Jinling common cold preparation and controlled releasing preparations and preparation method thereof
A technology of controlled-release preparations and extraction methods, which is applied in the direction of pill delivery, antiviral agents, anti-infective drugs, etc., can solve the problems of increasing efficiency but not increasing toxicity, slow onset of effect, and small toxic and side effects, so as to improve bioavailability, Fast onset, long-lasting effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
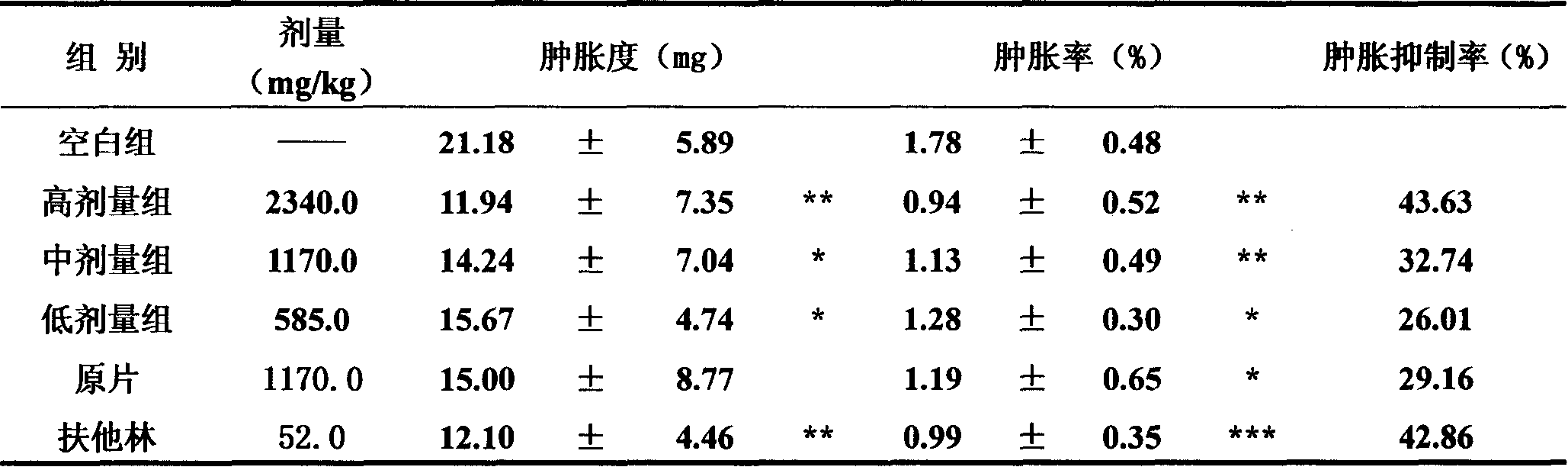
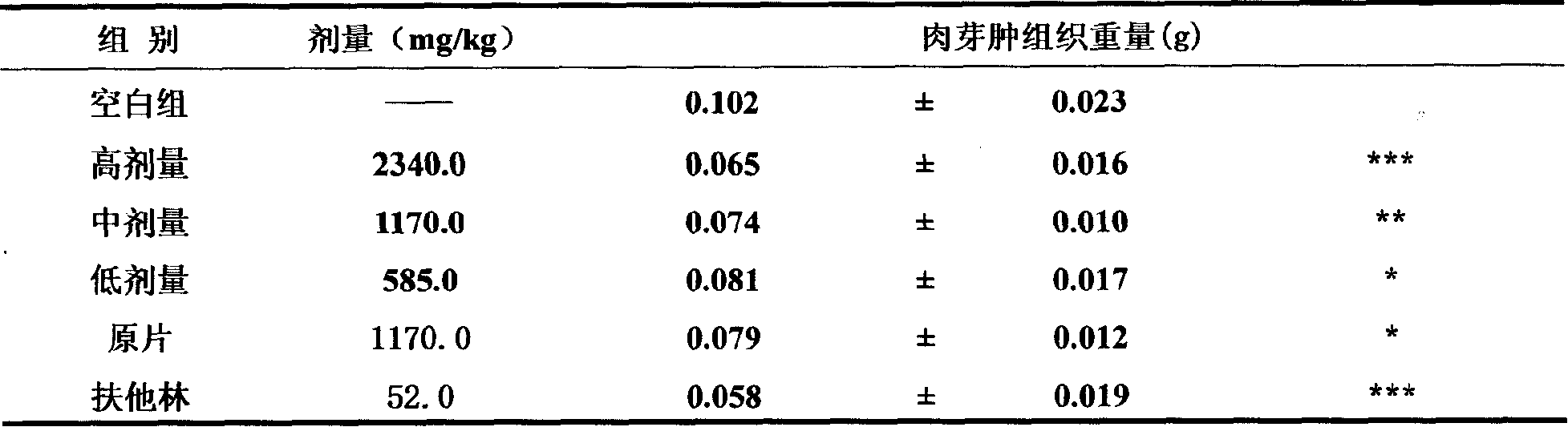
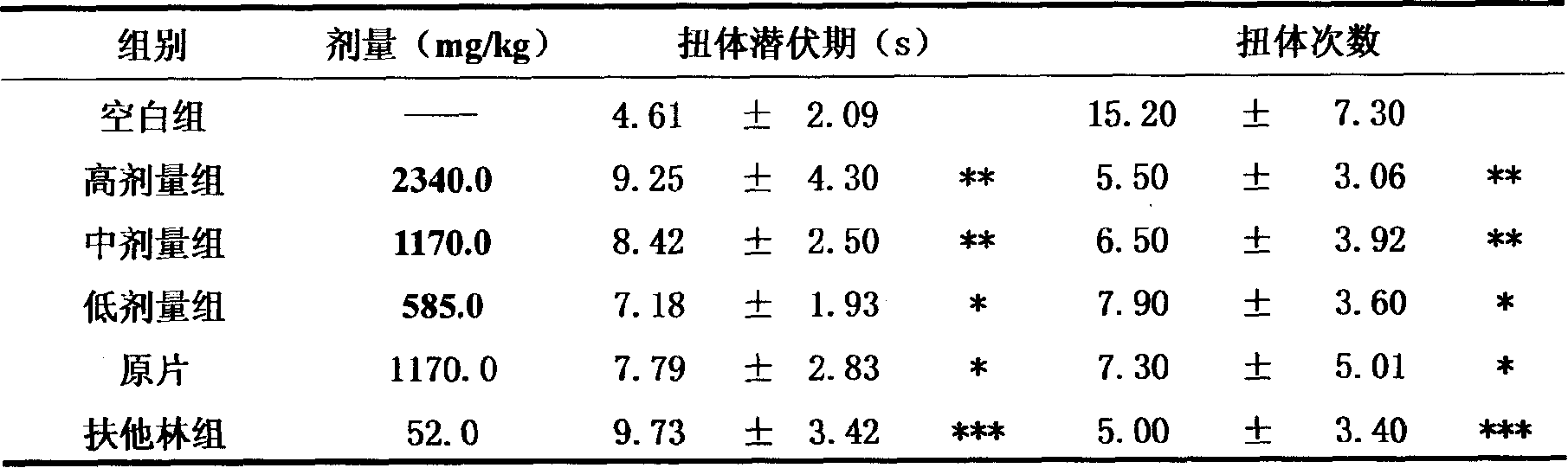
Image
Examples
Embodiment 1
[0164] The preparation method of tablet of the present invention (making 1000):
[0165] Quick release part:
[0166] Chlorpheniramine 1g Vitamin C10g
[0167] Aspirin 60g plus polyvinylpyrrolidone 10g
[0168] Lactose 10g Sodium hydroxymethyl starch 10g
[0169] Take honeysuckle vine, wild chrysanthemum, and pea root, add water and decoct twice, add 10 times the amount of water for the first time, decoct for 2 hours, add 8 times the amount of water for the second time, decoct for 2 hours, combine the decoction, Filter and concentrate the filtrate to a thick paste, dry and set aside.
[0170] Crush the antelope horn into a fine powder and mix it with the rest of the concentrated buffalo horn powder.
[0171] The above-mentioned drugs and auxiliary materials were mixed and granulated with 95% ethanol, dried, and passed through a 16-mesh sieve for granulation.
[0172] Sustained release part:
[0173] Antelope Horn 1g Concentrated Buffalo Horn Powder 3g
[0174] Honeysuck...
Embodiment 2
[0180] The preparation method of capsule of the present invention (making 1000):
[0181] Quick release part:
[0182] Chlorpheniramine 1g Vitamin C10g
[0183] Aspirin 60g Croscarmellose Sodium 16g
