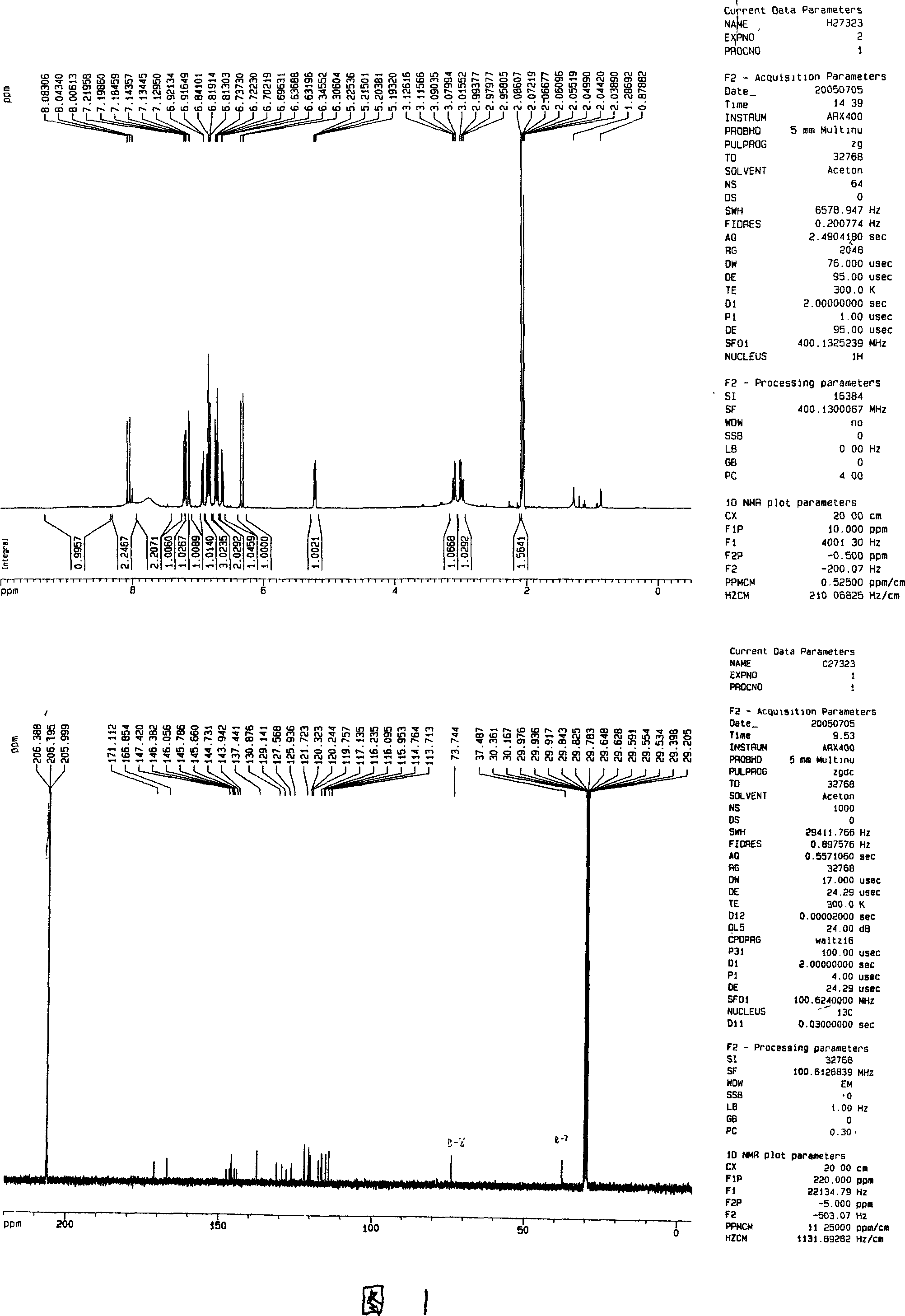
Salvia minium phenolic acid A and process of preparing preparation and use
A technology of salvianolic acid and salvianolic acid, applied in the field of medicine, can solve the problems of low yield of salvianolic acid A, low content of salvianolic acid A, and high requirements for purified samples
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0201] Embodiment one: the preparation method of salvianolic acid A, its processing step is:
[0202] 1. Take 100kg of Danshen crude powder, add 8 times the amount of 70% ethanol to reflux and extract 3 times, each time for 1.0 hour, and filter;
[0203] 2. Concentrate the combined filtrate to medicinal material: medicinal liquid (1:4), adjust the pH value of the concentrated solution to 6.0, place it in a reaction tank at 110° C., 0.05 Pa, and react for 6 hours;
[0204] 3. Filter the reaction solution, pass the filtrate through the AB-8 type (manufactured by Nankai University Chemical Factory) macroporous adsorption resin column (the amount of medicinal material: resin is 1:2), wash with water until the sugar detection reaction is negative, and then use 10% ethanol Eluted to FeCl 3 If the reaction is negative, then elute with 30% ethanol, use HPLC to track and detect, collect the eluate containing salvianolic acid A, and concentrate under reduced pressure until there is no ...
