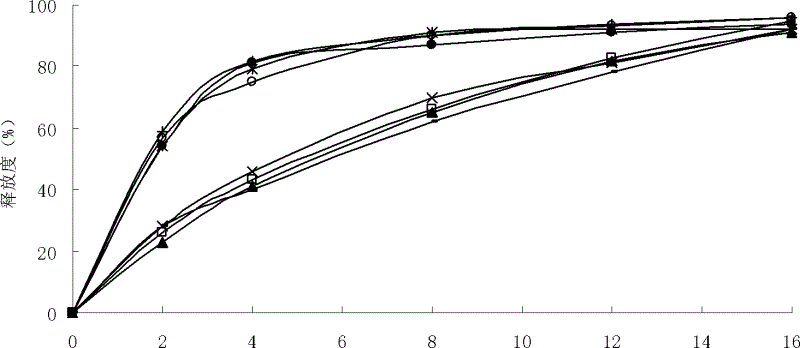
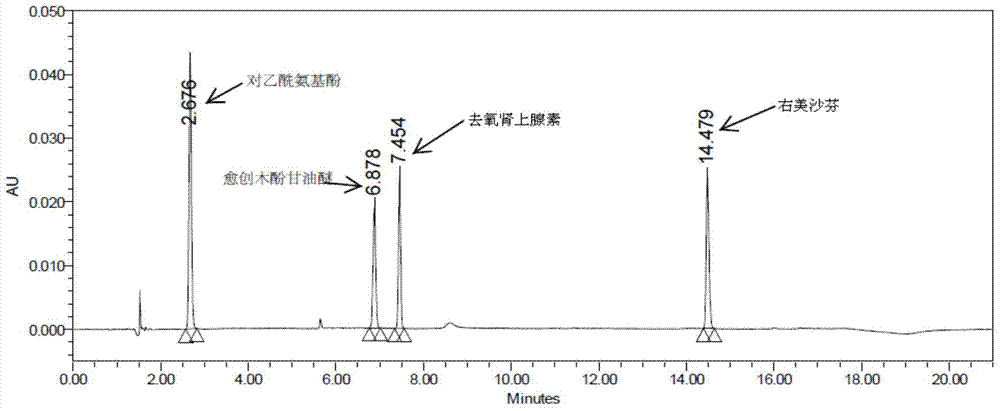
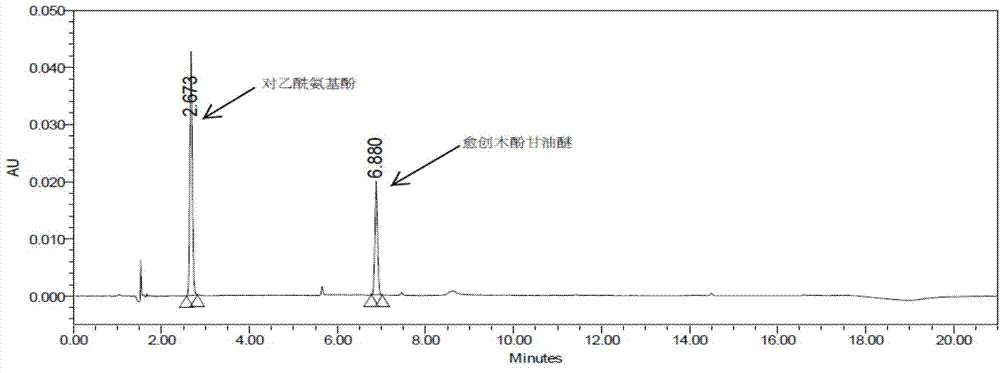
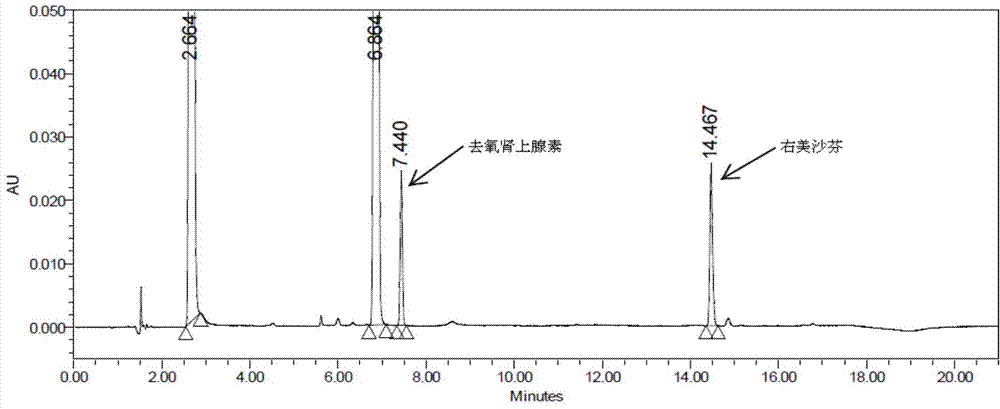
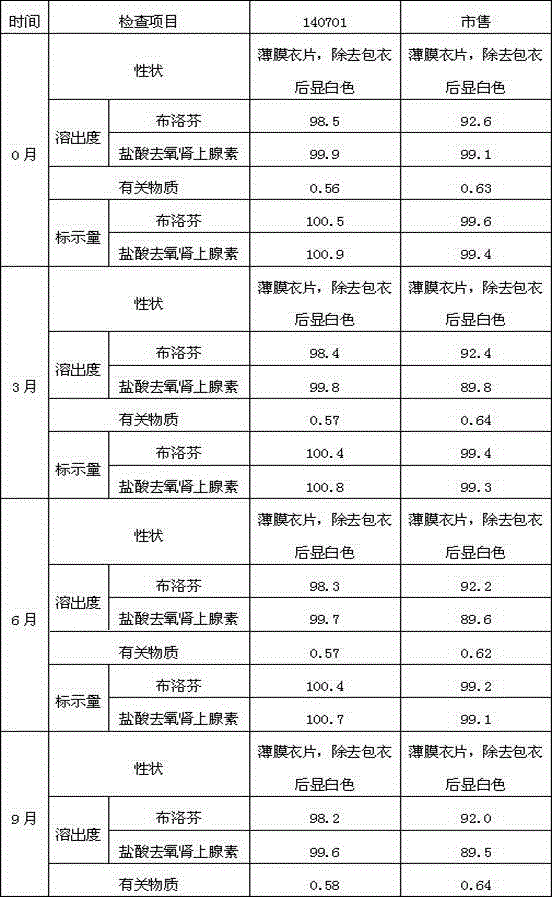
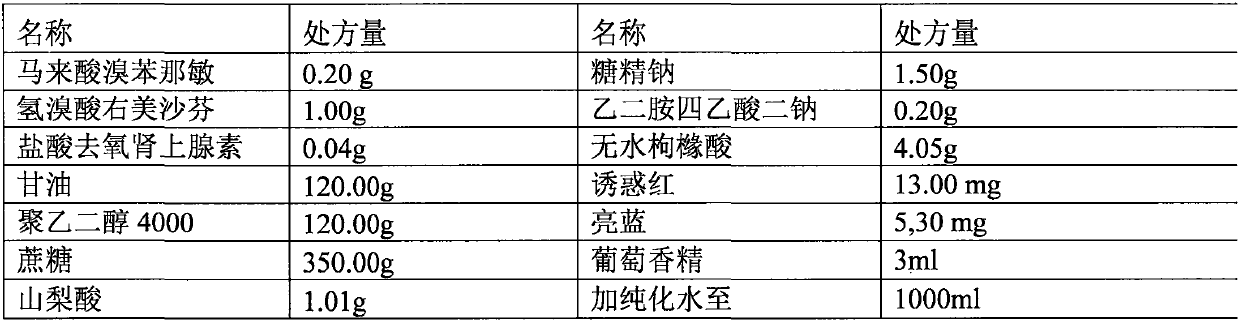
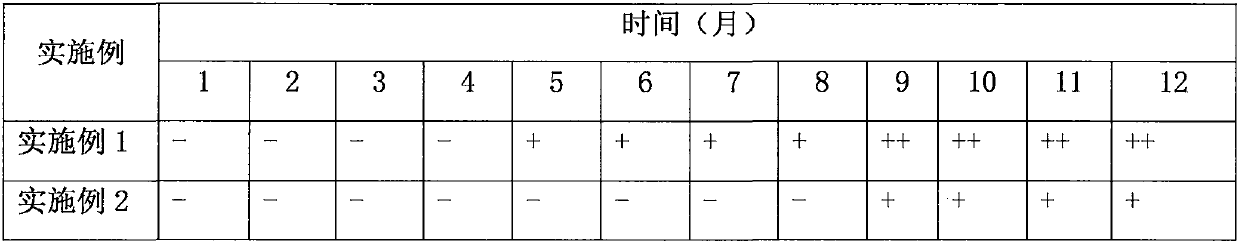
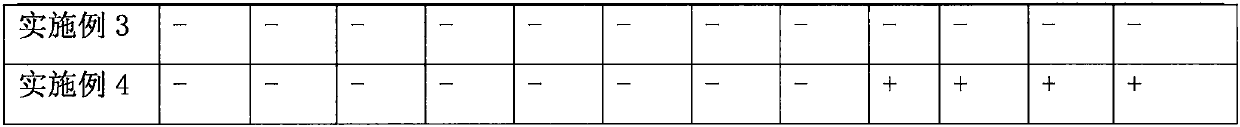
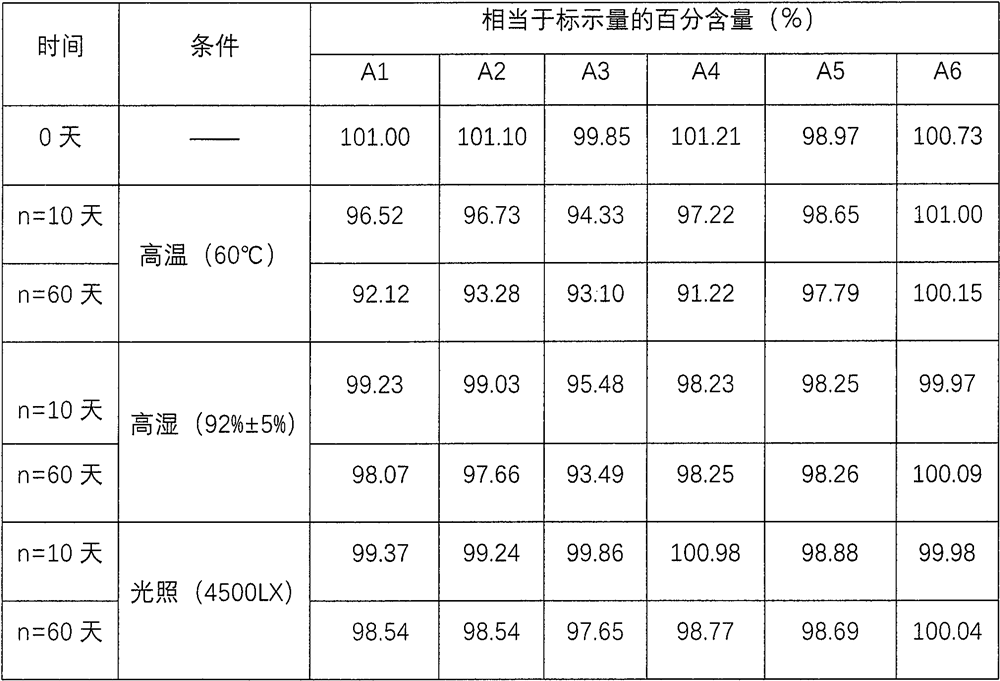
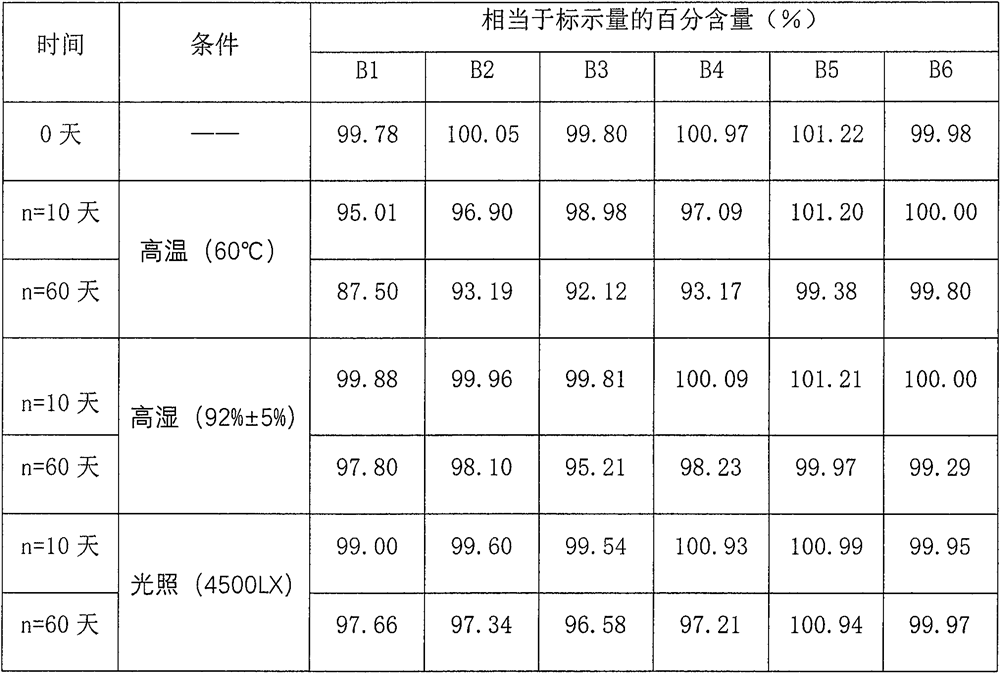
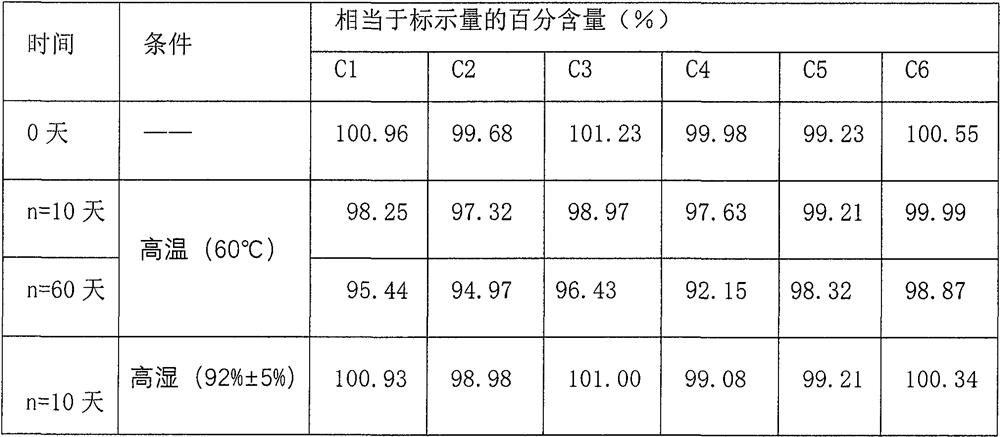
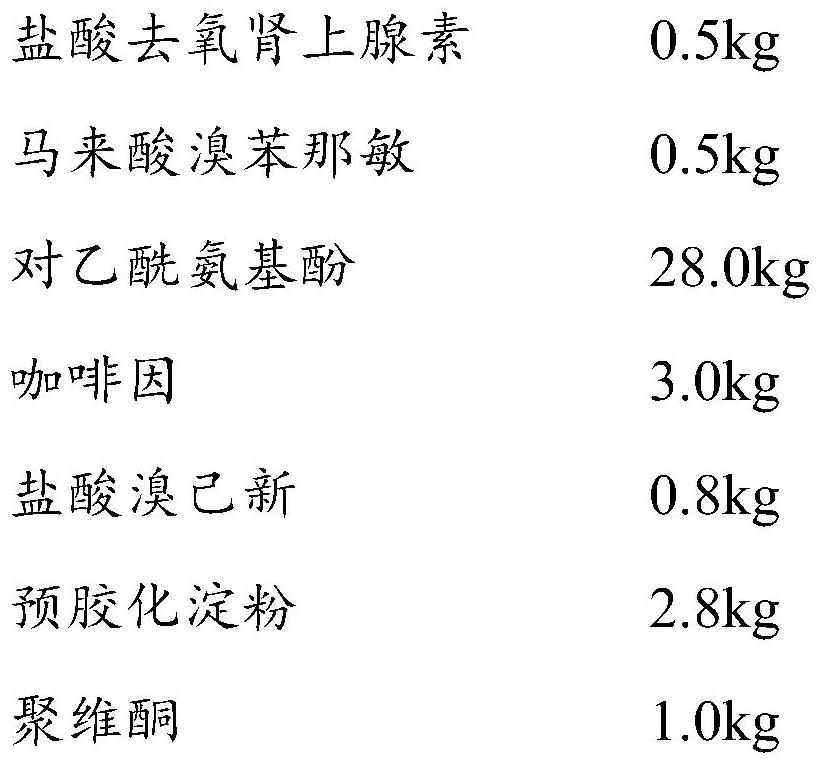
Patents
Literature
81 results about "Phenylephrine Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
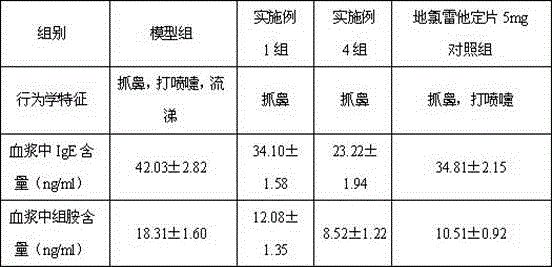
The hydrochloride salt form of phenylephrine, a direct-acting sympathomimetic amine chemically related to adrenaline and ephedrine with potent vasoconstrictor property. Phenylephrine is a post-synaptic alpha-adrenergic receptor agonist that causes vasoconstriction, increases systolic/diastolic pressures, reflex bradycardia, and stroke output.
