat leaves. On the basis of the original quality standard, the identification method is optimized, the detection period is shortened, the detection cost is saved, and the identification for simultaneously detecting pinecones, cotton roots and loquat leaves by using the same thin-layer plate is obtained; in addition, by adding the detection of heavy metals and harmful elements and adding thedetermination of oleanolic acid and ursolic acid content in loquat leaves, the effective components of the medicine are controlled from qualitative detection to qualitative and quantitative detection. The detection method is high in accuracy and good in stability and can be effectively used for controlling the quality of the cough and asthma relieving medicine combination and improving and perfecting the quality standard.
Owner:贵州大隆药业有限责任公司
Quality control method for Liuwei pomegranate preparation
The present invention discloses a quality control method for Liuwei pomegranate capsules, and belongs to the technical field of medicine. According to the present invention, the existing quality standard of the Liuwei pomegranate capsules is correspondingly increased, and the thin layer identification of cassia bark in the Liuwei pomegranate capsule is added on the basis of the original standard; the thin layer identification of piperine is improved, a negative control is added, an optimal developing system and an optimal color developing reagent are preferably selected, and the use of chemical reagents with large harm on the operator is avoided; the detection of a hydroxysafflor yellow A content in safflower is added, such that the safe, uniform, stable and controllable product quality is further ensured. The quality control method of the present invention can be provided for controlling the quality of the Liuwei pomegranate capsules, and for controlling the qualities of the other formulations in the same prescription, wherein the formulations comprise tablets, granules, and the like.
Owner:JINKE TIBETAN MEDICINE QINGHAI PROV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com