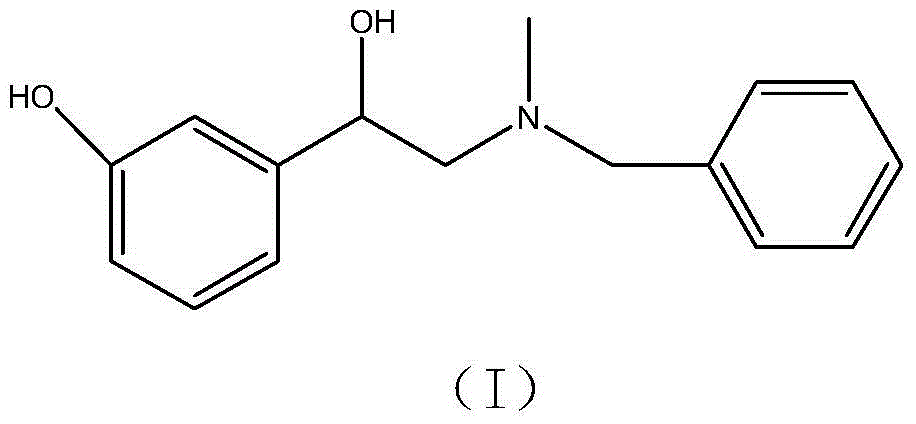
Preparation method of phenylephrine hydrochloride impurity
A technology for phenylephrine hydrochloride and epinephrine, applied in the field of 2--1-(3-hydroxyphenyl)ethanol, phenylephrine hydrochloride impurity D
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: the preparation method 1 of impurity D
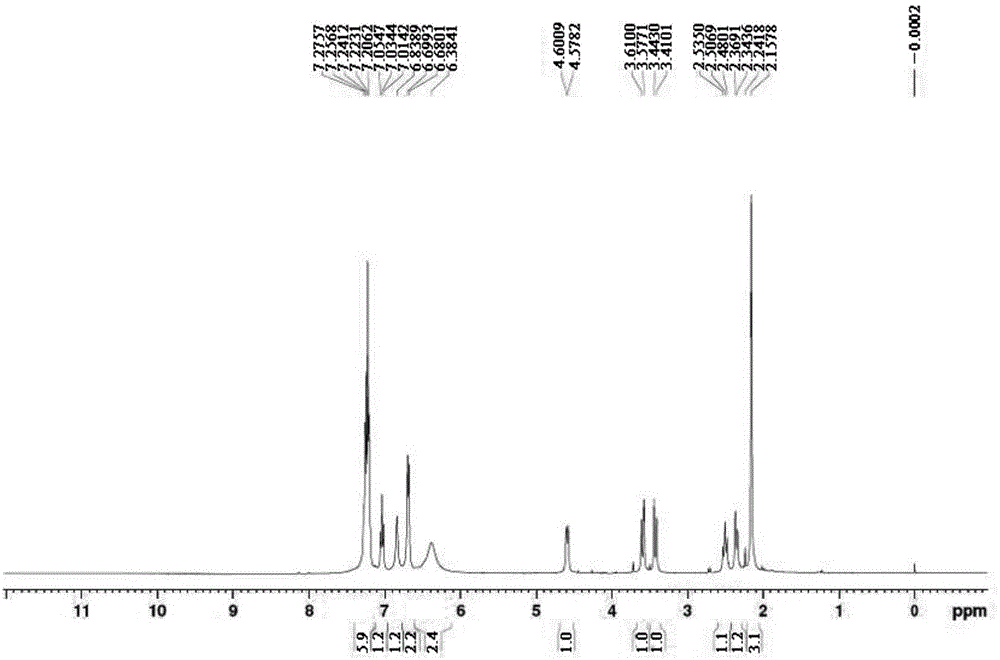
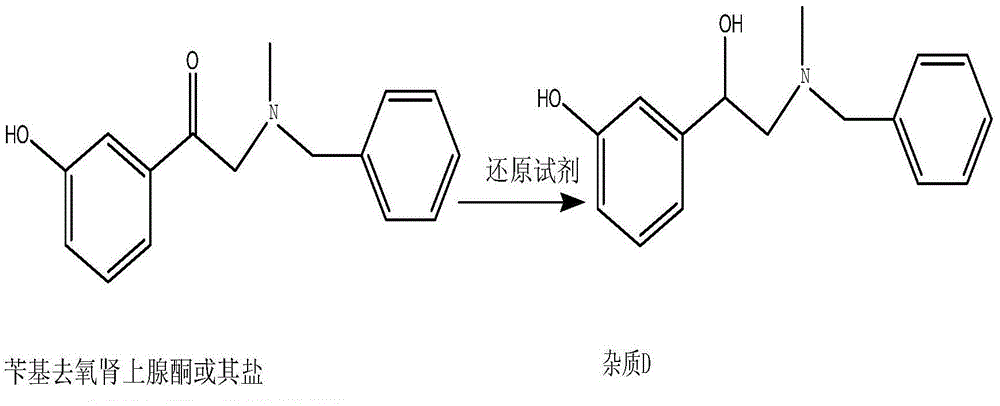
[0026] Add 300 mL of water and 30 g of benzyl phenylephrine hydrochloride to a 1L reaction flask, stir until dissolved, slowly add 10 g of sodium borohydride under stirring, gas is generated, after adding sodium borohydride, at room temperature (20-30 ℃) under stirring, liquid phase detection, impurity D peak area percentage 92%. After the reaction is completed, add ammonia water to adjust the pH value to 9-10, then add 200 mL of dichloromethane, stir for 15 minutes, let stand for 15 minutes, and perform layering, the upper layer is an aqueous phase, the lower layer is an organic phase, the organic phase is reserved, and the aqueous phase is Extract with dichloromethane twice, each time with 100 mL of dichloromethane, combine the organic phases, and concentrate under reduced pressure until there are no droplets to obtain the crude product of impurity D, which is a brown oil with a purity of 96%. Recrystallization w...
Embodiment 2
[0027] Embodiment 2: the preparation method 2 of impurity D
[0028] Add 3 mL of tetrahydrofuran and 0.5 g of benzyl phenylephrine to a 10-mL reaction flask, stir, and a white suspension is obtained. Slowly add 0.5 g of lithium aluminum hydride under stirring. After adding, stir at room temperature (20-30 °C). , liquid phase detection, impurity D peak area percentage 82%. After completion of the reaction, filter, concentrate the filtrate to dryness, add 10 mL of dichloromethane and 10 mL of water, carry out layering, retain the organic phase, and concentrate under reduced pressure until there are no droplets to obtain a crude product of impurity D, a brown oil with a purity of 84 %. Recrystallization from 2ml ethyl acetate gave impurity D, about 0.26g, yield 52%, purity 93.5%
Embodiment 3
[0029] Embodiment 3: Different recrystallization solvents are on the preparation yield of impurity D, the influence of quality:
[0030] According to the preparation method 1 of impurity D, the selected recrystallization reagents are respectively: the mixed solvent of ethanol, ethyl acetate, butyl acetate, ethanol and butyl acetate, complete 4 experiments, obtain 4 samples, the yield, the quality data See the table below:
[0031]
[0032]
[0033] Conclusion: The recrystallization reagent adopts the mixed reagent of ethanol and butyl acetate, and the yield and quality are relatively best.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com