Phenylephrine hydrochloride containing liquid composition
A technology of phenylephrine hydrochloride and phenylephrine, which is applied in the field of liquid compositions containing phenylephrine hydrochloride and liquid compositions containing oxyepinephrine hydrochloride. issues of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
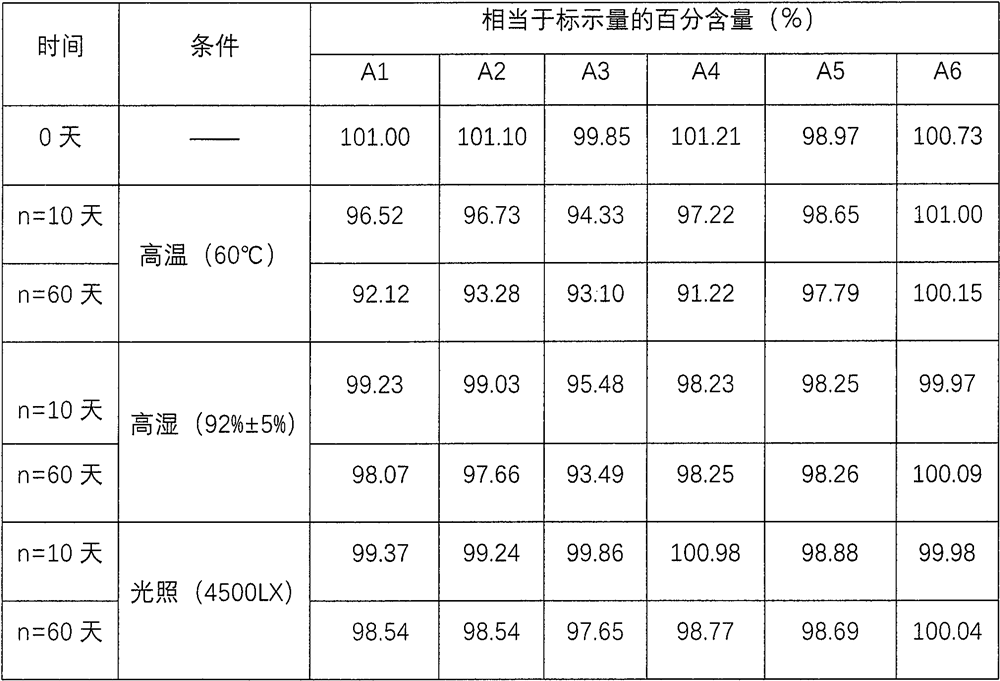
[0052] Embodiment 1 (investigate the stability of the medicinal liquid of composition 1 under different pH value conditions)
[0053] prescription:
[0054] name Prescription amount name Prescription amount brompheniramine maleate 0.30g Phosphate buffer Appropriate amount Dextromethorphan Hydrobromide 0.50g Disodium edetate 0.20g Phenylephrine Hydrochloride 0.15g Anhydrous citric acid 4.05g glycerin 120.00g allura red 13.00mg polyethylene glycol 4000 120.00g Bright blue 5,30mg sucrose 350.00g grape essence 3ml Sorbic acid 1.01g Add purified water to 1000ml
[0055] Prepare 1000ml according to the described preparation method: first, add co-solvent and thickener into a clean container, add brompheniramine maleate and dextromethorphan hydrobromide, stir until dissolved, and obtain an additional drug active substance solution ; in another container, add solvent, phenylephrine hydrochloride, flavor...