Compositions and methods for treatment of coughing, sneezing, rhinorrhea, and/or nasal obstruction
The present invention relates to compositions comprising an antitussive, a decongestant and an expectorant, and in a specific embodiment comprising hydrocodone, phenylephrine hydrochloride and guaifenesin, wherein the composition may be substantially free of added sugar and added alcohol, and methods for using these compositions for the treatment of patients suffering from, for example and without limitation, coughing, sneezing, rhinorrhea, and / or nasal obstruction.
Owner:EVERETT LAB
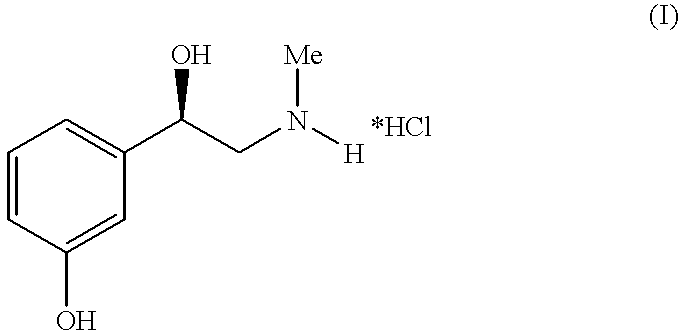
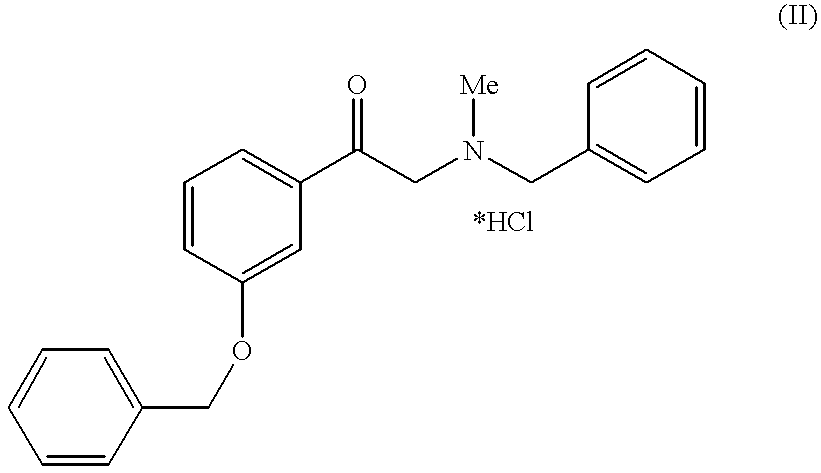
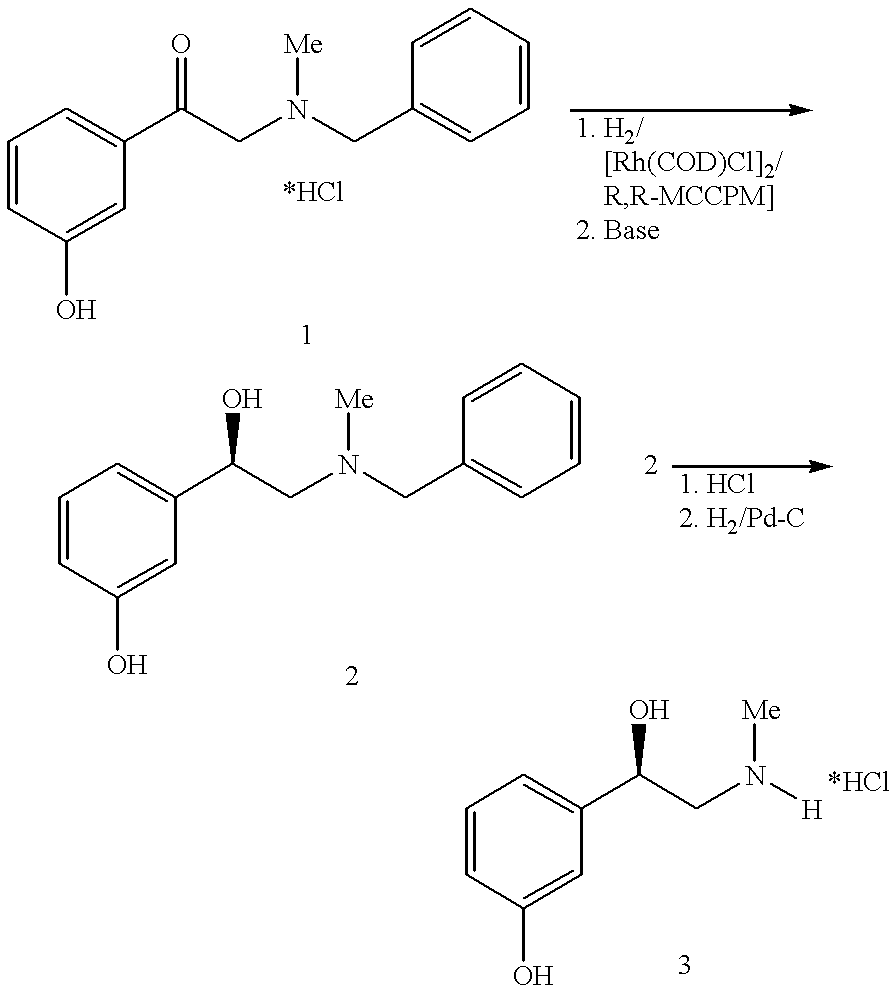
Method for preparing of L-phenylephrine hydrochloride
InactiveUS6187956B1Shorten the timeLow costSenses disorderOrganic compound preparationAsymmetric hydrogenationPyrrolidine
The present invention relates to an improved process for preparing L-phenylephrine hydrochloride 3 on an industrial scale by asymmetric hydrogenation as the key step and a special sequence of subsequent steps, using [Rh(COD)Cl]2 as catalyst and a chiral, two-pronged phosphine ligand such as (2R,4R)-4-(dicyclohexylphosphino)-2-(diphenylphosphino-methyl)-N-methyl-aminocarbonyl-pyrrolidine as the catalyst system.
Owner:BOEHRINGER INGELHEIM PHARM KG
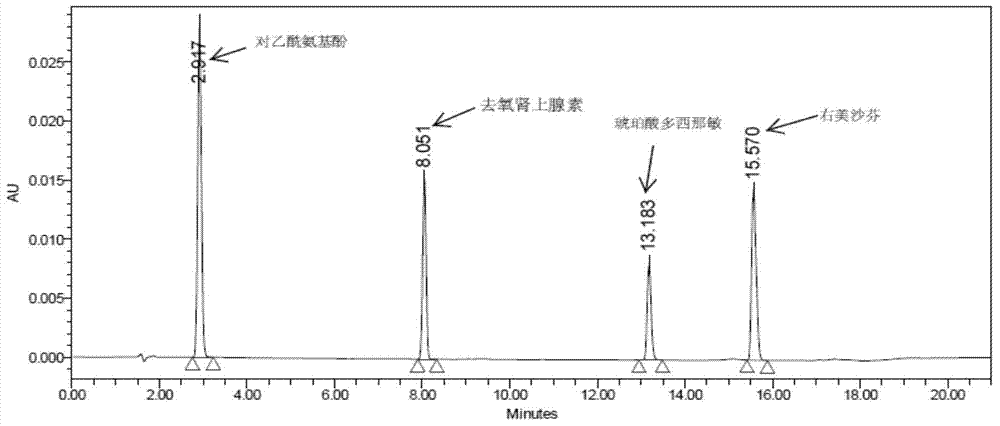
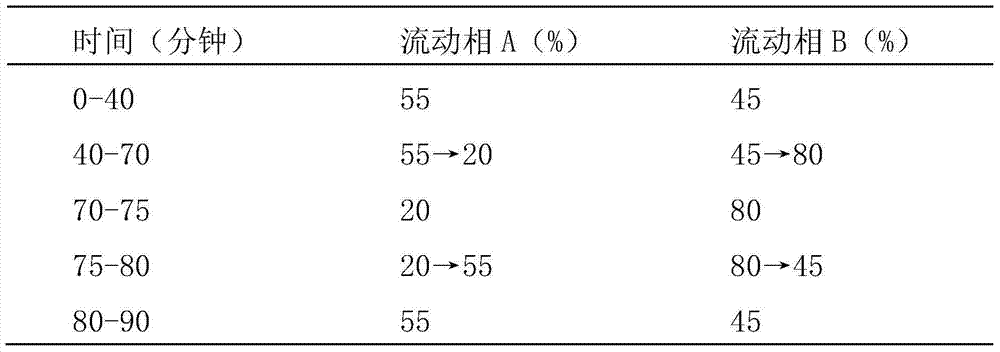
Method for analyzing night cold flu cough allergy capsule by utilizing HPLC (High Performance Liquid Chromatography)
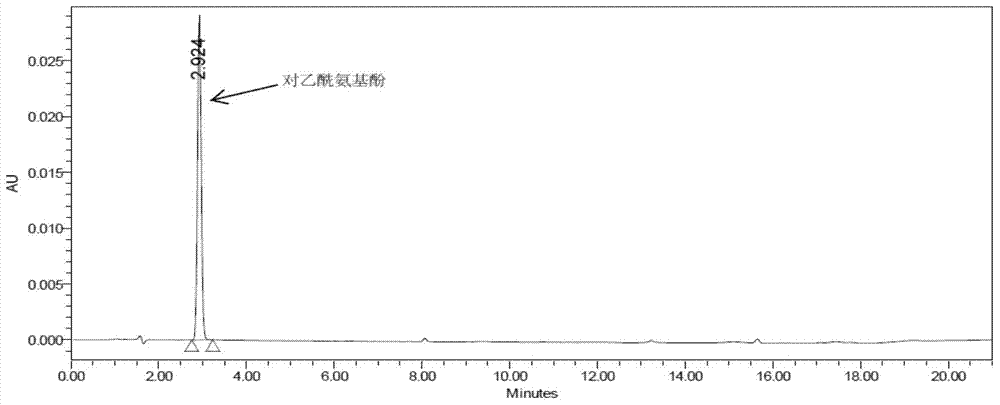
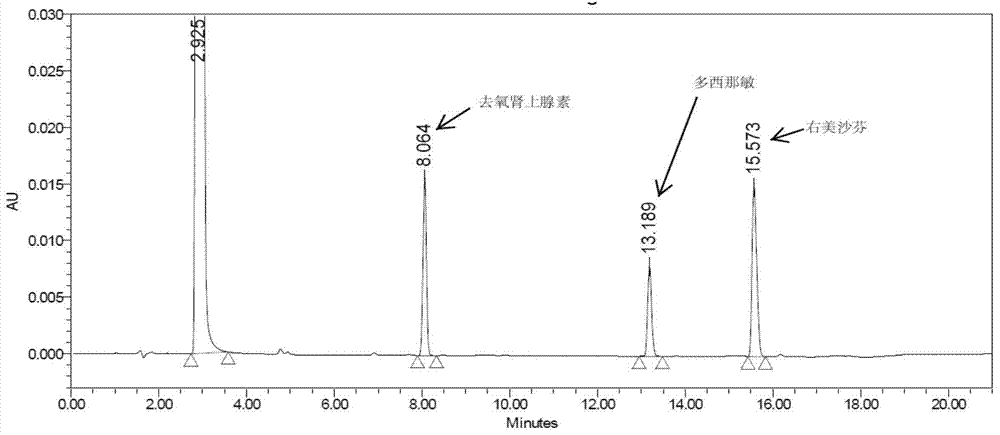
The invention discloses a method for analyzing a night cold flu cough allergy capsule by utilizing HPLC (High Performance Liquid Chromatography). The night cold flu cough allergy capsule contains acetaminophen, phenylephrine hydrochloride, succinic acid doxylamine and dextromethorphan hydrobromide. In the HPLC analysis, an octadecyl silane bonded silica gel column is adopted as a chromatographic column; a sodium 1-octanesulfonate-phosphate buffer solution with the pH of 2.0-3.0 acts as a mobile phase A; acetonitrile and a mixed solution of acetonitrile and methyl alcohol act as a mobile phase B. The method can be simultaneously and effectively used for detecting four effective ingredients in the night cold flu cough allergy capsule, is simple to operate, analyzes rapidly, is good in repeatability, has favorable specificity, and can effectively and comprehensively control the product quality of the night cold flu cough allergy capsule.
