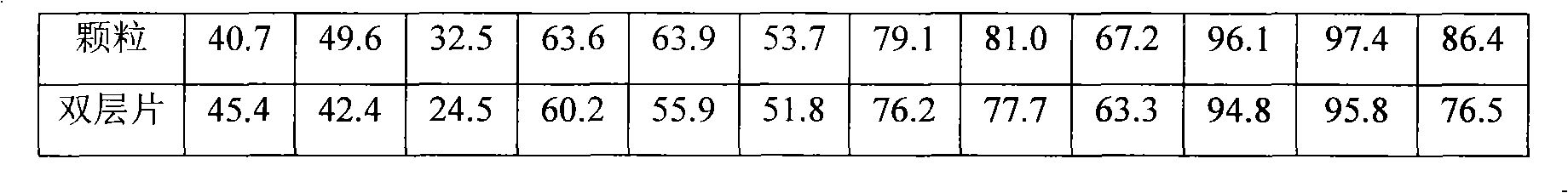Compound slow release preparation of pseudoephedrine, chlorphenamine and dextromethorphan
A slow-release preparation, pseudoephedrine technology, which is applied in the directions of non-active ingredients medical preparations, active ingredients-containing medical preparations, pharmaceutical formulas, etc. Side effects and other issues, to achieve the effect of less medication frequency, high safety, and reduced medication frequency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
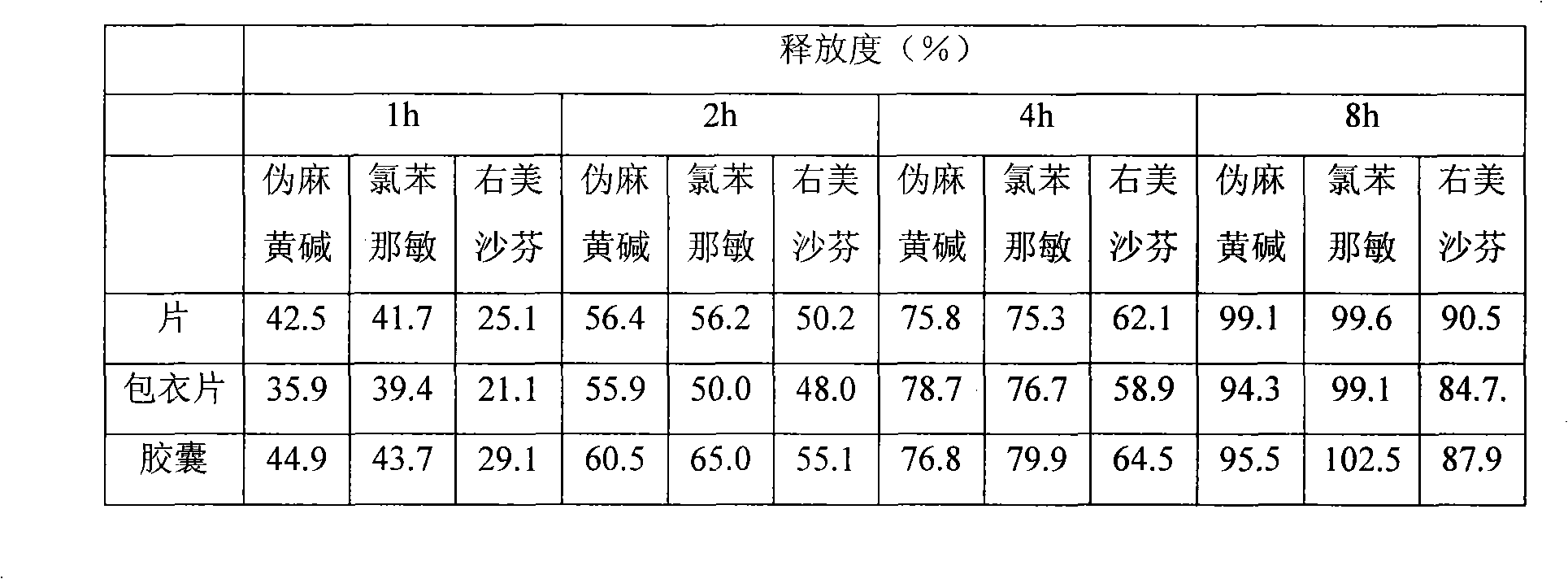
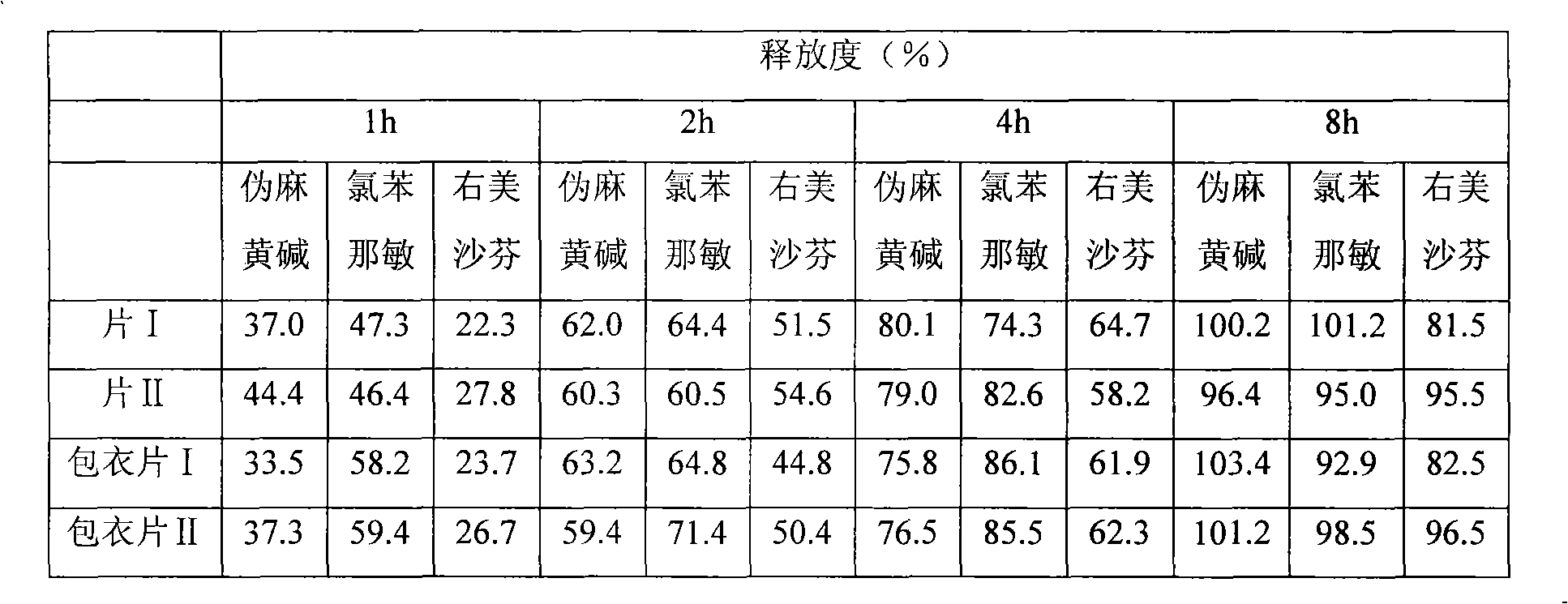
Image
Examples
Embodiment 1
[0020] prescription:
[0021] Pseudoephedrine hydrochloride
90g
4g
30g
45g
Acrylic
25g
46g
10% Povidone K 30 aqueous solution
Appropriate amount
Appropriate amount
[0022] Coating prescription:
[0023] Opadry
10g
pure water
Add to 1000ml
[0024] Made into 1000 grains (tablets)
[0025] Preparation method 1:
[0026] (1) Preparation of granules Carnauba wax, acrylic resin, and microcrystalline cellulose were respectively passed through an 80-mesh sieve and mixed uniformly. Then add pseudoephedrine hydrochloride, chlorpheniramine maleate, and dextromethorphan hydrobromide in turn, mix well, and add 10% povidone K 30 The aqueous solution is a soft material made of an adhesive, wet granules are prepared through a 20-me...
Embodiment 2
[0043] prescription:
[0044] Pseudoephedrine hydrochloride
60g
15g
2g
Cellulose acetate