Embodiment 2
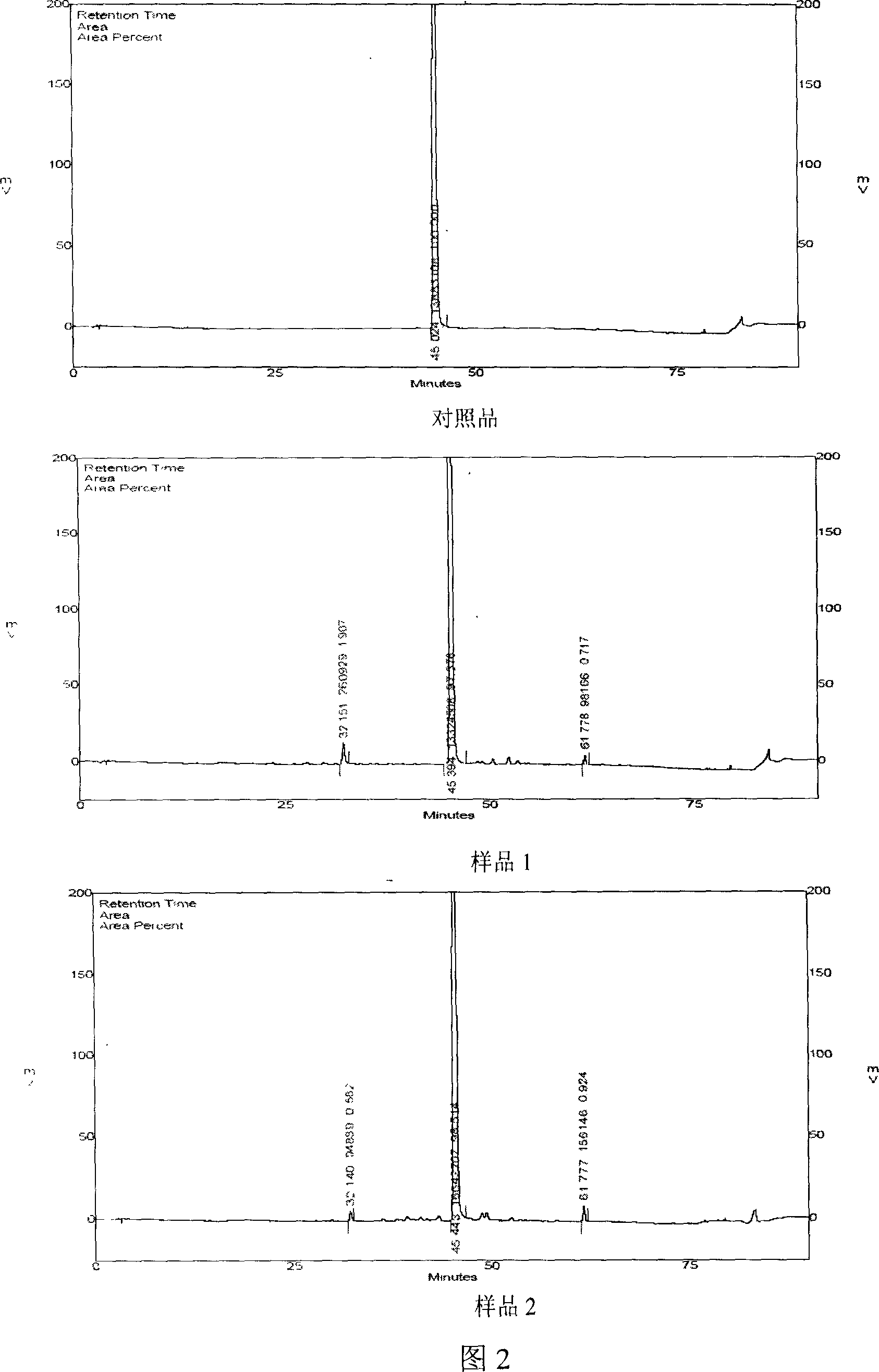
[0208] Embodiment two: basically the same as embodiment one, the difference is that in step 3, the macroporous resin model is changed to HPD-100 (produced by Cangzhou Baoen Chemical Co., Ltd.); Amide chromatography column (medicine material: resin ratio: 1:2), wash with water until the sugar detection reaction is negative, then use 40% ethanol to elute to FeCl 3 If the reaction is negative, it is eluted with 80% ethanol, followed by high performance liquid phase detection, and the eluate containing salvianolic acid A is collected and concentrated under reduced pressure until there is no organic solvent; in step 5, the pH value is adjusted to 5.