Owner:HUMANWELL PURACAP PHARM WUHAN CO LTD
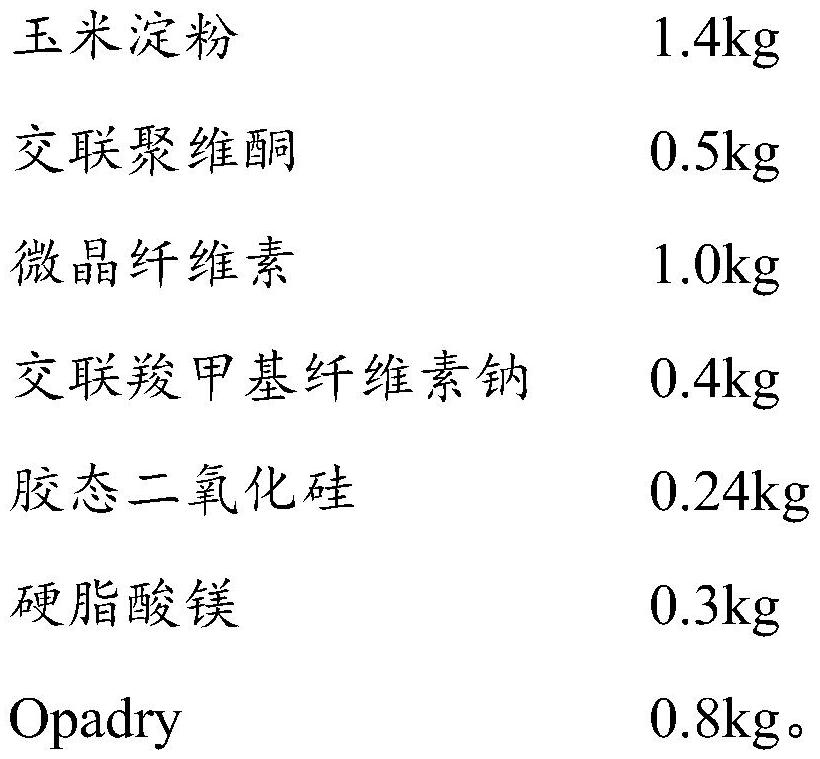
Allergy and congestion relief and preparation method thereof
ActiveCN104983732AImprove stabilitySimple preparation processOrganic active ingredientsPharmaceutical non-active ingredientsAllergySilicon dioxide
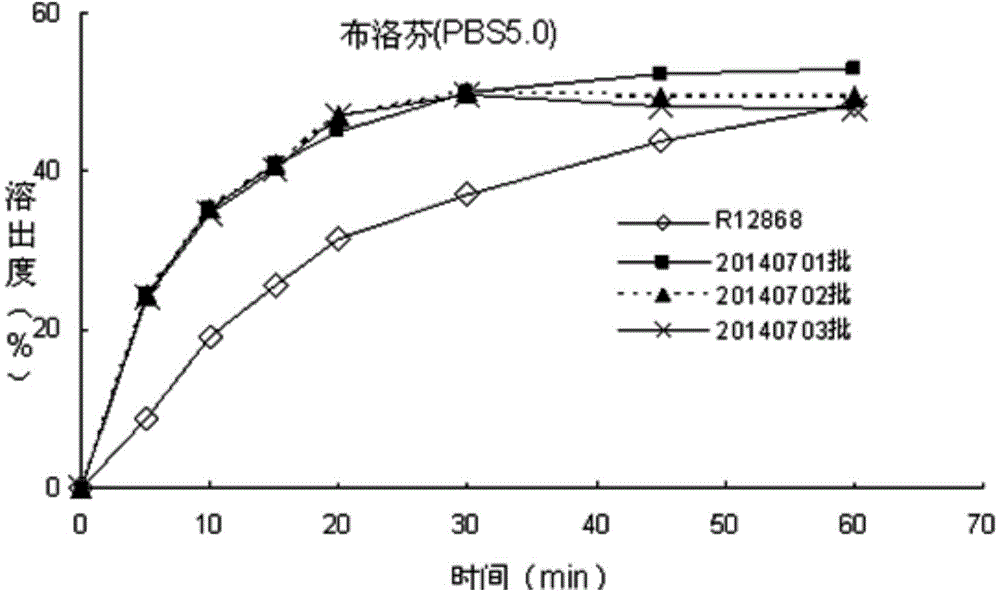
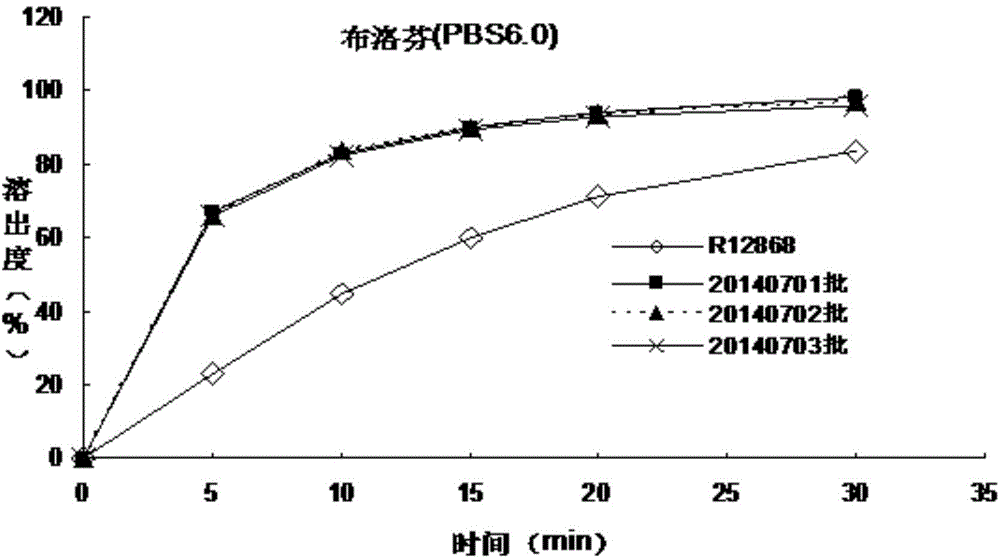
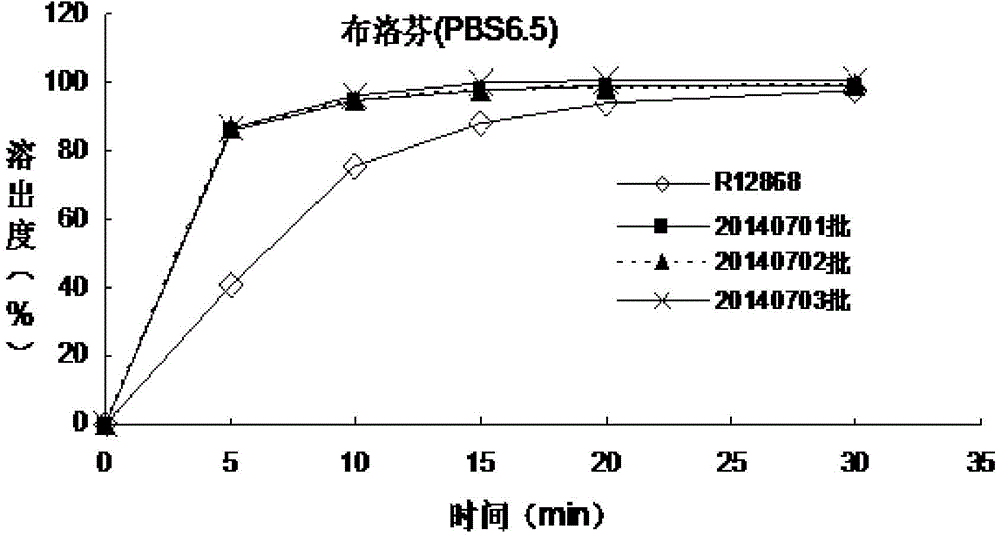
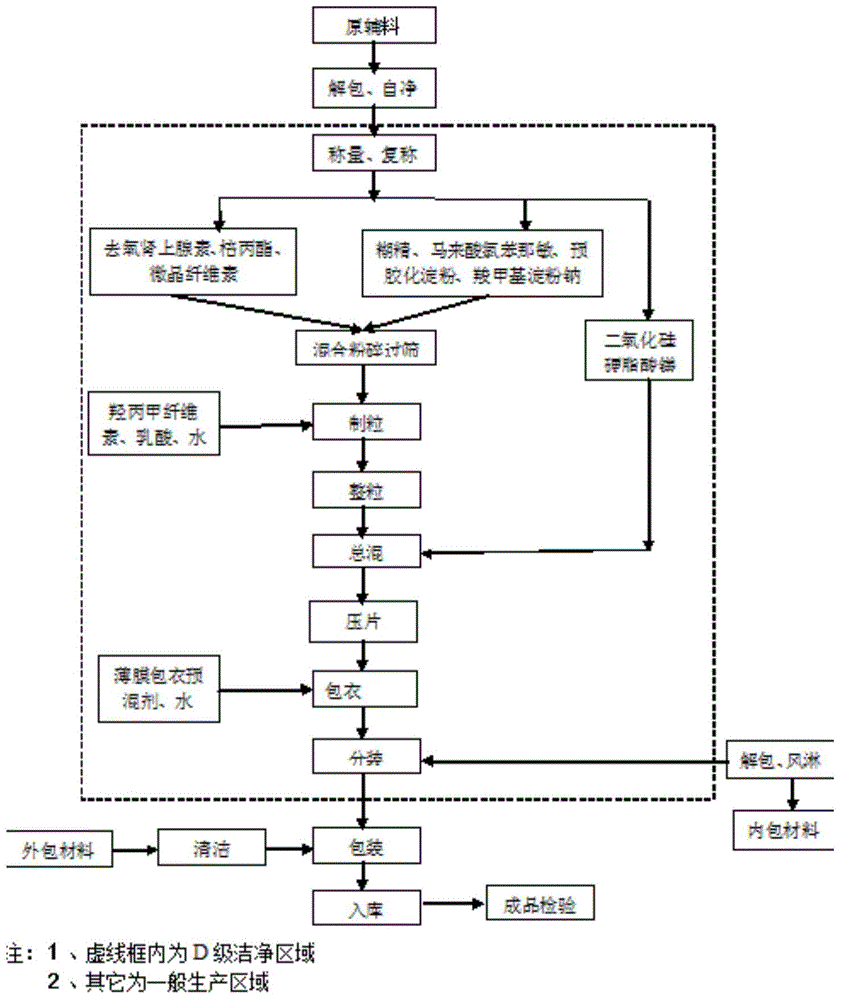
The invention discloses an allergy and congestion relief and a preparation method thereof. The prescription of the allergy and congestion relief comprises ibuprofen, phenylephrine hydrochloride, chlorpheniramine maleate, microcrystalline cellulose, pregelatinized starch, hydroxypropyl methylcellulose, sodium carboxymethyl starch, silicon dioxide, a film coating premixed agent, lactic acid and propyl gallate. Compared with the prior art, the prepared allergy and congestion relief has the beneficial effects that stability is good, the stability of key indexes such as impurities is obviously superior to that of launched products abroad, and the dissolution rate of medicines, especially the dissolution rate of the indissolvable medicine ibuprofen is equivalent to or slightly rapider than that of the launched products abroad.
Owner:CHINA RESOURCES SANJIU MEDICAL & PHARMA +1
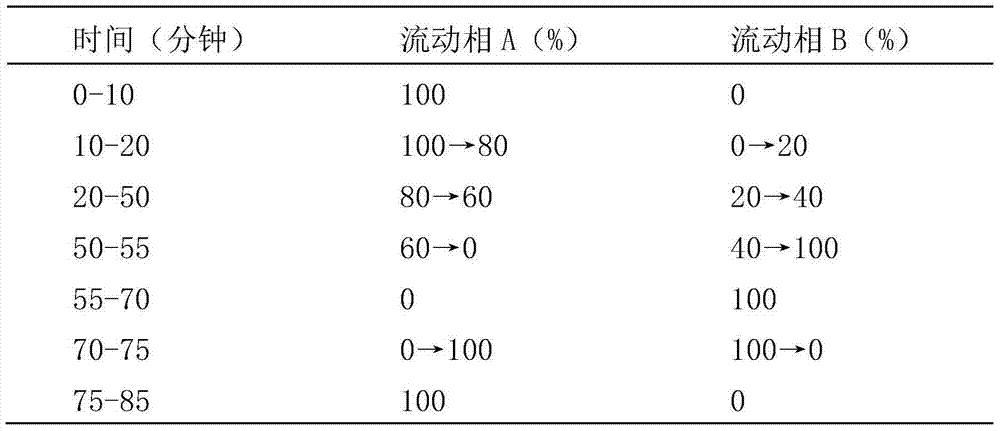
Method for determining content of three components comprising phenylephrine hydrochloride, chlorphenamine maleate and ibuprofen in compound cold treatment tablet
ActiveCN104251889AComprehensive quality inspection indicatorsGood repeatabilityComponent separationChlorphenamine maleateSulfonate
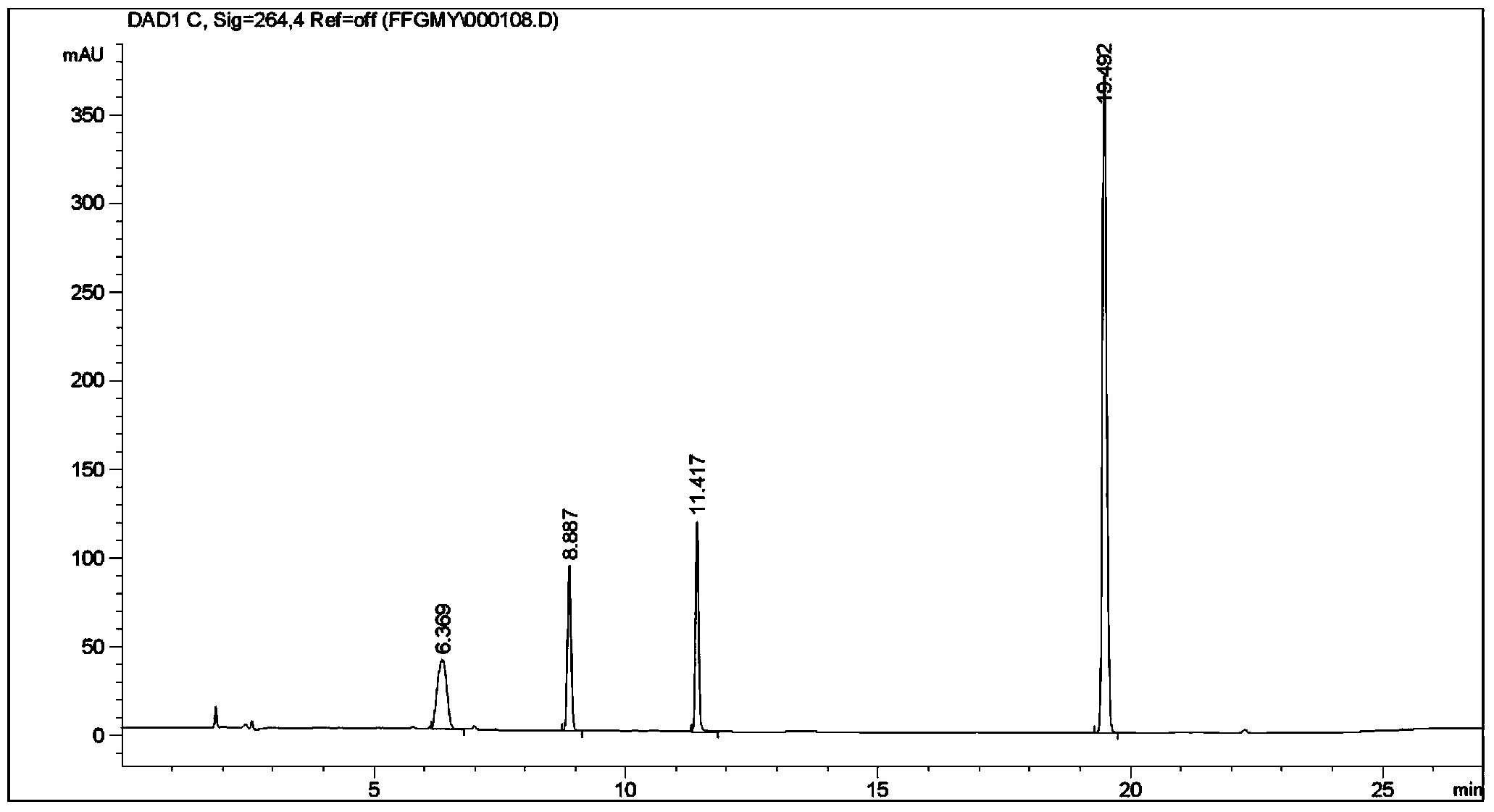
he invention discloses a method for simultaneously determining phenylephrine hydrochloride, chlorphenamine maleate and ibuprofen in a compound cold treatment medicine. The method comprises the following steps: respectively preparing a phenylephrine hydrochloride reference substance solution, a chlorphenamine maleate reference substance solution and an ibuprofen reference substance solution; preparing a compound cold treatment medicine sample solution; and determining through high performance liquid chromatography, wherein octadecyl silane-bonded silica gel (2504.0mm, 5mum) is used as a filler, a sodium octane sulfonate solution is used as a mobile phase A, acetonitrile is used as a mobile phase B, gradient elution is carried out, the column temperature is 35DEG C, the flow velocity is 1ml / min, and the detection wavelength is 264nm.