20g
Hypromellose E5
55g
65g
10% Povidone K 30 aqueous solution
Appropriate amount
Appropriate amount
[0045] Coating prescription:
[0046] Chlorpheniramine maleate
2g
Opadry II
10g
pure water
Appropriate amount until completely dissolved
[0047] Made into 1000 grains (tablets)
[0048] Preparation method 1:
[0049] (1) Preparation of granules: Cellulose acetate, hydroxypropylmethylcellulose E5, and lactose were respectively passed through an 80-mesh sieve, fully mixed with equal increments, and then added pseudoephedrine hydrochloride, dextromethorphan hydrobromide, Chlorpheniramine maleate, mix it well with 10% Povidone K 30 The aqueous solution is a so...
Embodiment 3
[0068] prescription:
[0069] pseudoephedrine sulfate
40g
1g
15g
sodium alginate
20g
28g
50g
10% Povidone K 25 aqueous solution
Appropriate amount
[0070] Coating prescription:
[0071] pseudoephedrine sulfate
5g
Chlorpheniramine maleate
1g
Cellulose diacetate
15g
Appropriate amount until completely dissolved
[0072] Made into 1000 grains (tablets)
[0073] Preparation:
[0074] (1) Preparation of granules Sodium alginate, gelatin, and pregelatinized starch were respectively passed through a 100-mesh sieve, fully mixed in equal increments, and then added with prescribed amounts of pseudoephedrine sulfate, chlorpheniramine maleate, and hydrobromic acid Dextromethorphan, mixed well with 10% Povidone K 25 The aqueous solution ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com