Method for investigation of release degree of ibuprofen-pseudoephedrin hydrochloride sustained-release preparation
A technology of sustained-release preparations and release rate, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., and can solve the problems of checking the release rate of unrecorded compound sustained-release preparations, affecting the release of active ingredients, and not being able to effectively control the pH value of the dissolution medium, etc. Achieve obvious social and economic benefits, high sensitivity, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
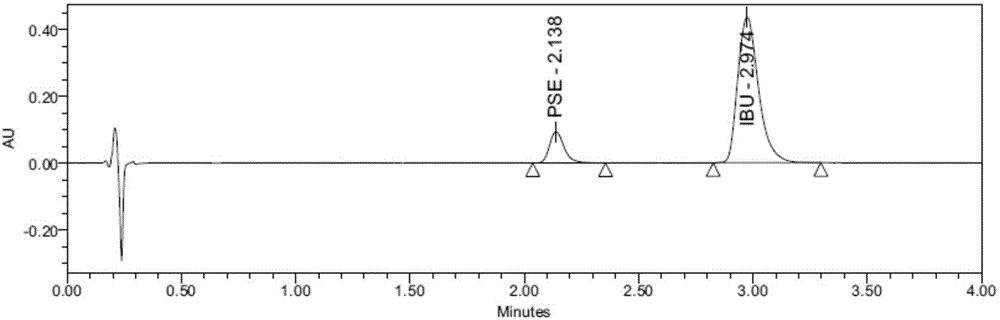
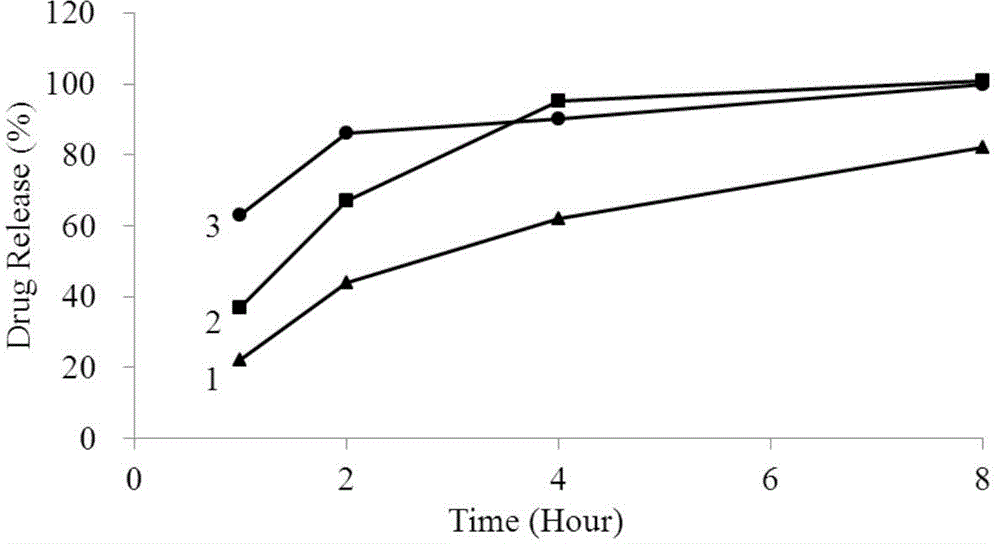
[0046] Example 1: Determination of the release rate of Buluo pseudoeba sustained-release capsules (batch number 11020340) (the release medium does not contain lecithin, and the concentration of phosphate buffer is 0.05mol / L)
[0047] (1), preparation of release medium: take 10.00g of lecithin and dissolve it in 100ml ethanol to obtain a lecithin stock solution with a concentration of 100mg / ml, accurately measure 60ml of lecithin stock solution and an appropriate amount of 0.05mol / L Put phosphate buffer (pH6.00±0.05) in a flask, and remove ethanol by rotary evaporation to obtain a lecithin solution. Add the lecithin solution in the flask to the phosphate buffer, supplement the phosphate buffer to 6L, and mix well , adjust the pH to 6.00±0.05 with 1mol / L sodium hydroxide solution, heat to 42.0°C and degas for 5 minutes before use. The preparation method of the 0.05 mol / L phosphate buffer solution is as follows: 68.05 g of potassium dihydrogen phosphate is weighed and dissolved i...
Embodiment 2
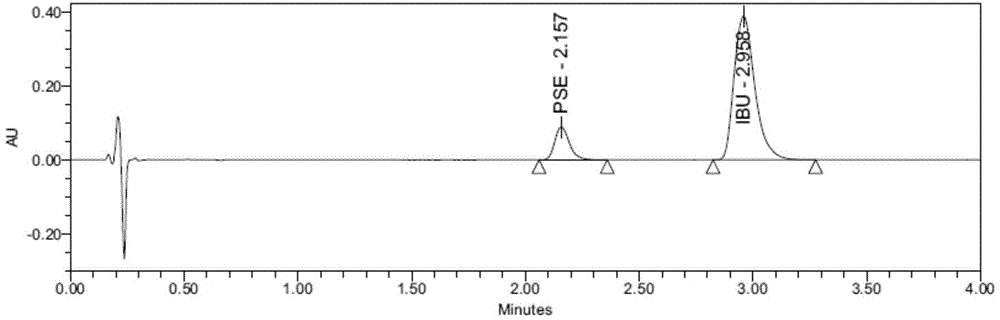
[0056] Example 2: Determination of the release rate of Buluo pseudoeba sustained-release capsules (batch number 11020340) (the release medium contains 0.1% lecithin, and the concentration of phosphate buffer is 0.05mol / L)
[0057] (1), preparation of release medium: take 10.00g of lecithin and dissolve it in 100ml ethanol to obtain a lecithin stock solution with a concentration of 100mg / ml, accurately measure 60ml of lecithin stock solution and an appropriate amount of 0.05mol / L Put phosphate buffer (pH6.00±0.05) in a flask, and remove ethanol by rotary evaporation to obtain a lecithin solution. Add the lecithin solution in the flask to the phosphate buffer, supplement the phosphate buffer to 6L, and mix well , adjust the pH to 6.00±0.05 with 1mol / L sodium hydroxide solution, heat to 42.0°C and degas for 5 minutes before use; Prepared by potassium dihydrogen, adjust the pH value with 1mol / L sodium hydroxide solution.
[0058] (2), take 6 Buluo pseudoephedrine sustained-releas...
Embodiment 3
[0066] Example 3: Determination of the release rate of Buluo pseudoeba sustained-release capsules (batch number 11020340) (the release medium contains 0.2% lecithin, and the concentration of phosphate buffer is 0.05mol / L)
[0067](1), preparation of release medium: take 20.00g of lecithin and dissolve it in 200ml ethanol to obtain a lecithin stock solution with a concentration of 100mg / ml, accurately measure 120ml of lecithin stock solution and an appropriate amount of 0.05mol / L Put phosphate buffer (pH6.00±0.05) in a flask, and remove ethanol by rotary evaporation to obtain a lecithin solution. Add the lecithin solution in the flask to the phosphate buffer, supplement the phosphate buffer to 6L, and mix well , adjust the pH to 6.00±0.05 with 1mol / L sodium hydroxide solution, heat to 42.0°C and degas for 5 minutes before use; Prepared by potassium dihydrogen, adjust the pH value with 1mol / L sodium hydroxide solution.
[0068] (2), take 6 Buluo pseudoephedrine sustained-releas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com