Phenylephrine hydrochloride and chlorphenamine maleate preparation and preparation method thereof
A technology of renin and preparations, which is applied in the field of renin phenamine preparations and its preparation, can solve the problems of impurities generated by addition reactions, etc., and achieve the effects of fast release speed, high stability, and rapid control of cold symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0047] The embodiment of the present invention provides a preparation method of renin iramine preparation, which comprises the following steps:
[0048] S1. Provide a first core, a first coating material, a second core and a second coating material, the first core is phenylephrine or a pharmaceutically acceptable salt thereof and the first pharmaceutically active agent and / or Or the mixture of the first auxiliary material, the second core is a mixture of chlorpheniramine maleate and / or brompheniramine maleate and the second pharmaceutically active agent and / or the second auxiliary material;
[0049] S2. Coating the first core with the first coating material to obtain the first coating;
[0050] S3. Coating the second core with the second coating material to obtain the second coating;
[0051] S4. Mixing the first coating and the second coating to obtain a reniniramine preparation.
[0052] In the preparation method provided by the embodiment of the present invention, the fir...
Embodiment 1
[0065] This embodiment provides a kind of renin iramine preparation (capsule) and preparation method thereof, and the steps are as follows:
[0066] (11) Phenylephrine hydrochloride coating:
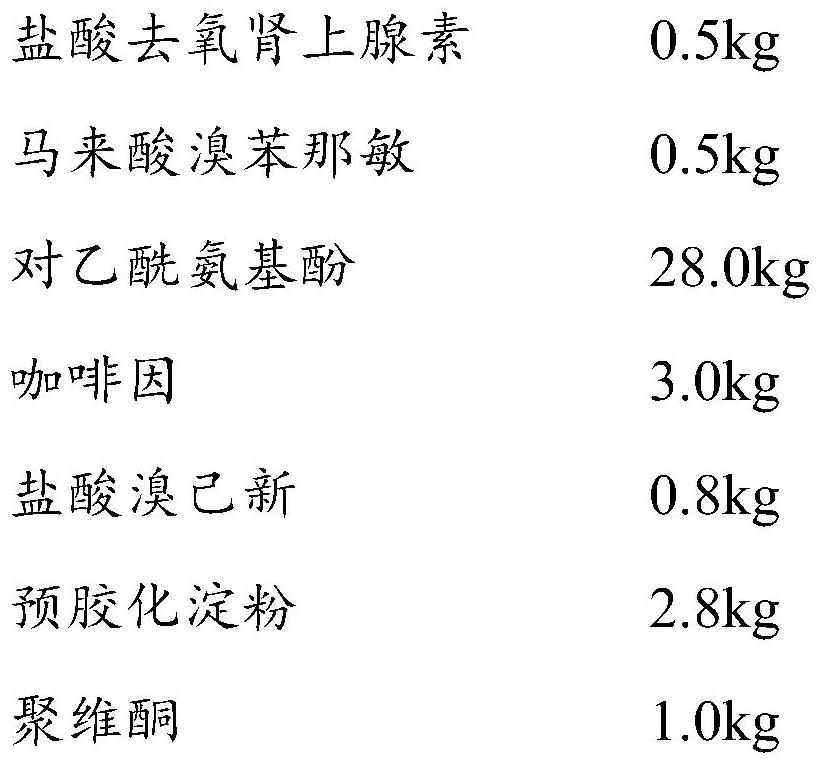
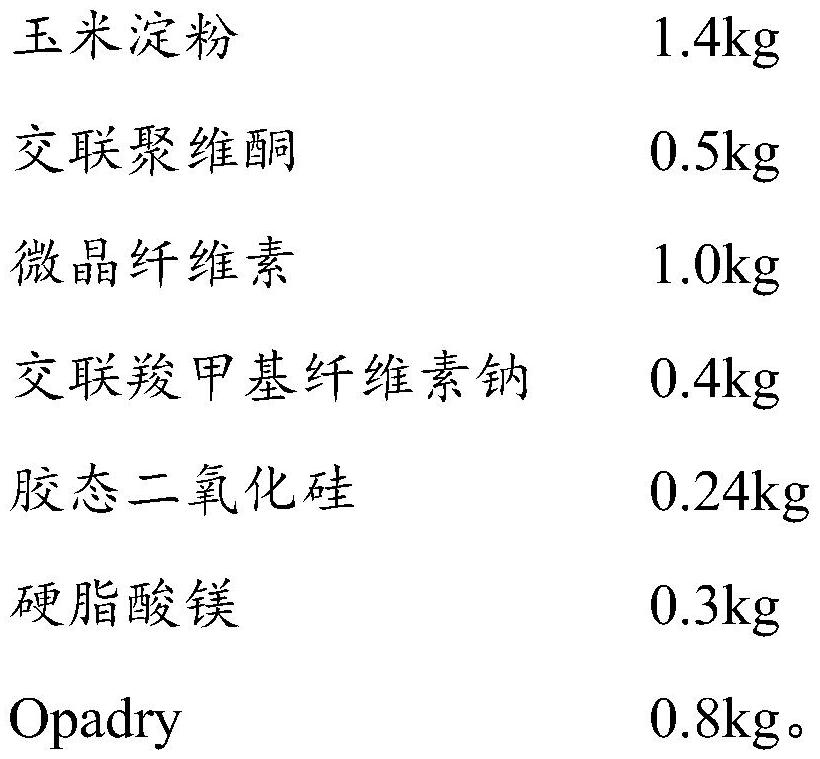