Owner:SHENZHEN NEPTUNUS PHARMA RES INST CO LTD
Solid preparation of compound ammonia phenol renin medicine composition liposome
The invention discloses a solid preparation of a compound ammonia phenol renin medicine composition liposome and a preparation method thereof. The method comprises that the compound ammonia phenol renin medicine composition liposome is prepared by acetaminophen, anhydrous caffein, phenylephrine hydrochloride, chlorpheniramine maleate, vitamin B1, egg yolk lecithin acyl serine, phosphatidyl ethanolamine and octadecylamine which are selected according to specified weight ratio, and then the solid preparation is prepared by the compound ammonia phenol renin medicine composition liposome through an ordinary preparation method. The solid preparation of the liposome is high in encapsulation and even in particle size, improves quality of a preparation product, reduces toxic and side effects, andis suitable for industrialized production. In addition, the preparation method is simple, and the medicine is reserved in blood circulation for a long time.
Owner:HAINAN MEIDA PHARMA
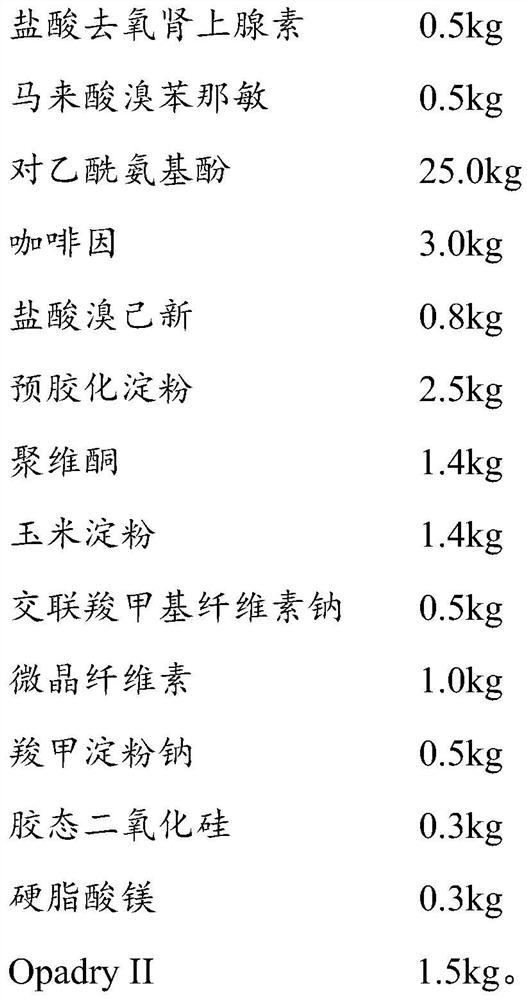
Method for analyzing daytime severe cold and flu capsules by high performance liquid chromatography
The invention discloses a method for analyzing daytime severe cold and flu capsules by high performance liquid chromatography. The daytime severe cold and flu capsules contain acetaminophen, phenylephrine hydrochloride, dextromethorphan hydrobromide and guaifenesin; high performance liquid chromatography analysis conditions are as follows: a chromatographic column adopts an octadecyl silane bonded silica gel column, a sodium octanesulfonate containing 0.1v% triethylamine is used as a mobile phase A, and acetonitrile is used as a mobile phase B. The method can efficiently and simultaneously detect four effective components in the daytime severe cold and flu capsules; furthermore, the method is easy to operate, high in analysis speed, high in repetitiveness and extremely high in specificity; the product quality of a daytime severe cold and flu capsule preparation can be relatively efficiently and comprehensively controlled.
Owner:HUMANWELL PURACAP PHARM WUHAN CO LTD
Phenylephrine hydrochloride oral instant membrane and preparation method thereof
InactiveCN102871984AGreat tasteDissolve fastOrganic active ingredientsPharmaceutical delivery mechanismAllergic reactionNon-allergic rhinitis
The invention belongs to the field of a medicine technology which relates to a phenylephrine hydrochloride oral instant membrane and a preparation method of the phenylephrine hydrochloride oral instant membrane. The phenylephrine hydrochloride oral instant membrane is good in taste, capable of being quickly dissolved or disintegrated in an oral cavity, quickly released, and rapidly absorbed and taken effect in gastrointestinal tracts after the phenylephrine hydrochloride oral instant membrane is swollen. The phenylephrine hydrochloride oral instant membrane provided by the invention is utilized to eliminate nasopharyngeal mucosa hyperemia, relieve nasal mucosa hyperemia and swelling, and alleviate a nasal obstruction symptom caused by cold, influenza, an allergic reaction or non-allergic rhinitis and the like.
Owner:天津市聚星康华医药科技有限公司
Composition for improving stability of compound ibuprofen preparations and preparation method of composition
InactiveCN104758935AImprove stabilityReduce riskOrganic active ingredientsPowder deliveryTabletingOrganic chemistry
The invention discloses a composition for improving stability of compound ibuprofen preparations. The composition comprises the following components in parts by weight: 200 parts of ibuprofen, 10 parts of phenylephrine hydrochloride, 4 parts of chlorpheniramine maleate, 14-56 parts of glyceryl behenate and 50-800 parts of additives. The compound ibuprofen preparation composition can be directly used for preparing compound ibuprofen solid preparations such as tablets, capsules or granules or can be mixed with a proper quantity of additives to prepare the compound ibuprofen solid preparations such as tablets, capsules or granules by virtue of processes of tabletting, filling capsules or split charging. The compound ibuprofen preparation composition can be used for greatly improving the stability of ibuprofen, phenylephrine hydrochloride and chlorpheniramine maleate; the quality of products can be improved; the effective periods of the products can be prolonged; the risk of clinical medication is reduced.
Owner:铂镁医学临床研究(上海)有限公司
Preparation method for daytime-taken softgel for treating cold
ActiveCN103462927ANice appearanceStrong complianceOrganic active ingredientsAntipyreticMelting tankAlcohol
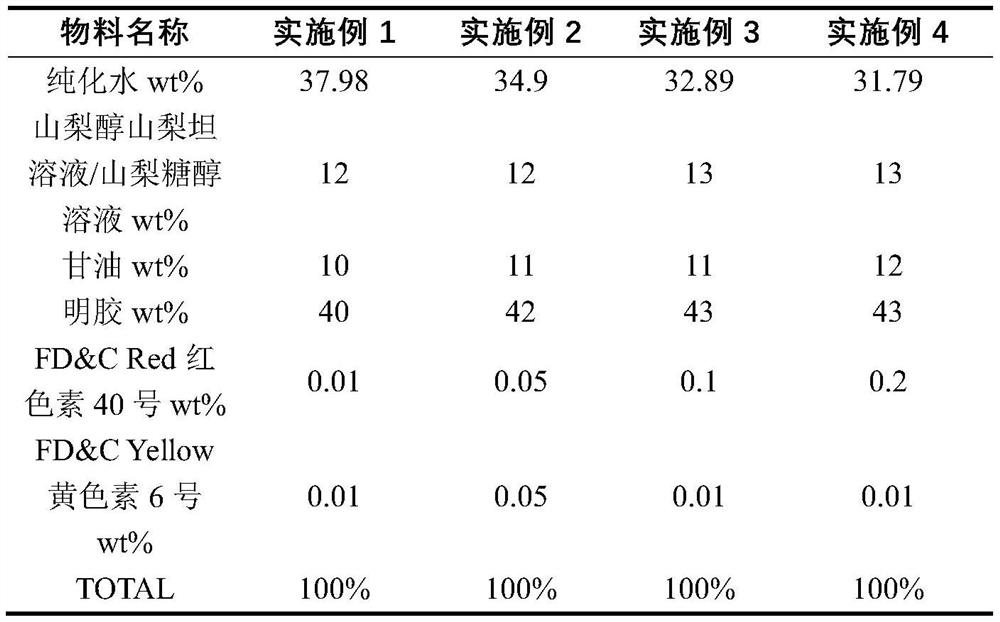
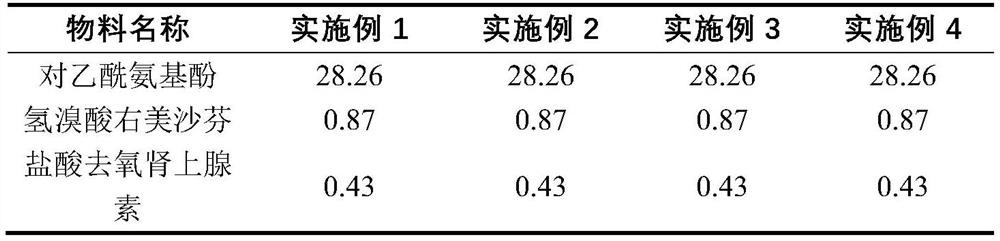
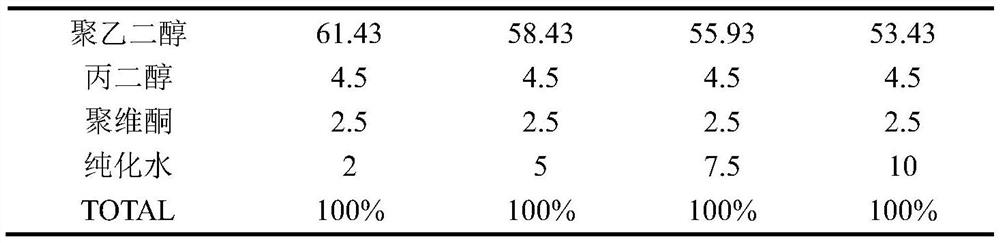