Embodiment 2
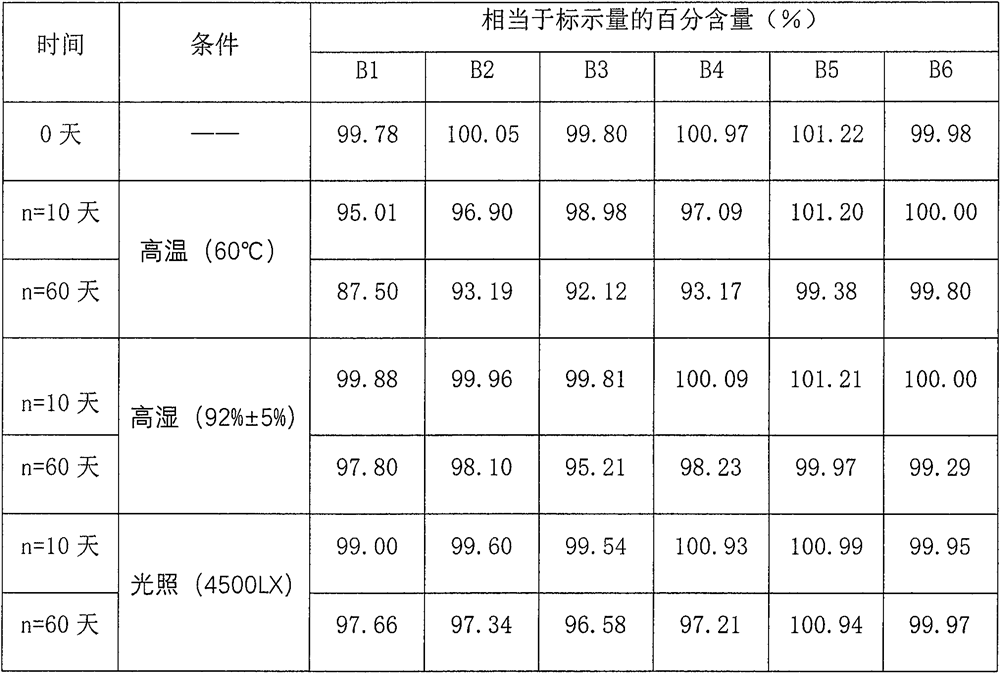
[0062] Embodiment 2 (investigate the stability of the medicinal solution of composition 2 when different pH values)
[0063] name Prescription amount name Prescription amount Acetaminophen 28.00g Phosphate buffer Appropriate amount Dextromethorphan Hydrobromide 0.15g Disodium edetate 0.20g Phenylephrine Hydrochloride 0.35g Propyl gallate 1.55g Guaifenesin 18.00g allura red 11.00mg glycerin 100.00g Bright blue 3,30mg sucrose 250.00g apple flavor 2.00ml Sorbic acid 0.75g Add purified water to 1000ml
[0064] Prepare 1000ml according to the described preparation method. First, add cosolvent and thickener into a clean container, add paracetamol, guaiacol glyceryl ether, and dextromethorphan hydrobromide, and stir until dissolved to obtain additional Pharmaceutical active substance solution; in another container, add solvent, phenylephrine hydrochloride, flavoring agent, preservative, complexing agen...
Embodiment 3
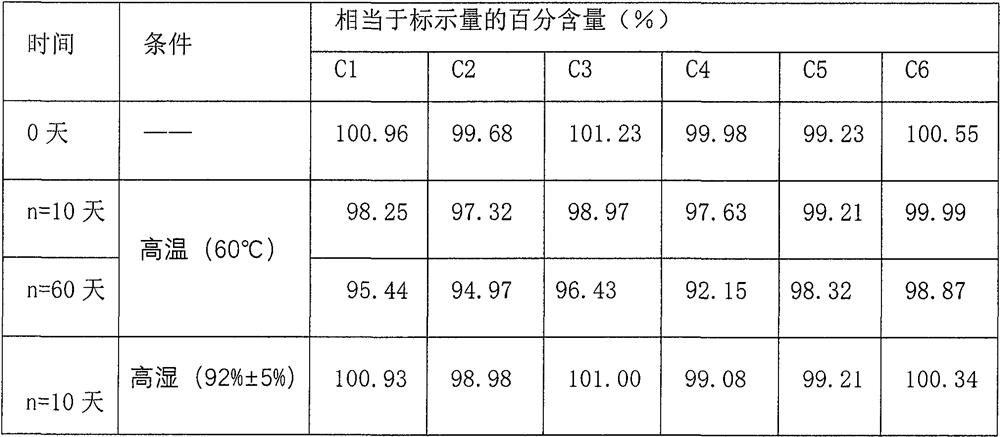
[0071] Example 3 (to investigate the stability of the medicinal solution of composition 3 at different pH values).
[0072] prescription:
[0073] name Prescription amount name Prescription amount Chlorpheniramine maleate 0.30g Phosphate buffer Appropriate amount Dextromethorphan Hydrobromide 1.20g microcrystalline cellulose 8.0g Phenylephrine Hydrochloride 0.50g sodium benzoate 1.55g Acetaminophen 0.05g allura red 11.00mg glycerin 100.00g Sorbitol 650g Sucralose 2,36g apple flavor 2.00ml xanthan gum 1.50g Add purified water to 1000ml Disodium edetate 2.3g
[0074] Prepare 1000ml according to the preparation method, at first, join solubilizer, thickener in the clean container, add chlorpheniramine maleate, acetaminophen, dextromethorphan hydrobromide, stir until dissolving, obtain Additional drug active substance solution; in another container, add solvent, phenylephrine hydrochloride,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com