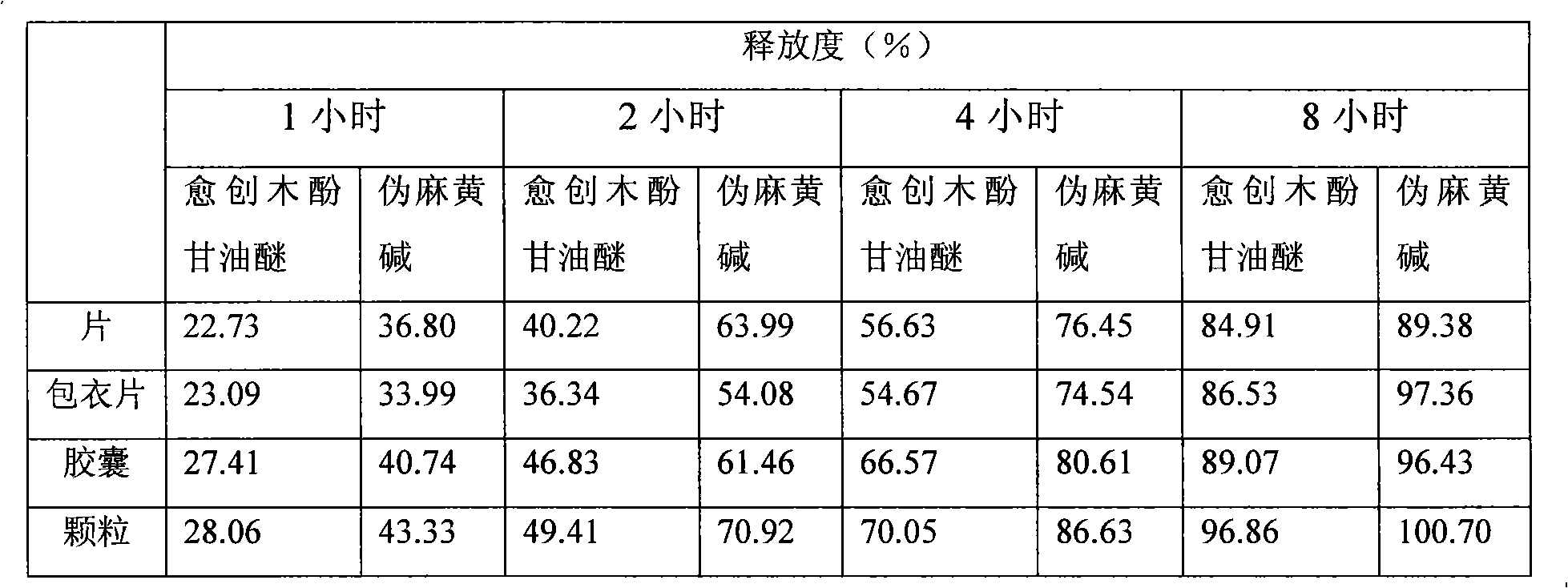
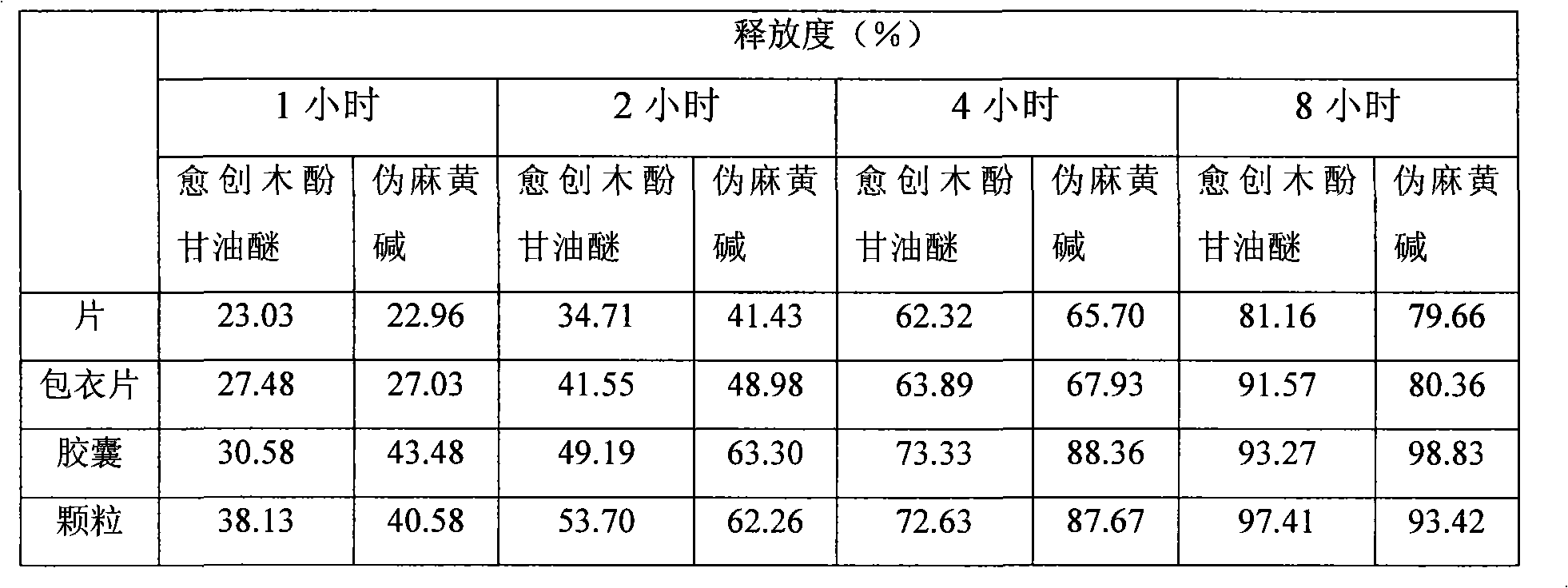
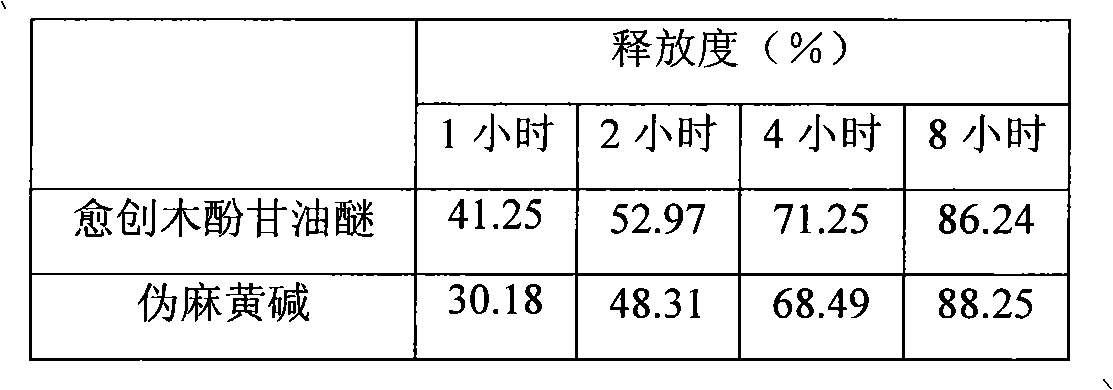
Glyceryl guaiacolate and pseudoephedrine compound sustained release preparation
A technology of guaiacol glyceryl ether and pseudoephedrine, which is applied in the field of medicine and achieves the effects of small fluctuation, less medication frequency and reduced medication frequency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] prescription:
[0028] Guaifenesin
800g
90g
Hypromellose E5
40g
25g
36g
Povidone K 30 aqueous solution
Appropriate amount
Appropriate amount
[0029] Coating prescription:
[0030] Opadry
15g
pure water
Add to 100ml
[0031] Preparation:
[0032] (1) Preparation of granules Hypromellose E5 and microcrystalline cellulose were sieved respectively, mixed evenly with guaiacol glyceryl ether, pseudoephedrine hydrochloride and carnauba wax, and mixed with 5% povidone K 30 The aqueous solution is a soft material made of adhesive, wet granules are prepared through a 20-mesh sieve, dried at 60°C, granulated through a 20-mesh sieve, and set aside.
[0033] (2) Preparation of coating liquid Add Opadry to pure water, and add pure water to 100ml, stir for 1 hour, ...
Embodiment 2
[0043] prescription:
[0044] Guaifenesin
400g
60g
Hypromellose K100
6g
Hypromellose E5
75g
[0045] microcrystalline cellulose
45g
Povidone K 30 aqueous solution