The invention discloses a preparation method for a daytime-taken softgel for treating a cold. The preparation method comprises the following steps: A, preparation of a gelatin liquor: dissolving plasticizer in water, adding into a gelatin melting tank, adding gelatin, stirring to dissolve, adding coloring agent into water, stirring uniformly to obtain a pigment solution, adding the pigment solution into an obtained gelatin solution, stirring and vacuumizing to exhaust air bubbles, so as to obtain the gelatin liquor; B, preparation of contents: adding mixed polyethylene glycol and polyhydric alcohol into polyvidone, stirring to dissolve, adding acetaminophen, stirring to obtain a suspension, adding into a proportioning tank, vacuumizing, filling with inert gas, heating to dissolve, cooling, adding dextromethorphan hydrobromide and phenylephrine hydrochloride, vacuumizing, and keeping the temperature to dissolve, so as to obtain a content solution; C preparation of the softgel: adding the gelatin liquor and the content solution into a pelleting machine respectively, pelleting, drying, pellet plastering and packaging. The invention aims to provide the preparation method for the daytime-taken softgel which is used for treating the cold, and is simple in process, convenient to manufacture and good in product stability.
Owner:安士制药(中山)有限公司
Allergy and Congestion Relief composition and preparation method thereof
InactiveCN107929247AImprove stabilityDissolution completeOrganic active ingredientsPill deliveryMedicineAllergy
The invention belongs to the technical field of medicine, and particularly relates to an Allergy and Congestion Relief composition and a preparation method thereof, wherein the Allergy and CongestionRelief composition contains ibuprofen, phenylephrine hydrochloride, chlorpheniramine maleate, lysine, instant sorbitol, a binder, a lubricant, and a disintegrant. According to the present invention, the product has advantages of good stability, complete dissolution and good quality; and the production method has the simple and easy-performing operation, and is suitable for industrial production.
Owner:长春海悦药业股份有限公司
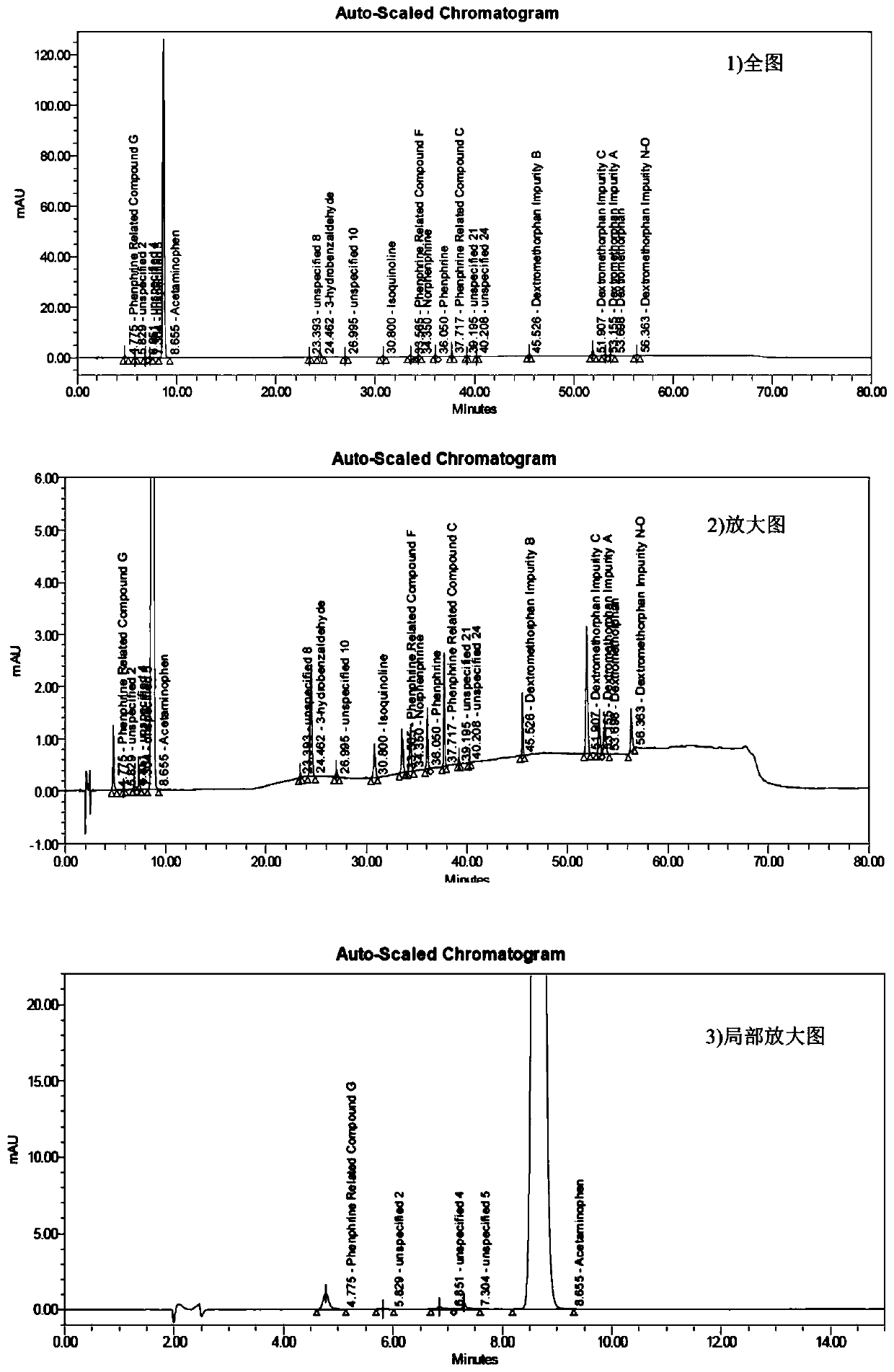
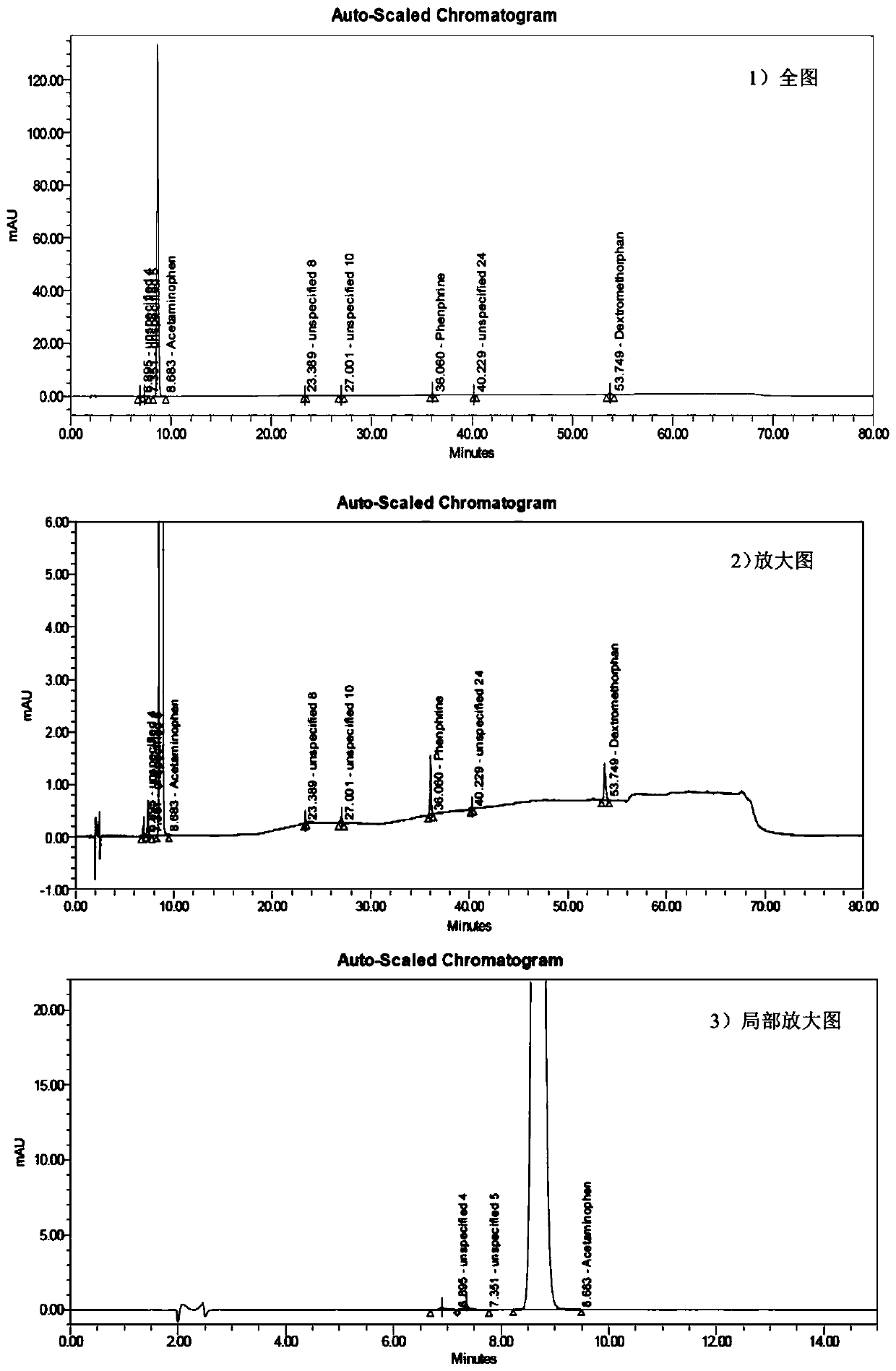
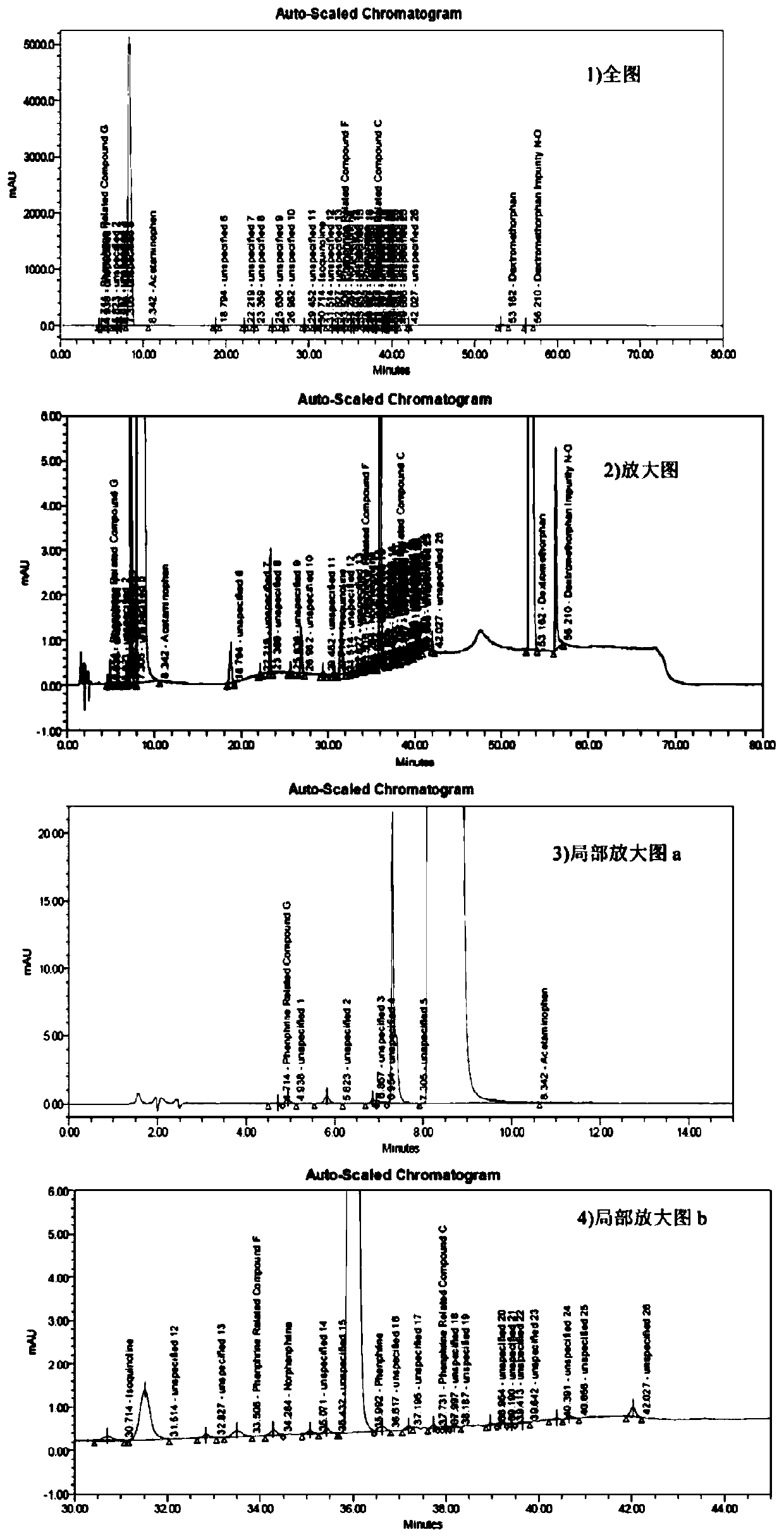
Method for determining related substances of pharmaceutical preparation containing acetaminophen, dextromethorphan hydrobromide and phenylephrine hydrochloride
The invention discloses a method for determining related substances of a pharmaceutical preparation containing acetaminophen, dextromethorphan hydrobromide and phenylephrine hydrochloride. The determination method disclosed by the invention is performed by adopting a high performance liquid chromatography; proper HPLC chromatographic conditions are screened out; and 10 related substances of dextromethorphan impurities I, dextromethorphan impurities II, dextromethorphan impurities III, dextromethorphan impurities IV, norepinephrine, a phenylephrine related substance F, a 4,6 diol isoquinoline analogue, a phenylephrine related substance C, 3-hydroxybenzaldehyde and a phenylephrine related substance G in the pharmaceutical preparation of acetaminophen, dextromethorphan hydrobromide and phenylephrine hydrochloride can be simultaneously determined under the same condition. Effective separation of a variety of impurities can be achieved, and detection time and detection cost are greatly saved. The method can be used for quality research and quality control of pharmaceutical preparation products containing the acetaminophen, the dextromethorphan hydrobromide and the phenylephrine hydrochloride.
Owner:安士制药(中山)有限公司
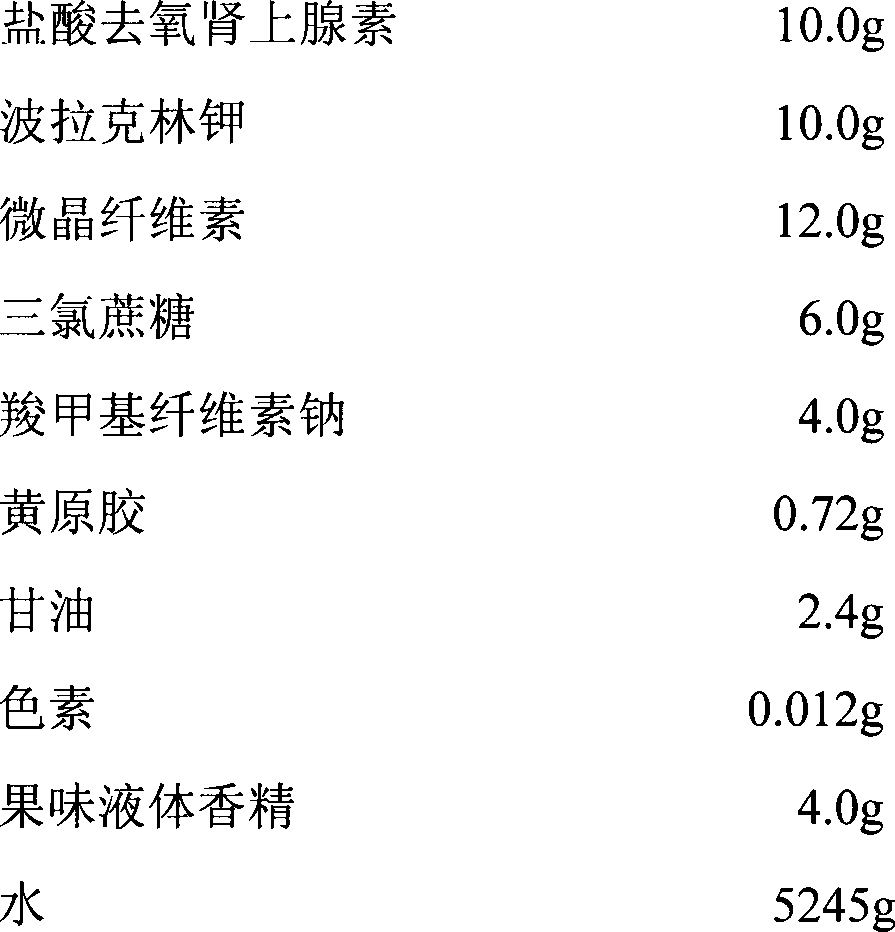
Phenylephrine hydrochloride injection and preparation process thereof
ActiveCN102525893ALow costOrganic active ingredientsInorganic non-active ingredientsFormularyPhenylephrine Injection
The invention discloses a formula of phenylephrine hydrochloride injection and a preparation process thereof. The phenylephrine hydrochloride injection contains, per 10000 mL, 100 g of phenylephrine hydrochloride, 40 g to 80 g of sodium chloride, 0.8 g to 1.2 g of disodium ethylenediaminetetraacetate, and water for injection added up to 10000 mL. The invention provides a low-cost prescription of phenylephrine hydrochloride injection and a preparation process thereof. The quality of the phenylephrine hydrochloride injection conforms to the regulations of notice on issue of chemical injection and multi-component biochemical injection basic technical requirements ([2008] No.7 file) issued by State Food and Drug Administration in 2008.
Owner:上海禾丰制药有限公司
Instant film preparation containing phenylephrine hydrochloride
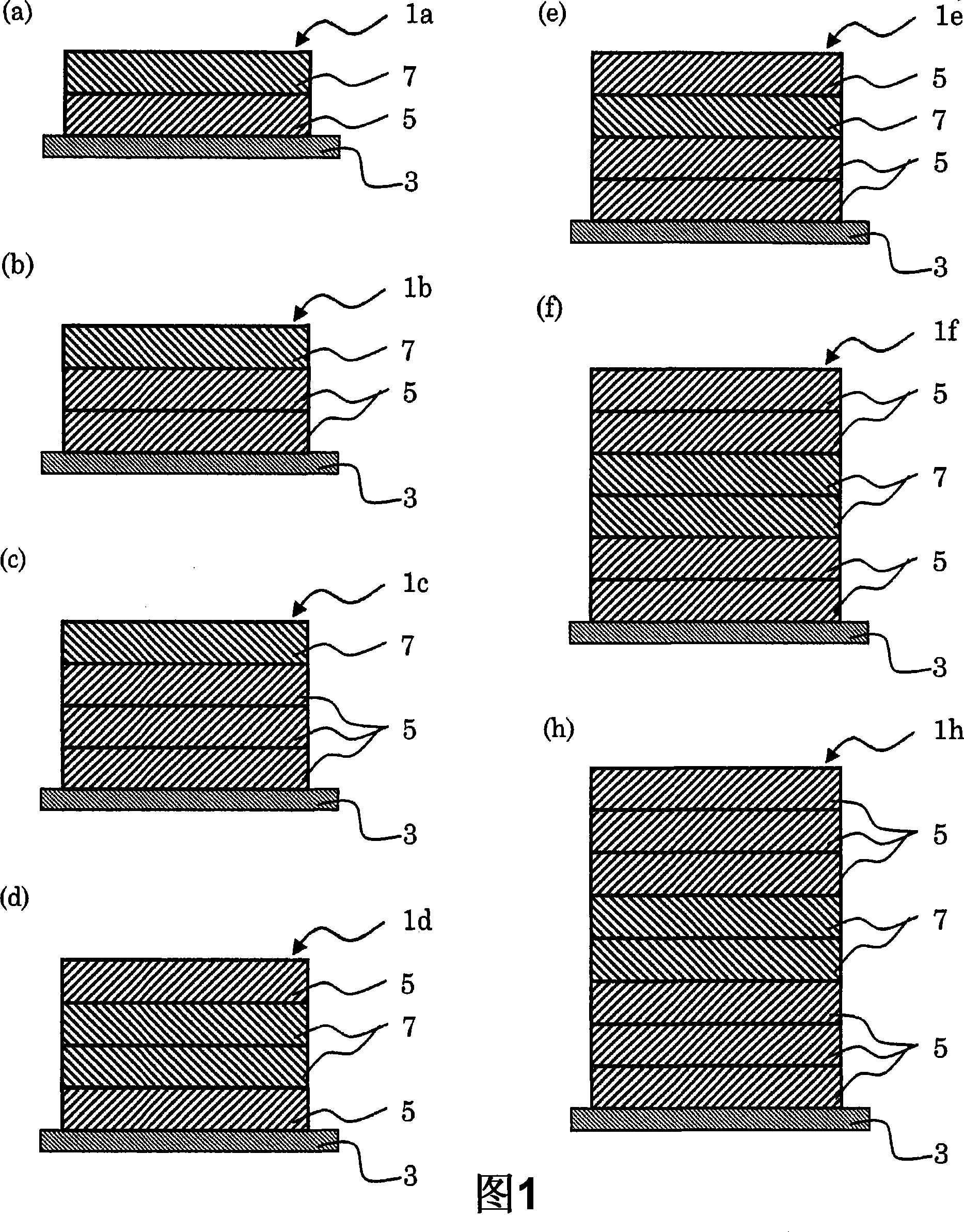
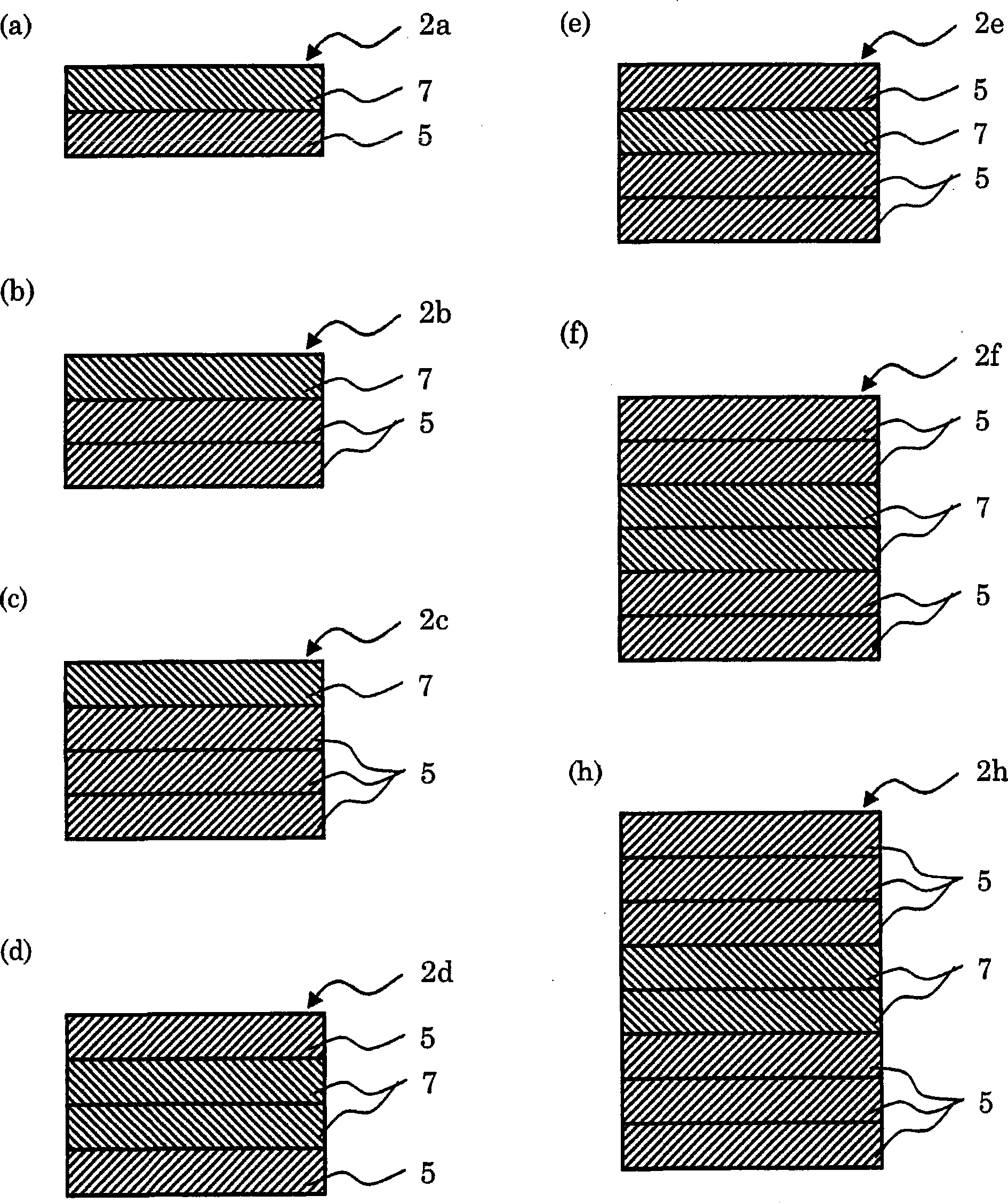
InactiveCN101422447AEasy to peelEasy to manufactureOrganic active ingredientsNervous disorderMethyl celluloseEpinephrine
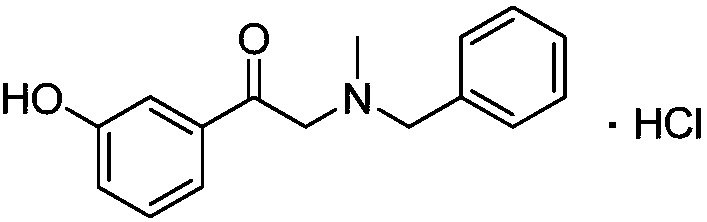
The present invention provides a membrane preparation which is combined with phenylephrine hydrochloride, hydroxypropyl cellulose and / or hydroxypropyl methyl cellulose and can be quickly dissolved in oral cavity. The rapidly soluble membrane preparation 1 comprising phenylephrine hydrochloride 1 according to the invention is characterized by comprising a resin film 3, one layer or more than two layers of shielding layer 5, and one layer or more than two layers of medicine-containing layer 7. The shielding layer 5 is formed on the resin film 3 and comprises edible macromolecular substance and does not contain phenylephrine hydrochloride. Additionally the medicine-containing layer 7 is formed on the shielding layer 5 and comprises at least one component selected from hydroxypropyl cellulose and hydroxypropyl methyl cellulose. Furthermore the rapidly soluble membrane preparation 1 comprising phenylephrine hydrochloride 1 according to the invention can comprise one layer or more than two layers of shielding layer 5 that comprises edible macromolecular substance and does not contain phenylephrine hydrochloride according to expectation.
