Compound acrivastine sustained release tablets, and preparation method thereof
A technology of atorvastatin and sustained-release tablets, which is applied in the directions of pharmaceutical formulations, medical preparations containing active ingredients, and pill delivery, etc., can solve the problems of poor patient tolerance and low bioavailability, and achieve less adverse reactions, Tolerance-improving, action-modifying effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0039] specific implementation plan
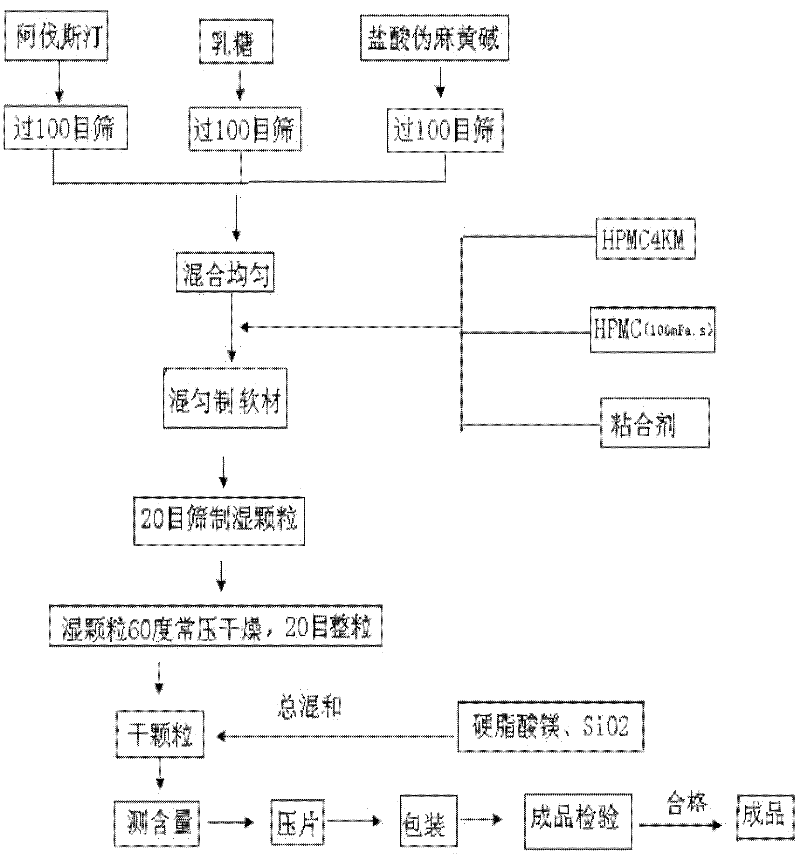
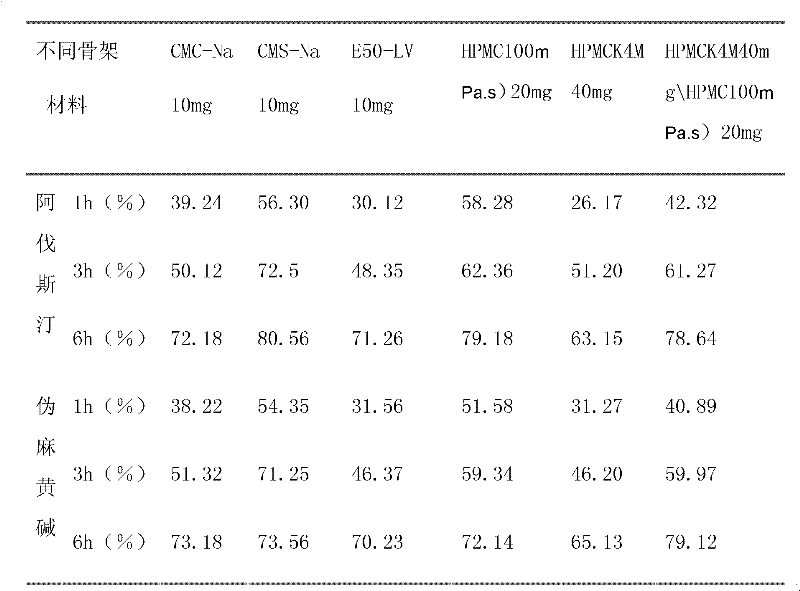
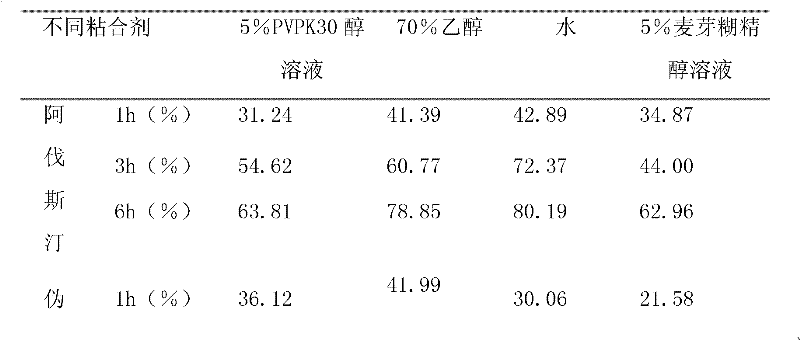
[0040] The formula and process of compound Avastin sustained-release tablets are as follows:
[0041] ① Prescription
[0042] Avastin: 16g
[0043] Pseudoephedrine hydrochloride: 120g
[0044] Hypromellose K4M: 40g
[0045] Hydroxypropyl methylcellulose (100mPa.s): 20g
[0046] Lactose: 100g
[0048] SiO 2 : 2g
[0049] 70% ethanol: appropriate amount,
[0050] The above prescription ingredients were made into 1000 tablets.
[0051] Preparation Process
[0052] (1) Protect from light, take each raw and auxiliary material according to the prescription, and set aside;
[0053] (2) Avastatin bulk drug, pseudoephedrine hydrochloride, and lactose are respectively passed through a 100-mesh sieve and fully mixed uniformly by the method of equal increment;
[0054] (3) Add hydroxypropyl methylcellulose K4M and hydroxypropyl methylcellulose (100mPa.s) to the above-mentioned mixed medicinal powder and mix ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com