Quality control method of Shangke bone-knitting medicine
A detection method and drug technology, applied in drug combination, pharmaceutical formula, bone disease, etc., can solve the problems of large pollution, inability to reach the dissolved state, light weight and large volume, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] The quality control method of embodiment 1 traumatology osteosynthesis medicine
[0101] The weight proportion of each crude drug in the described traumatology osteosynthesis medicine:
[0102] 6 parts of safflower, 20 parts of ground beetle, 5 parts of cinnabar
[0103] Nyx powder 10 parts Sunburned myrrh 2 parts Panax notoginseng 40 parts
[0104] 20 parts of roasted starfish 20 parts of roasted chicken bone 1 part of icicles
[0105] 10 parts of calcined natural copper, 2 parts of roasted frankincense, 2 parts of melon seeds
[0106] Character identification: For tablets: the product core is taupe to tan; taste bitter, fishy; for capsules: the product content is light brown to brown; Brown; bitter, fishy.
[0107] Microscopic identification: Take the medicine of the present invention and observe under a microscope: the pollen grains are round or oval, with a diameter of 43-66 μm, with tooth-like protrusions on the outer wall and 3 germination holes; the resin can...
Embodiment 2
[0118] The quality control method of embodiment 2 traumatology osteosynthesis medicine
[0119] In addition to the content described in the above-mentioned embodiment 1, the following content is also included:
[0120] Identification of Nuxseed Powder
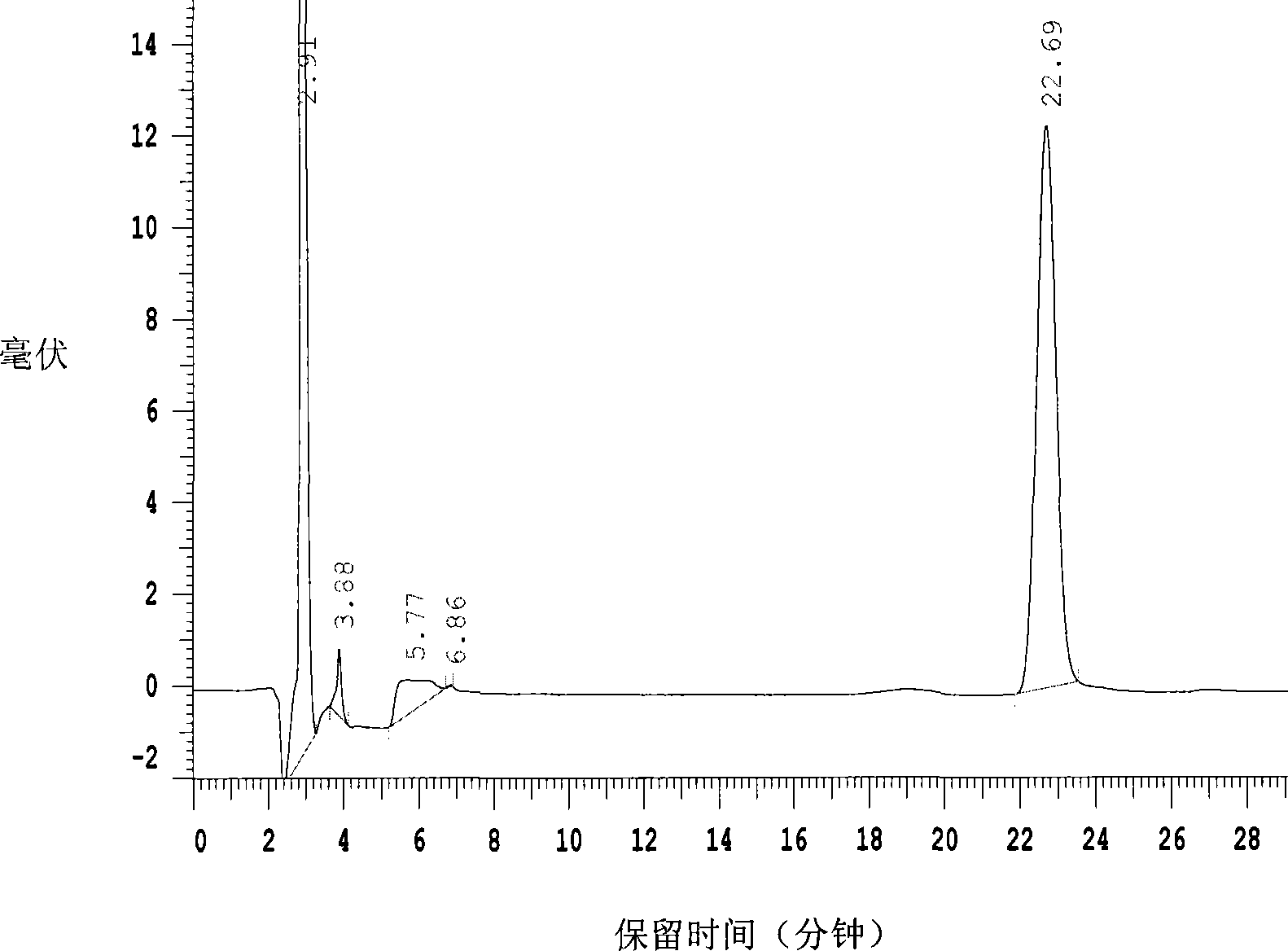
[0121] Get 4.0g of the pharmaceutical powder of the present invention, add 10:1 chloroform-ethanol mixed solution 15ml and concentrated ammonia water test solution 1.5ml, close up, ultrasonic treatment 20 minutes (160w, 50kHz), filter, and filtrate is as need testing sample Solution; Get strychnine and strychnine reference substance in addition, add chloroform to make the mixed solution that each 1ml contains 2mg, as reference substance solution; According to thin layer chromatography (Chinese Pharmacopoeia 2005 edition one, appendix VI B) For the test, draw 15 μl of the above-mentioned test solution and 5 μl of the reference solution, and place them on the same silica gel G thin-layer plate with sodium carboxymethylcellulose ...
Embodiment 3
[0124] The quality control method of embodiment 3 traumatology osteosynthesis medicine
[0125] Except the content described in embodiment 2, also comprise the identification of starfish, specifically as follows:
[0126] Extraction method: Take 2.0g of the drug powder of the present invention and 0.5g of the starfish reference medicinal material, add 6ml of hot water respectively, cover with plastic wrap, cook at 100°C for 30min, let cool and then centrifuge at 4000rpm for 20 minutes, take 10μl of the supernatant; according to electrophoresis Method (Chinese Pharmacopoeia 2005 edition three, appendix IV C) test, carry out SDS-polyacrylamide gel electrophoresis according to the following electrophoresis conditions, promptly obtain; The same protein band appears on the corresponding position of test sample and contrast medicinal material;
[0127] The electrophoresis conditions described therein are: separating gel: 12.5%; stacking gel: 5%; constant current: 30mA; voltage: 200V...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com