Owner:KOWA CO LTD +1
Phenylephrine formulations with improved stability
A pharmaceutical composition includes a pharmaceutical polysaccharide and phenylephrine hydrochloride. The ratio of said polysaccharide to phenylephrine hydrochloride is sufficient to dilute the composition such that phenylephrine hydrochloride is stable at high temperature and humidity.
Owner:NOVARTIS AG
Medical composition for treating cold and preparation method of medical composition
InactiveCN105476971APromote dissolutionImprove stabilityOrganic active ingredientsAntipyreticFreeze-dryingDissolution
The invention relates to a medical composition for treating cold and a preparation method of the medical composition. The preparation method consists of the following components by weight: 150-250 parts of ibuprofen, 5-15 parts of phenylephrine hydrochloride, 150-250 parts of mannitol, 20-60 parts of croscarmellose sodium, 50-90 parts of saccharose, 100-200 parts of microcrystalline cellulose, 10-20 parts of maltodextrin, 0.5-1.5 parts of propyl gallate, 2.5-7.5 parts of magnesium stearate and 3-5 parts of chlorpheniramine maleate. A preparation method for the two-prescription or three-prescription medical composition comprises the following steps: dissolving ibuprofen into pure water, heating to 70 DEG C, adding mannitol, dissolving, carrying out freeze drying in a freezer dryer, smashing into powder to obtain an ibuprofen and mannitol mixture, then mixing the obtained mixture with other components, and compressing by adopting a direct powder tabletting method to obtain tablets. According to the method, ibuprofen can be dissolved out quickly to achieve the quick effective effect. Through detection, dissolution of ibuprofen, chlorpheniramine maleate and phenylephrine hydrochloride in tablets prepared by adopting the method in 15 min can reach 90% or higher.
Owner:海南高升医药科技开发股份有限公司
Phehylephrine formulations with improveds stability
A pharmaceutical composition includes a pharmaceutical polysaccharide and phenylephrine hydrochloride. The ratio of said polysaccharide to phenylephrine hydrochloride is sufficient to dilute the composition such that phenylephrine hydrochloride is stable at high temperature and humidity.
Owner:NOVARTIS AG
Medicine composition for treating cold symptoms such as running noses and nasal obstruction and preparation method and application of medicine composition
ActiveCN110279695AStable blood concentrationGood treatment effectOrganic active ingredientsPharmaceutical delivery mechanismIrritationNose
The invention discloses a medicine composition for treating cold symptoms such as running noses and nasal obstruction and a preparation method and application of the medicine composition. The medicine composition is an oral liquid preparation consisting of brompheniramine maleate, dextromethorphan hydrobromide, phenylephrine hydrochloride as well as certain amounts of auxiliary materials such as a cosolvent, a preservative, a complexing agent, a pH value adjusting agent, a flavoring agent and an aromatic. The medicine composition has functions of alleviating and treating nasal obstruction caused by cold, cough, sore throats, headache, slight ache and pain and fever caused by slight irritation of throats and bronchi, and the like. The invention provides a cold medicine for children of 6-12 years old and adults and children greater than 12 years old.
Owner:北京博智绿洲医药科技有限公司
Compound pharmaceutical composition for treating rhinitis in children
InactiveCN105796578ASuitable for industrial productionEasy to prepareOrganic active ingredientsRespiratory disorderOrally disintegrating tabletSuppository
The invention discloses a compound pharmaceutical composition for treating rhinitis in children. The active ingredients consist of chlorpheniramine maleate, phenylephrine hydrochloride and dipotassium glycyrrhizinate. The pharmaceutical composition disclosed by the invention can be made into different dosage forms according to different clinical needs, including granules, orally disintegrating tablets, orally dissolving films, oral liquid and suppositories.
Owner:天津市聚星康华医药科技有限公司
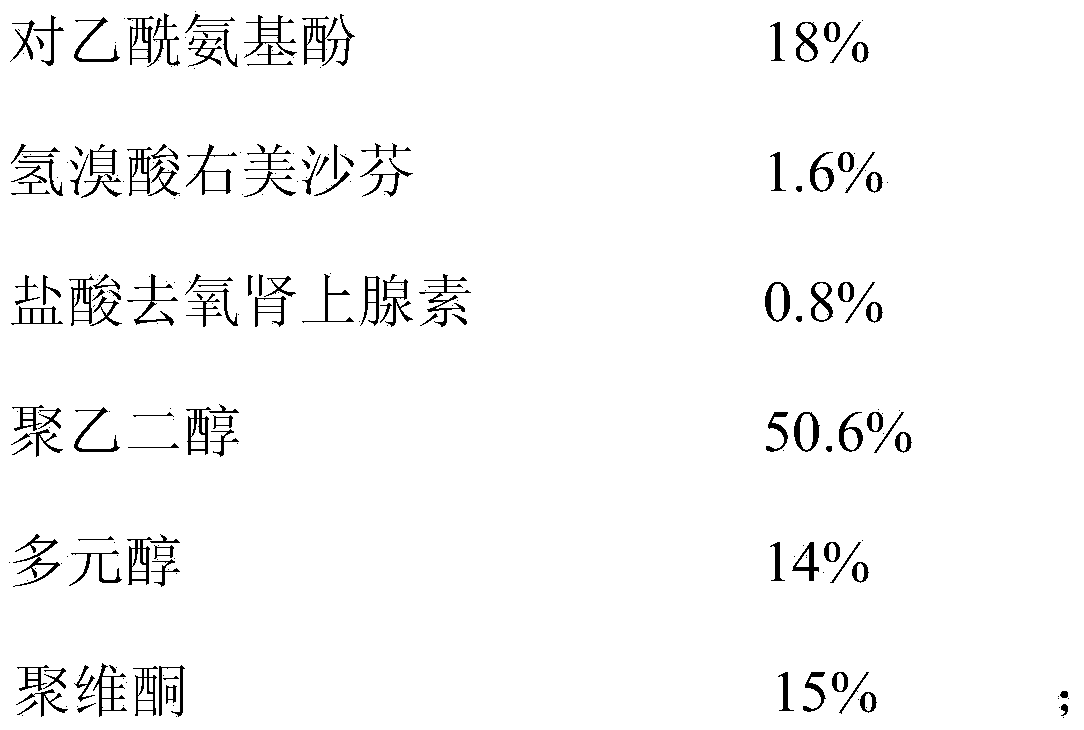
Daily compound preparation for treating cold and preparation method thereof
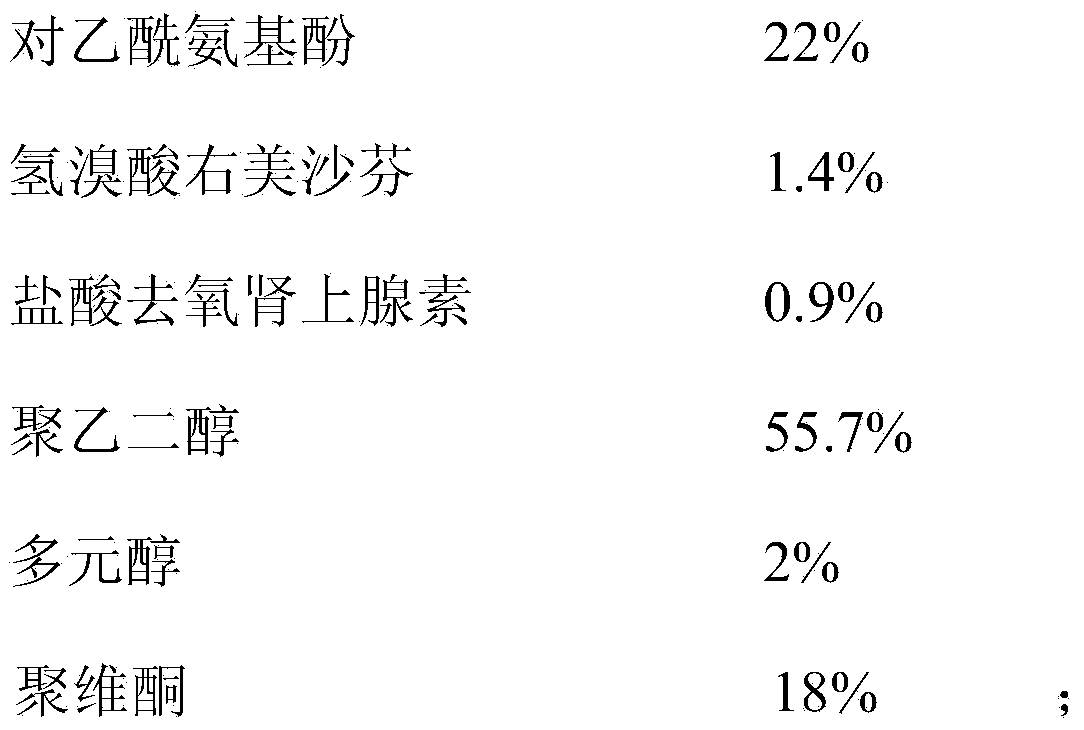
ActiveCN112716956APromote absorptionImprove efficacyOrganic active ingredientsAntiviralsPolyethylene glycolGlycerol
The invention discloses a daily compound preparation for treating cold, and particularly belongs to the technical field of pharmaceutics. The daily compound preparation is in a capsule form and comprises a capsule shell and a content in the capsule shell; the content comprises the following chemical raw materials: acetaminophen, dextromethorphan hydrobromide, phenylephrine hydrochloride, a solubilizer, purified water and one or a mixture of two or more of polyethylene glycol, propylene glycol or glycerol. The content is a clarified liquid composition, and the clarified liquid composition is good in stability, high in bioavailability, rapid in effect and capable of well treating clinical cold.
