Determination method of phenylephrine hydrochloride, chlorpheniramine maleate and ibuprofen in compound cold medicine tablets
A technology of phenylephrine hydrochloride and chlorpheniramine acid is applied in the field of medical testing to achieve the effects of controlling product quality, comprehensive quality testing indicators and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] [Example 1] Preparation of reference solution
[0032] Take phenylephrine hydrochloride, chlorpheniramine maleate and ibuprofen reference substances respectively, accurately weigh them, and add a solvent to make each 1ml solution containing phenylephrine hydrochloride, chlorpheniramine maleate and Ibuprofen is a mixed solution of 6.64, 0.332, and 0.132 mg, respectively, to obtain.
Embodiment 2
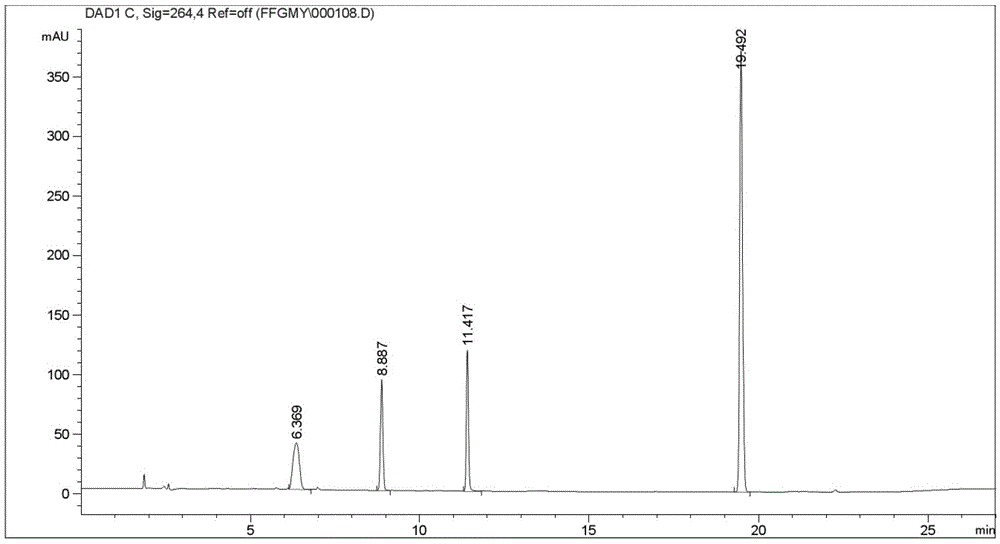
[0033] [Example 2] Screening of chromatographic conditions and system applicability
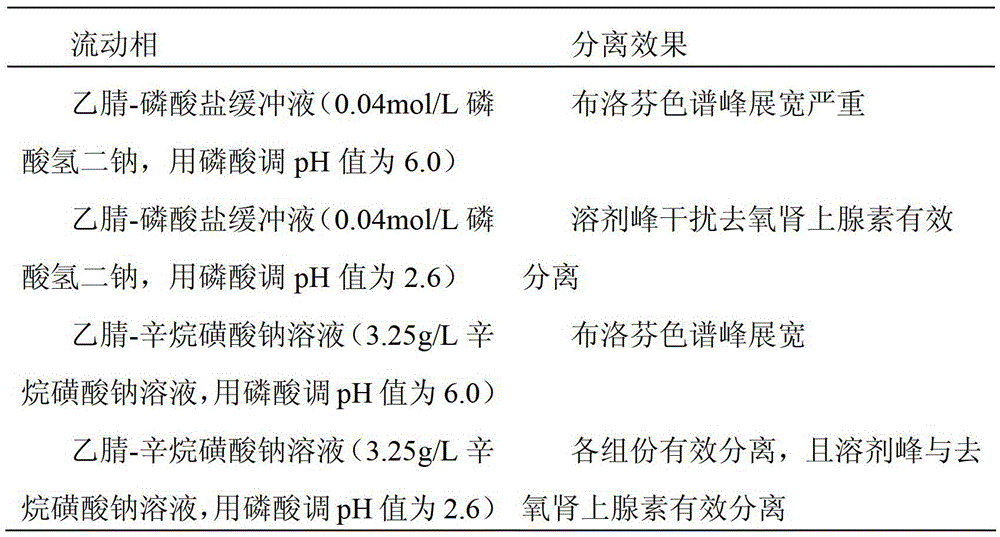
[0034] 2.1 Selection of mobile phase
[0035] According to the relevant literature and the specific conditions of the experiment, acetonitrile-phosphate buffer (0.04mol / L disodium hydrogen phosphate, adjusted to pH 6.0 with phosphoric acid), acetonitrile-phosphate buffer (0.04mol / L hydrogen phosphate dibasic Sodium, adjust the pH to 2.6 with phosphoric acid, acetonitrile-sodium octane sulfonate solution (3.25g / L sodium octane sulfonate solution, adjust the pH to 6.0 with phosphoric acid), acetonitrile-octane sulfonate sodium solution (3.25 g / L sodium octane sulfonate solution, adjust the pH to 2.6 with phosphoric acid), the separation effect under each mobile phase condition is as follows (see Table 1)
[0036] Table 1. Mobile phase selection results
[0037]
[0038] 2.2 Selection of Column
[0039] Try different brands and models of chromatographic columns, and finally decide to use Kromasil (C1825...
Embodiment 3
[0057] [Example 3] Preparation of test solution
[0058] Take the test compound cold tablets (batch number 20130416), accurately weigh it, and prepare a solution with a concentration of 4 mg / ml. The specific steps are to take the compound cold tablet, grind it finely, take about 20mg of the powder, accurately weigh it, place it in a 5ml volumetric flask, sonicate for 10 minutes, let it cool, add solvent to dilute to the mark, shake well, filter, and take the subsequent filtrate. Immediately.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com