[0184] Hypromellose 10g Microcrystalline Cellulose 12g
[0185] Take wild chrysanthemum, add 12 times the amount of water, use a Soxhlet extractor to extract the volatile oil until the volatile oil no longer increases, add water and decoct the remaining medicinal residues and honeysuckle for 3 times, add 10 times the amount of water for the first time, and decoct Boil for 3 hours; add 8 times the amount of water for the second time, and decoct for 2 hours; add 8 times the amount of water for the third time, decoct for 1 hour, filter, combine the decoction, and concentrate the filtrate to a thick paste, that is, .
[0186] Take bean root, soak in acid water with pH 3.5 for 1 hour, decoct twice, add 10 times the amount of acid water each time, decoct for 2 hours each time, filt...
Embodiment 3
[0197] The preparation method of granule of the present invention (making 1000 bags):
[0198] Quick release part:
[0199] Chlorpheniramine 1g Vitamin C10g
[0200] Aspirin 60g Hydroxymethylcellulose 14g
[0201] Polyvinylpyrrolidone 15g Magnesium stearate 1g
[0202] Take the wild chrysanthemum, add 12 times the amount of water, use a Soxhlet extractor to extract the volatile oil until the volatile oil no longer increases, add water and decoct the remaining medicinal residues and honeysuckle for 3 times, add 12 times the amount of water for the first time, and decoct Boil for 2 hours; add 10 times the amount of water for the second time, decoct for 1 hour; add 8 times the amount of water for the third time, decoct for 1 hour, filter, combine the decoction, and concentrate the filtrate to a thick paste, that is, .
[0203] Take bean root, soak in pH3 acid water for 1.5 hours, decoct twice, add 12 times the amount of acid water for the first time, decoct for 2 hours; add 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com