Owner:HUMANWELL PURACAP PHARM WUHAN CO LTD
Preparation method of phenylephrine hydrochloride impurity
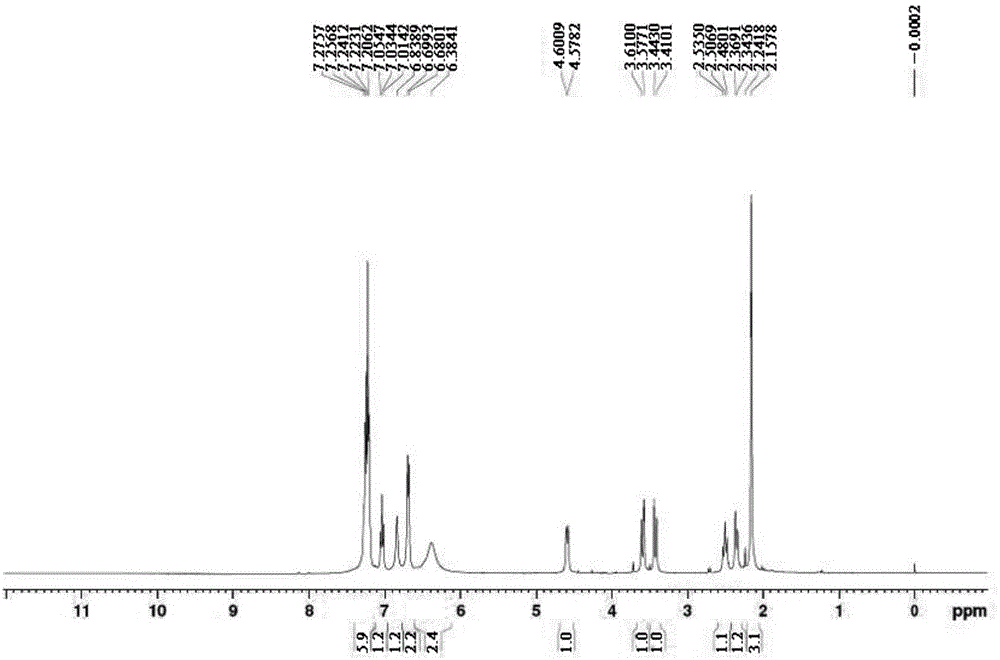
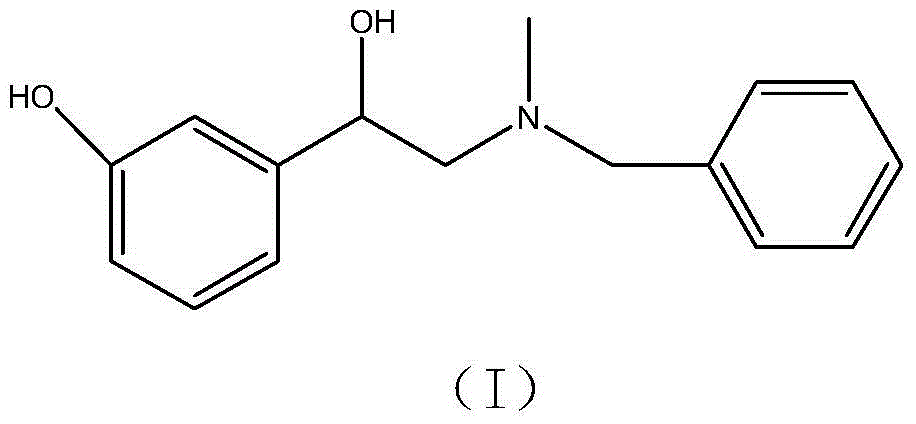
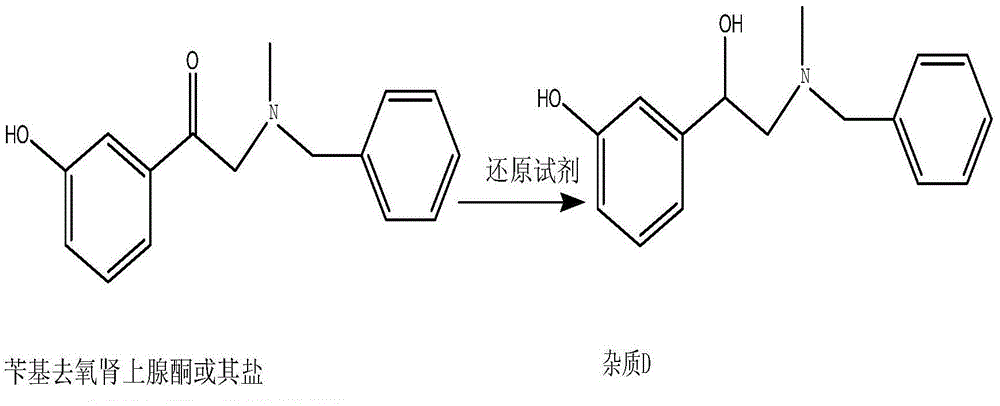
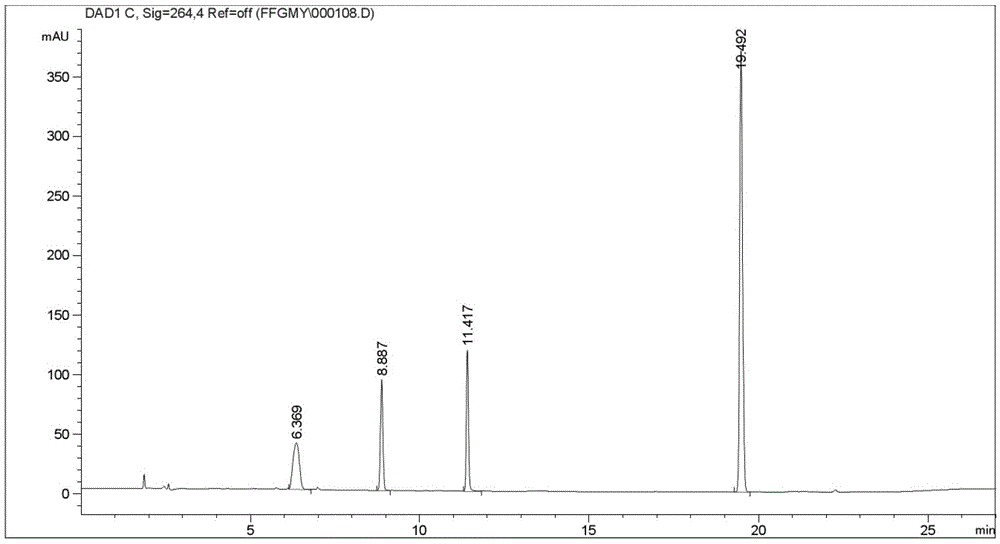
ActiveCN103553942AOrganic compound preparationAmino-hyroxy compound preparationPhenylephrine HydrochlorideNMR - Nuclear magnetic resonance
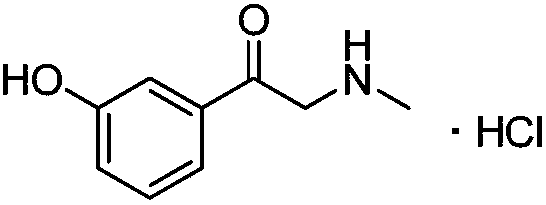
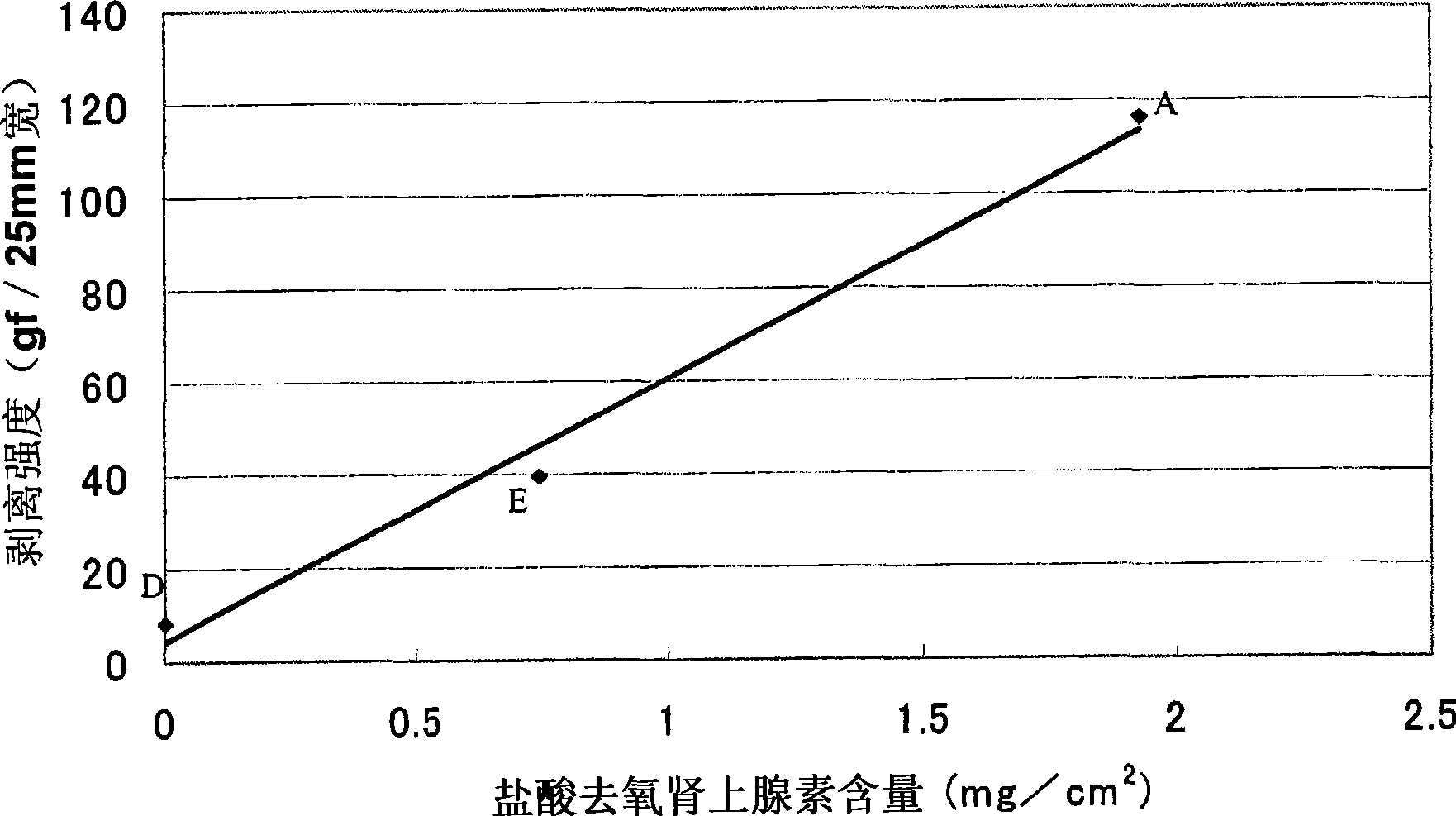
The invention belongs to the field of pharmaceutical chemistry and discloses a phenylephrine hydrochloride impurity D, namely 2-(N-benzyl methylamino)-1-(3-hydroxyl phenyl) alcohol and a preparation method thereof. An impurity D is one of main impurities of crude drugs of phenylephrine hydrochloride; the phenylephrine hydrochloride impurity D is synthesized, a reference substance is provided for examination and quantitative and qualitative analysis of the phenylephrine hydrochloride impurity, so that the quality standards of phenylephrine hydrochloride are improved, and guidance is provided for safe medication of phenylephrine hydrochloride; meanwhile, an effective test basis is provided for obtaining phenylephrine hydrochloride which satisfies EP (European Pharmacopeia) quality standards; the figure 1 is an impurity D H-nuclear magnetic resonance spectrogram.
Owner:WUHAN WUYAO SCI & TECH
Determination method of phenylephrine hydrochloride, chlorpheniramine maleate and ibuprofen in compound cold medicine tablets
ActiveCN104251889BComprehensive quality inspection indicatorsGood repeatabilityComponent separationSulfonateCold treatment
he invention discloses a method for simultaneously determining phenylephrine hydrochloride, chlorphenamine maleate and ibuprofen in a compound cold treatment medicine. The method comprises the following steps: respectively preparing a phenylephrine hydrochloride reference substance solution, a chlorphenamine maleate reference substance solution and an ibuprofen reference substance solution; preparing a compound cold treatment medicine sample solution; and determining through high performance liquid chromatography, wherein octadecyl silane-bonded silica gel (2504.0mm, 5mum) is used as a filler, a sodium octane sulfonate solution is used as a mobile phase A, acetonitrile is used as a mobile phase B, gradient elution is carried out, the column temperature is 35DEG C, the flow velocity is 1ml / min, and the detection wavelength is 264nm.
Owner:SHENZHEN NEPTUNUS PHARMA RES INST CO LTD
Compound eye drops used before ophthalmologic operation and preparing method thereof
InactiveCN105213418AImprove comfortReduced source ocular surface toxicityOrganic active ingredientsSenses disorderTropicamideMydriasis
The invention discloses compound eye drops used before an ophthalmologic operation and a preparing method thereof. The eye drops are prepared from, by weight, 5 mg / ml proparacaine hydrochloride, 5 mg / ml tropicamide, 5 mg / ml phenylephrine hydrochloride, 1-3 mg / ml sodium hyaluronate, buffer salt, isoosmotic adjusting agent, pH modifier and water for injection. By the adoption of the compound eye drops used before an ophthalmologic operation, mydriasis and surface anesthesia can be achieved, the workload of nurses is effectively relieved, and the working efficiency of nurses is improved.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Medicament
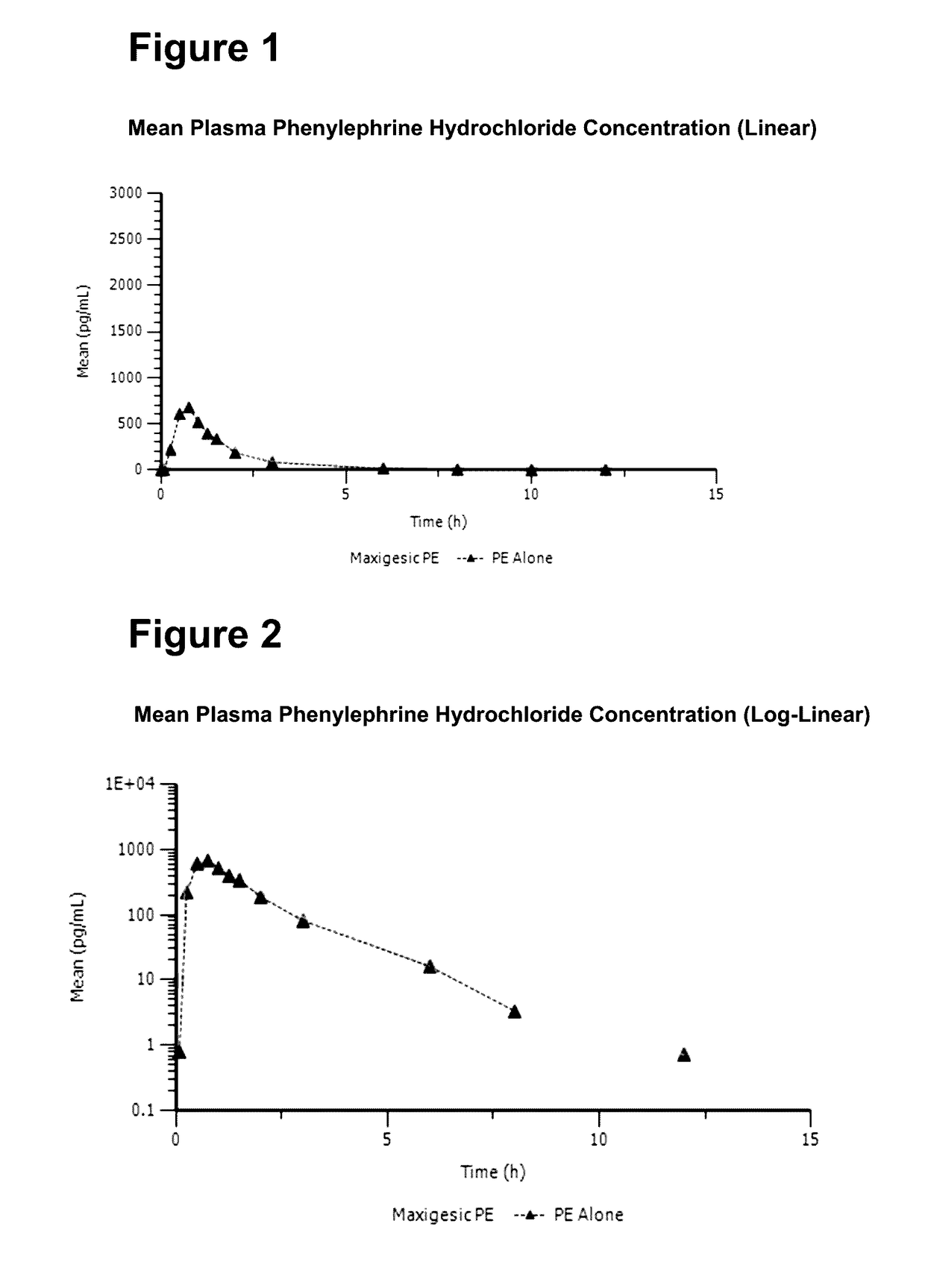
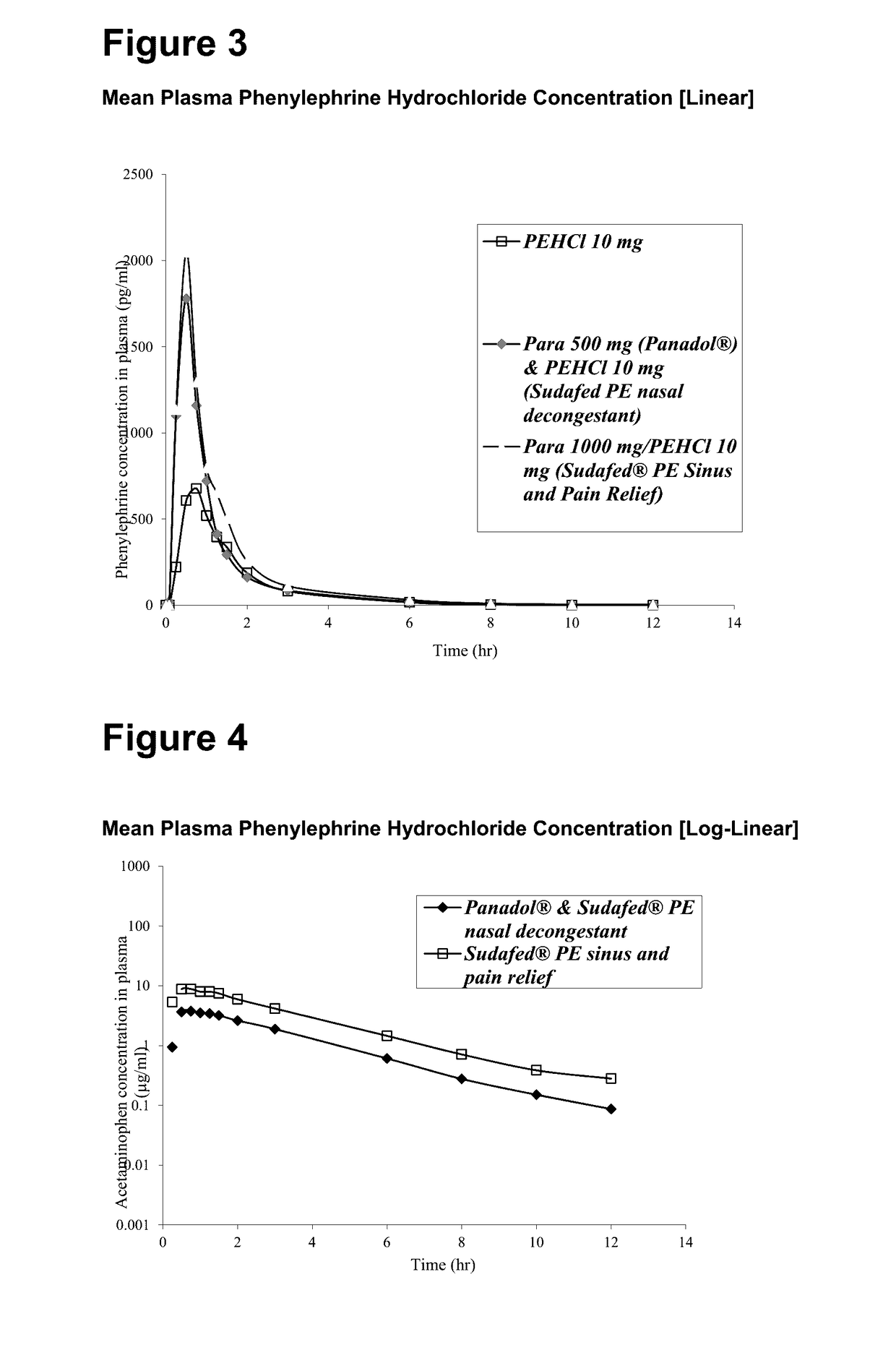
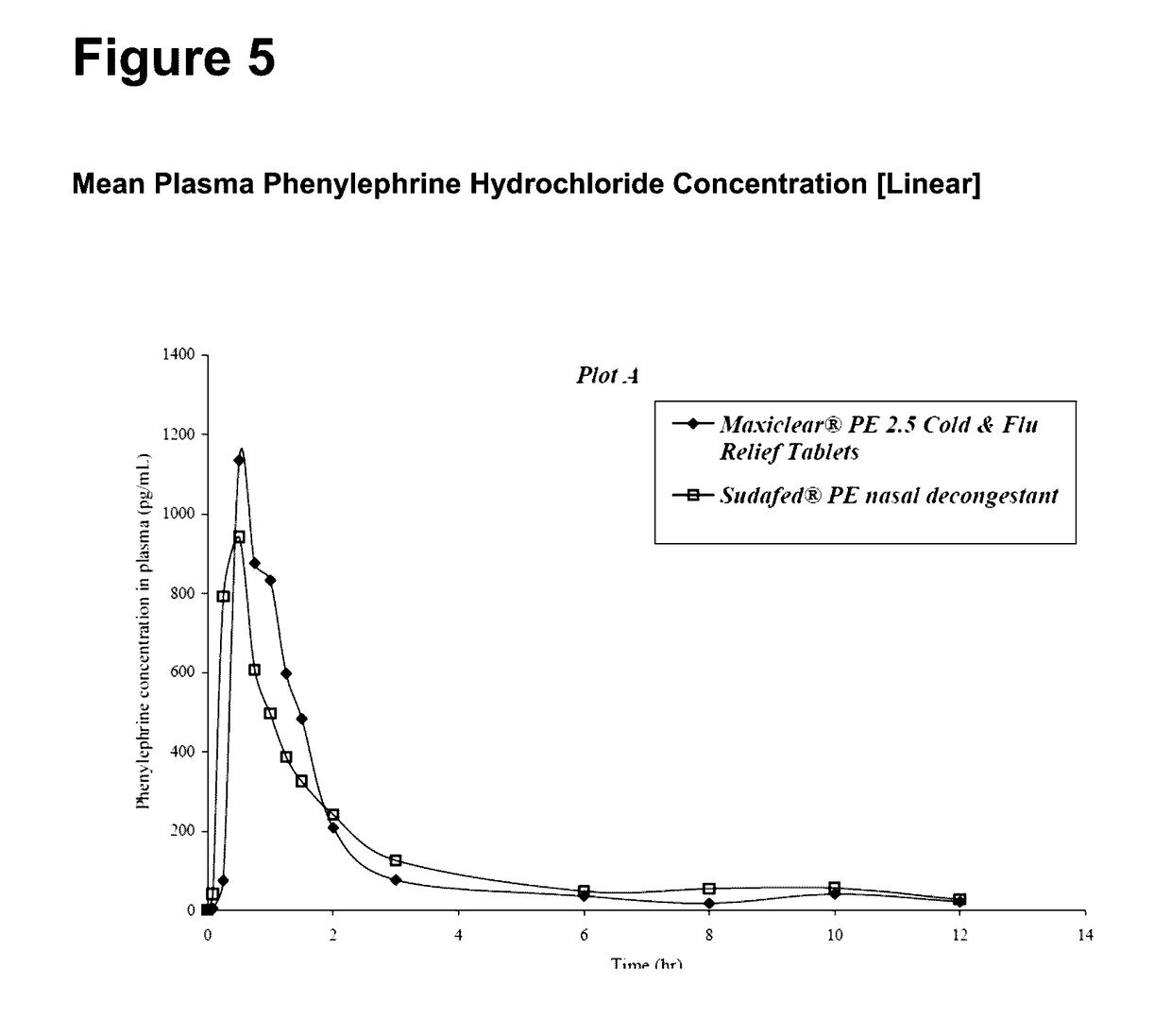
A medicament for treating upper respiratory mucosal congestion, the medicament having a combination of:approximately 2 mg to approximately 4 mg phenylephrine hydrochloride (or an equivalent amount of a pharmaceutically acceptable alternative form of phenylephrine); andapproximately 250 mg to approximately 500 mg paracetamol;for providing an adult with a total dose of:approximately 4 mg to approximately 8 mg phenylephrine hydrochloride (or an equivalent amount of a pharmaceutically acceptable alternative form of phenylephrine); andapproximately 500 mg to approximately 1,000 mg paracetamol.
Owner:AFT PHARM LTD
Medicament
Owner:AFT PHARM LTD
Phenylephrine hydrochloride-chlorpheniramine maleate tablet and preparation method thereof
ActiveCN104922124AImprove stabilitySimple preparation processOrganic active ingredientsPharmaceutical delivery mechanismSilicon dioxidePharmacology
The invention discloses a phenylephrine hydrochloride-chlorpheniramine maleate tablet and a preparation method thereof. The prescription of the phenylephrine hydrochloride-chlorpheniramine maleate tablet comprises phenylephrine hydrochloride, chlorpheniramine maleate, microcrystalline cellulose, dextrin, pregelatinized starch, sodium carboxymethyl starch, a silicon dioxide, a film coating premixing agent, lactic acid, propyl gallate and magnesium stearate. Compared with the prior art, the phenylephrine hydrochloride-chlorpheniramine maleate tablet prepared by the invention has the characteristics that the stability is good, the stability of key indicators of impurities and the like is obviously superior to that of a product which has been marketed abroad, and the dissolution rate of a medicine is equivalent to or slightly quicker than that of the product which has been marketed abroad.
Owner:CHINA RESOURCES SANJIU MEDICAL & PHARMA +1
Preparation method of m-hydroxybenzaldehyde