[0067] (111) Material pretreatment: 0.5kg phenylephrine hydrochloride, 14kg acetaminophen, 1.5kg caffeine, 0.4kg bromhexine hydrochloride, 1.4kg pregelatinized starch, 0.5kg povidone, 0.7kg corn Starch, 0.25kg crospovidone, and 0.5kg microcrystalline cellulose are respectively passed through a 40-60 mesh sieve;
[0068] (112) Fluidized mixing: when the air inlet temperature is 50-60°C, the air inlet volume is 100-300m 3 Under the condition of / h, the sieved material is sucked into the fluidized bed, and fluidized and mixed for 5 minutes to obtain the material in a fluidized state;
[0069] (113) Spray granulation: when the air inlet temperature is 50-60°C, the atomization pressure is 1.0-3.0bar, and the air inlet volume is 100-300m 3 / h, spray speed is under the condition of 0.1-0.3kg...
Embodiment 2
[0091] This embodiment provides a kind of renin iramine preparation (capsule) and preparation method thereof, and the steps are as follows:
[0092] (21) Phenylephrine hydrochloride coating:
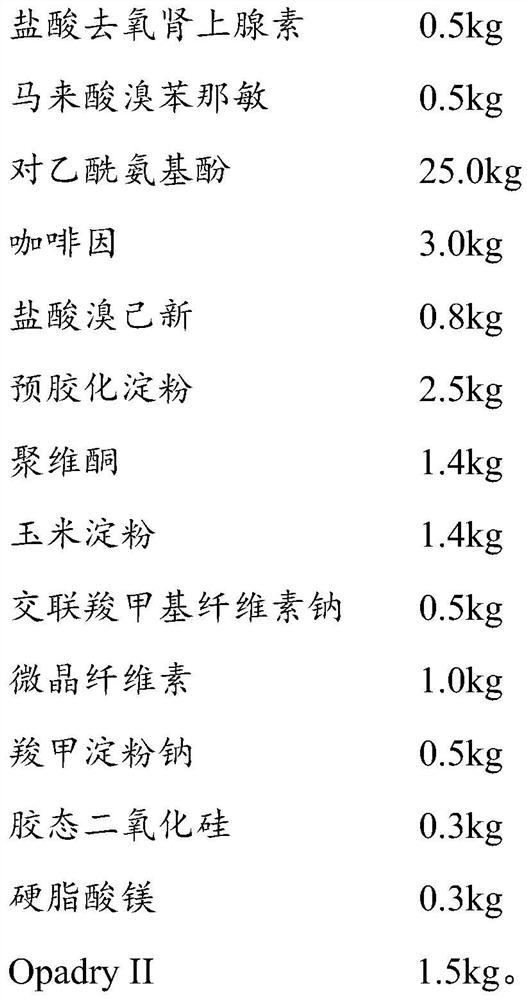
[0093] (211) Material pretreatment: 0.5kg phenylephrine hydrochloride, 12.5kg acetaminophen, 1.5kg caffeine, 0.4kg bromhexine hydrochloride, 1.25kg pregelatinized starch, 0.7kg povidone, 0.7kg Corn starch, 0.25kg croscarmellose sodium, and 0.5kg microcrystalline cellulose are respectively passed through a 40-60 mesh sieve;
[0094] (212) Fluidized mixing: when the air inlet temperature is 50-60°C, the air inlet volume is 100-300m 3 Under the condition of / h, the sieved material is sucked into the fluidized bed, and fluidized and mixed for 5 minutes to obtain the material in a fluidized state;
[0095] (213) Spray granulation: when the air inlet temperature is 50-60°C, the atomization pressure is 1.0-3.0bar, and the air inlet volume is 100-300m 3 / h, spray speed is under the condition ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com