Appropriate amount
Appropriate amount
[0046] Coating prescription:
[0047] Pseudoephedrine Hydrochloride
30g
Opadry
25g
water
Appropriate amount until completely dissolved
[0048] Preparation:
[0049] (1) Preparation of granules Hypromellose and microcrystalline cellulose are sieved respectively, mixed and evenly mixed, then mixed evenly with guaiacol glyceryl ether and pseudoephedrine hydrochloride, and mixed with 8% povidone K 30 The aqueous solution is a soft material made of adhesive, wet granules are prepared through a 20-mesh sieve, dried at 60°C, granulated through a 20-mesh sieve, and set aside.
[0050] (2) ...
Embodiment 3
[0060] prescription:
[0061] Guaifenesin
600g
pseudoephedrine sulfate
60g
110g
20g
Hypromellose K4
7g
72g
60g
50g
Povidone K 30 aqueous solution
Appropriate amount
20g
[0062] Coating prescription:
[0063] Opadry II
30g
pure water
1000ml
[0064] Preparation:
[0065] (1) Each component in the prescription is passed through a 100-mesh sieve respectively, and set aside;
[0066] (2) Preparation of immediate-release granules: Fully mix the prescription amount of pregelatinized starch and 1 / 2 amount of sodium carboxymethyl starch, mix evenly with guaiacol glyceryl ether, and mix with 5% povidone K 30 The aqueous solution is a soft material made of adhesive, 20-mesh sieve is used ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com