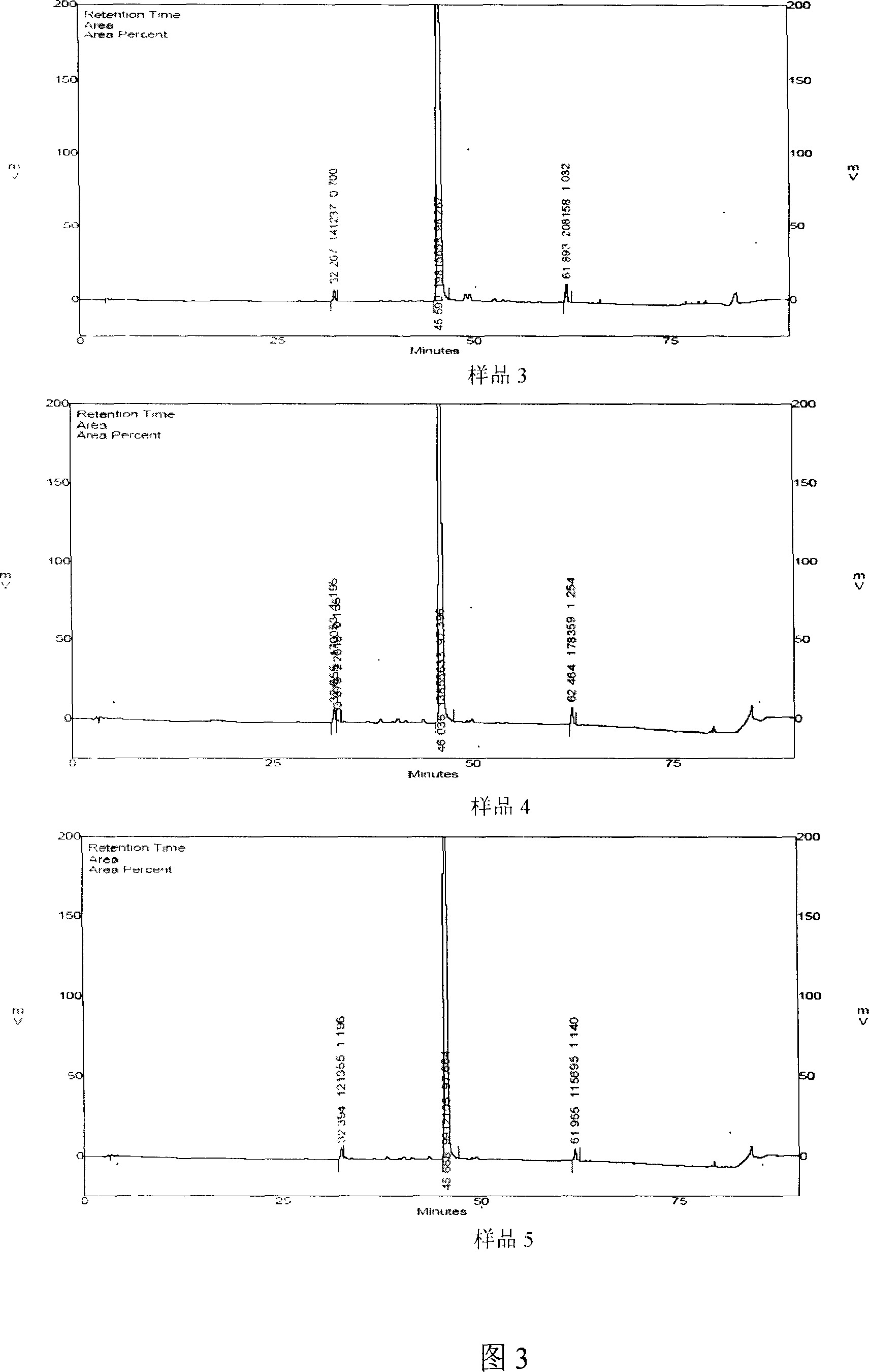
[0209] It was determined that the content of salvianolic acid A was 97.97, the retention time of salvianolic acid A was 45.44 minutes, the retention time of impurity 1 was 32.14 minutes, the relative retention time of impurity 1 was 0.707, the content of impurity 1 was 0.57; the retention time of impurity 2 was 61.78 minutes, and the r...
Embodiment 3
[0210] Embodiment three: basically the same as embodiment one, the difference is that the macroporous resin in step 3 is changed to HPD-300 (produced by Cangzhou Baoen Chemical Co., Ltd.); step 4 is changed to filter the concentrated solution, and the polyamide layer on the filtrate Analyze the column (the amount of medicinal material: resin is 1:2), first wash with water until the sugar detection reaction is negative, then use 50% ethanol to elute until salvianolic acid A appears in the eluent, then elute with 95% ethanol, and use High-performance liquid phase tracking detection, collecting the eluate containing salvianolic acid A, and concentrating under reduced pressure until there is no organic solvent.
[0211] It was determined that the content of salvianolic acid A was 97.87, the retention time of salvianolic acid A was 45.594 minutes, the retention time of impurity 1 was 32.27 minutes, the relative retention time of impurity 1 was 0.708, the content of impurity 1 was 0....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com