Quality control method of pharmaceutical composition for preventing and treating coronary heart disease
A quality control method and composition technology, which is applied in the direction of drug combination, pharmaceutical formula, cardiovascular system diseases, etc., can solve the problem of no quality control index, etc., and achieve the effect of preventing and treating angina pectoris
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0102] Embodiment 1 The quality control method of pharmaceutical composition of the present invention
[0103] 【prescription】
[0104] Bezoar 100 parts Musk 10 parts Pearl 400 parts
[0105] Toad cake 100 parts Red ginseng 60 parts Panax notoginseng 300 parts
[0106] 100 parts of borneol, 100 parts of pig gall paste, 50 parts of ocher
[0107] Concentrated Buffalo Horn Powder 130 parts Danshen Extract 100 parts
[0108] 【Method】
[0109] (1) taking the bulk drug of the weight ratio for subsequent use;
[0110] (2) pearl, salvia miltiorrhiza extract and ocher are pulverized into the finest powder respectively;
[0111] (3) Red ginseng and Panax notoginseng are crushed and added to pig gall paste, mixed evenly, and crushed into the finest powder;
[0112] (4) Crush bezoar, musk, toad venom, buffalo horn concentrated powder and borneol into the finest powder, mix with the above-mentioned powders except ochre, sieve, mix well, pour water into pills, coat ochre, and dry, tha...
Embodiment 2
[0130] Embodiment 2 The quality control method of pharmaceutical composition of the present invention
[0131] Except the content in embodiment 1, also include following content:
[0132] TLC identification of toad venom:
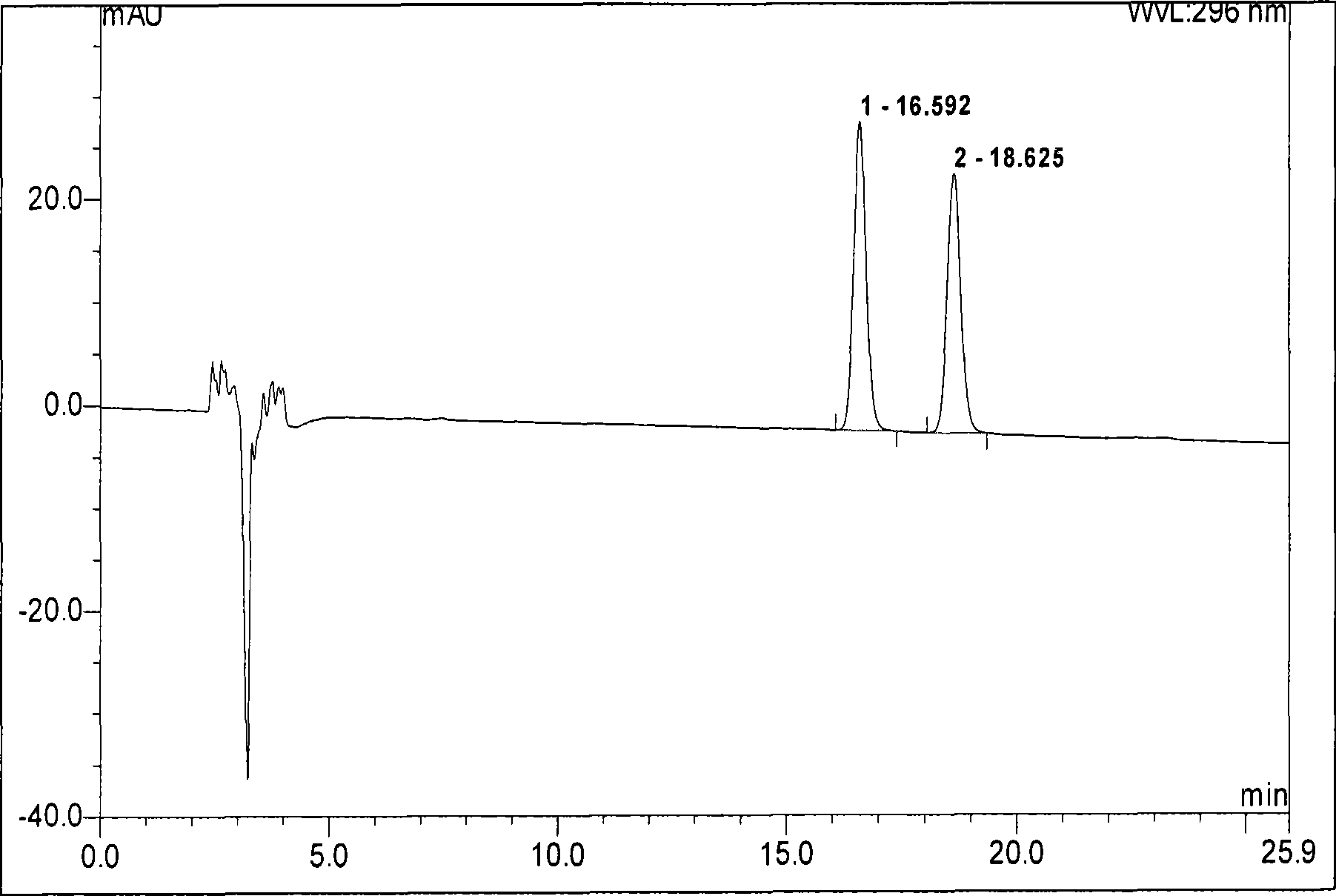
[0133] Take 0.7g of the medicine of the present invention, grind it finely, add 1ml of chloroform, shake it, leave it for 1 hour, and use the supernatant as the test solution; take 0.05g of the reference medicinal material of toadstool, and make a solution of the reference medicinal material in the same way; Add chloroform to each 1ml containing 1mg of the reference substance of Dugenji and Cinobufaction base, respectively, as the reference substance solution; according to the thin-layer chromatography test in Appendix VI B of the Chinese Pharmacopoeia 2005 edition, absorb the above four 4 μl of each solution was spotted on the same silica gel G thin-layer plate, and cyclohexane: chloroform: acetone was used as a developing agent at a ratio of 4:3:3, and d...
Embodiment 3
[0146] Embodiment 3 The quality control method of pharmaceutical composition of the present invention
[0147] Except the content in embodiment 2, also include following content:
[0148] TLC identification of Salvia miltiorrhiza: take 3g of the drug powder of the present invention, add 10ml of ether, shake, place for 1 hour, filter, evaporate the filtrate to dryness, add 1ml of ethyl acetate to the residue to dissolve, and use it as the test solution; take another tanshinone II A reference substance, add ethyl acetate to make a solution containing 5mg per 1ml, as the reference substance solution; according to the thin-layer chromatography test in Appendix VI B of the Chinese Pharmacopoeia version in 2005, draw 5 μl of the reference substance solution and 10 μl of the test solution. , point respectively on the same silica gel G thin-layer plate, with benzene:ethyl acetate as 19:1 as developing agent, develop, take out, and dry; Spots of the same color; for TLC see figure 2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com