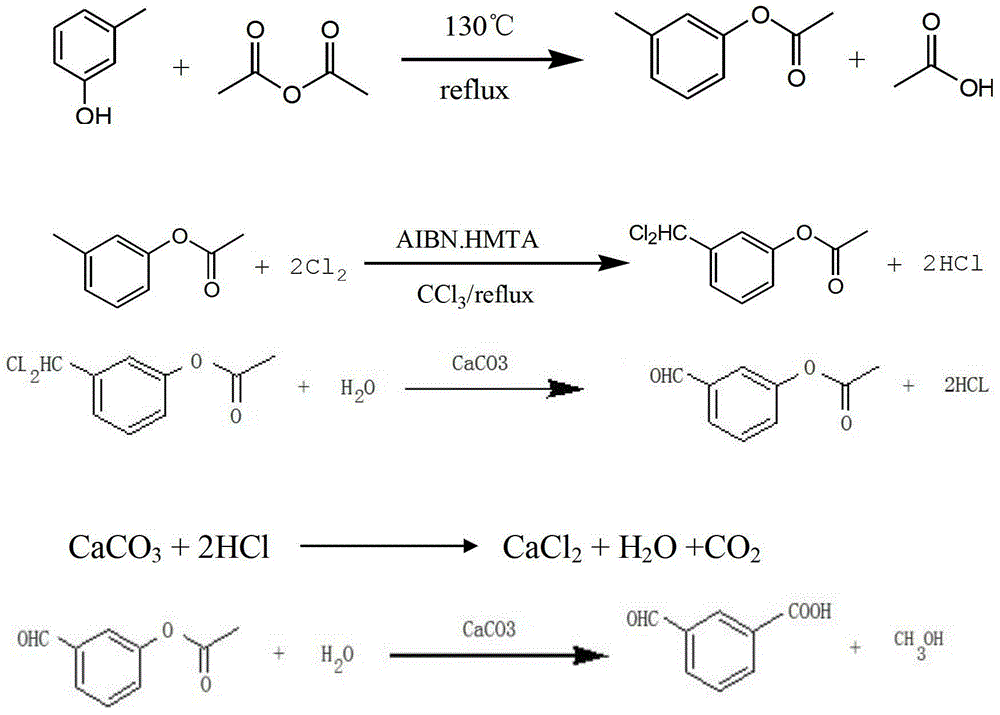
ActiveCN103333062ASimple production processRaw materials are simple and easy to obtainOrganic compound preparationCarbonyl compound preparationMetals industryDistillation
The invention relates to the technical field of m-hydroxybenzaldehyde, particularly to a preparation method of m-hydroxybenzaldehyde. The product is prepared by an esterification section, a chlorination section, a distillation section, a hydrolysis section, a refining section and a drying section. The raw materials and auxiliary materials of the product are easily available, the technological steps are simplified, pollutants are fewer, the product yield is up to more than 85%, the weight of the product is easy to control, and the product cost is reduced by about 20% compared with the past. The product can be used for making epinephrine and its derivatives, phenylephrine hydrochloride and the like in medicine; in the metal industry, the product can serve as a nickel plating brightener, and can be used for corrosion prevention and decoration of steel, copper and copper alloys, zinc die castings, aluminum alloys and the like; in the spice industry, the product can be directly used as a spice, or can be used for making spices after esterification, etherification and derivative reactions; and also, a small amount of the product can be used as a quantitative analysis reagent of sugar.
Owner:怀化泰通新材料科技有限公司
Anti-cold drug suitable for children aged 4-11 years old and preparation method of drug
InactiveCN110302149AImprove complianceRelieve nasal congestionOrganic active ingredientsDispersion deliveryCommon coldDextromethorphan Hydrobromide
The invention relates to an anti-cold drug suitable for children aged 4-11 years old and a preparation method of the drug. Dextromethorphan hydrobromide and phenylephrine hydrochloride are taken as active ingredients, a pH regulator is added to regulate the pH value to 3.8 + / - 2, and auxiliary ingredients such as a cosolvent, a stabilize, a thickening agent, a bacteriostat and the like are added,so that the defects in the prior art are effectively solved, and the stability of the drug is increased. At the same time, a certain amount of flavoring agent and aromatic agent are added to improve the compliance of children. The drug is mainly used for relieving nasal obstruction and cough caused by common cold of children aged 4-11 years old.
Owner:北京博智绿洲医药科技有限公司
Phenylephrine hydrochloride containing liquid composition
InactiveCN112076183AInorganic non-active ingredientsPharmaceutical delivery mechanismSinusitisDisease
The invention provides six liquid compositions containing phenylephrine hydrochloride. The six liquid compositions contain the following active substances: a composition 1: phenylephrine hydrochloride, brompheniramine maleate and dextromethorphan hydrobromide; a composition 2: phenylephrine hydrochloride, acetaminophen, guaiacol glyceryl ether and dextromethorphan hydrobromide; a composition 3: phenylephrine hydrochloride, chlorpheniramine maleate, acetaminophen and dextromethorphan hydrobromide; a composition 4: phenylephrine hydrochloride, acetaminophen and dextromethorphan hydrobromide; a composition 5: phenylephrine hydrochloride, acetaminophen and diphenhydramine hydrochloride; and a composition 6: phenylephrine hydrochloride, acetaminophen and guaiacol glyceryl ether. The inventor finds that the stability of the six liquid compositions is related to the pH value through research. The liquid composition can be used for treating respiratory diseases caused by cold, including virusinfection, such as influenza and cold, as well as allergy, sinusitis, rhinitis and the like.
Owner:北京博智绿洲医药科技有限公司
Phenylephrine hydrochloride and chlorphenamine maleate preparation and preparation method thereof
InactiveCN112472677ASolve the technical problem of impurities generated by addition reactionOrganic active ingredientsPharmaceutical non-active ingredientsChlorobenzeneActive agent
The invention belongs to the technical field of pharmaceutical preparations, and particularly relates to a phenylephrine hydrochloride and chlorphenamine maleate preparation and a preparation method thereof. The phenylephrine hydrochloride and chlorphenamine maleate preparation comprises a mixture formed by a first coating and a second coating, wherein the first coating is composed of a first coreand a first coating film coating the surface of the first core, the second coating is composed of a second core and a second coating film coating the surface of the second core, the first core is a mixture of phenylephrine or a pharmaceutically acceptable salt thereof and a first pharmaceutical active agent and / or a first auxiliary material, and the second core is a mixture of chlorpheniramine maleate and / or bromopheniramine maleate and a second pharmaceutical active agent and / or a second auxiliary material. According to the phenylephrine hydrochloride and chlorphenamine maleate preparation,the first core and the second core are not in contact with each other, so that the phenylephrine is thoroughly isolated from chlorpheniramine maleate and / or bromopheniramine maleate, and the obtainedphenylephrine hydrochloride and chlorphenamine maleate preparation has higher product stability, safety and effectiveness.
Owner:BRIGHT FUTURE PHARMA LAB LTD (CN)
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com