Patents
Literature
96 results about "Dl-Epinephrine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methods of treatment of patients at increased risk of development of ischemic events and compounds hereof
InactiveUS20130040898A1Impair thrombus formationIncreased riskElcosanoid active ingredientsInorganic active ingredientsBeta blockerPlatelet inhibitors
The present invention relates to compounds for treatment that protects the endothelium, prevents pathologic thrombus formation in the microcirculation and preserves platelet number and function and thus may be related to treatment or prevention of ischemic events in patients with cardiovascular disease. The present invention is particularly useful for patients having or being at increased risk of development of an ischemic event such as an acute myocardial infarction and / or no-reflow phenomena and / or ischemia-reperfusion injury by administration of agent(s) modulating and / or preserving endothelial integrity. The compounds may be administered in combination with standard treatment of acute cardiovascular ischemic events such as Platelet inhibitors such as aspirin (ASA), Thienopyridins, GPIIb / IIIa inhibitors), Parenteral anticoagulants such as unfractioned heparin (UFH), bivalirudin, enoxaparin, and fondaparinux, Verapamil, Adenosine, Sodium nitroprusside, Nitroglycerin, Epinephrine, Beta-blockers and surgical methods such as percutaneous coronary intervention (PCI), PCI with thrombus aspiration, PCI with stents.
Owner:THROMBOLOGIC
Stabilization of quinol composition such as catecholamine drugs
InactiveUS20120029085A1Stabilizing compoundReduced form requirementsBiocidePharmaceutical delivery mechanismCatecholaminePh buffering
Compositions and methods are provided for obtaining stabilized quinol compositions, such as catecholamine drugs (e.g., epinephrine solutions), and also for obtaining stable pharmaceutical formulations that comprise a stabilized quinol composition and a second pharmacologically active component such as a local anesthetic or other active drug ingredient having a reversibly protonated amine group. Stability is achieved through the inclusion of an appropriately selected pH buffer and a thiol agent, based on redox and pH buffering principles including pKa of the buffer and of the reversibly protonated amine group.
Owner:MACKAY JON
Epinephrine dosing regimens comprising buccal, lingual or sublingual and injectable dosage forms
The present invention relates to methods of administering a series of epinephrine doses for the treatment of allergic emergencies, including anaphylaxis, comprising buccal, lingual or sublingual epinephrine dosage forms and injectable epinephrine dosage forms. Also provided herein are kits and packaging systems useful in these methods.
Owner:SCIELE PHARMA
Methods for Buccal, Lingual or Sublingual Dosing Regimens of Epinephrine for the Treatment of Allergic Emergencies
InactiveUS20070293581A1Patient compliance is goodReduce worriesBiocideOrganic active ingredientsDosing regimenDosage form
The present invention relates to methods of administering dosage forms which comprise epinephrine, including buccal, lingual, sublingual or transmucosal dosage forms comprising epinephrine for treatment of allergic emergencies, including anaphylaxis. Also provided herein are kits and packaging systems useful in these methods.
Owner:SCIELE PHARMA
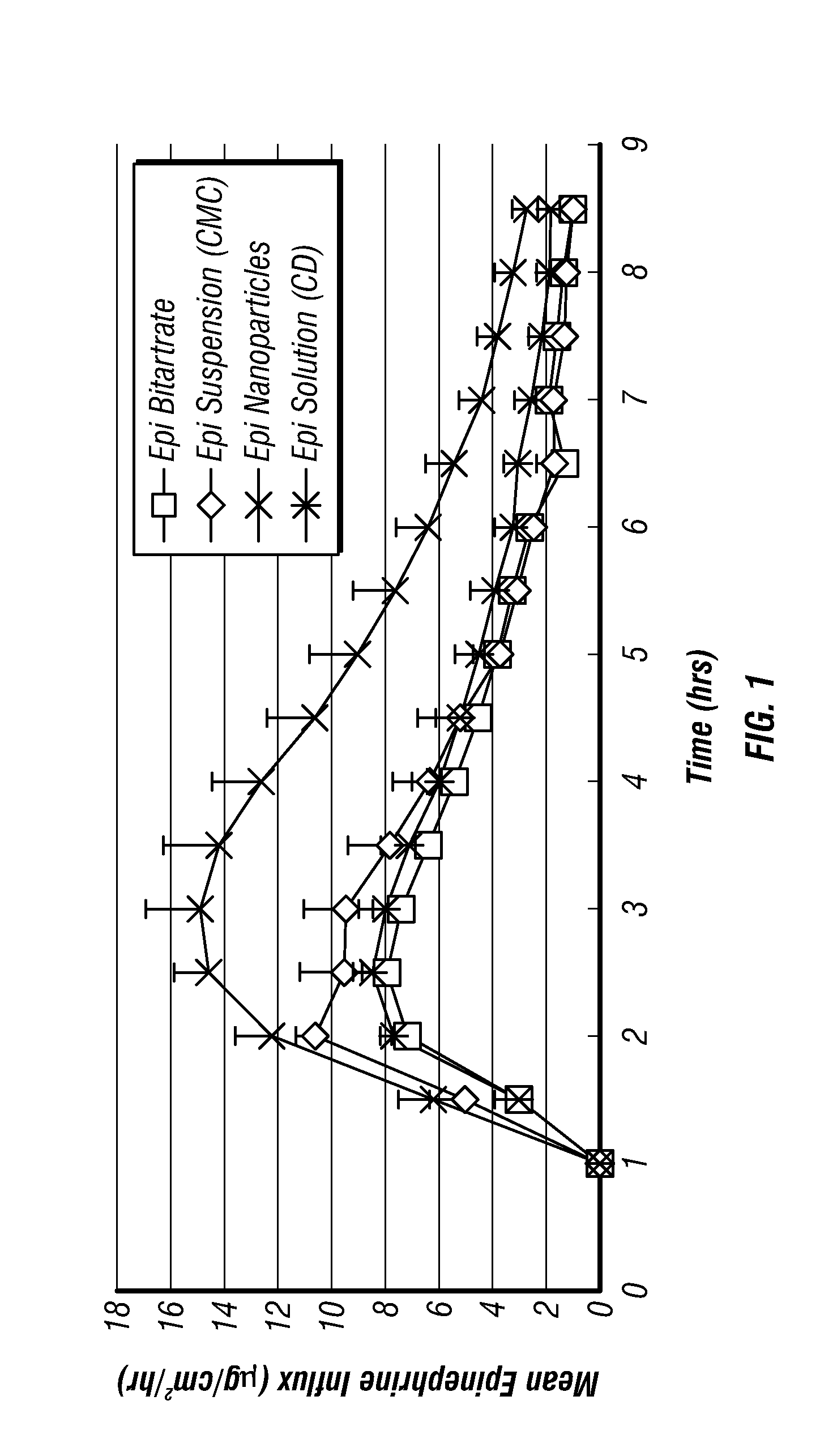
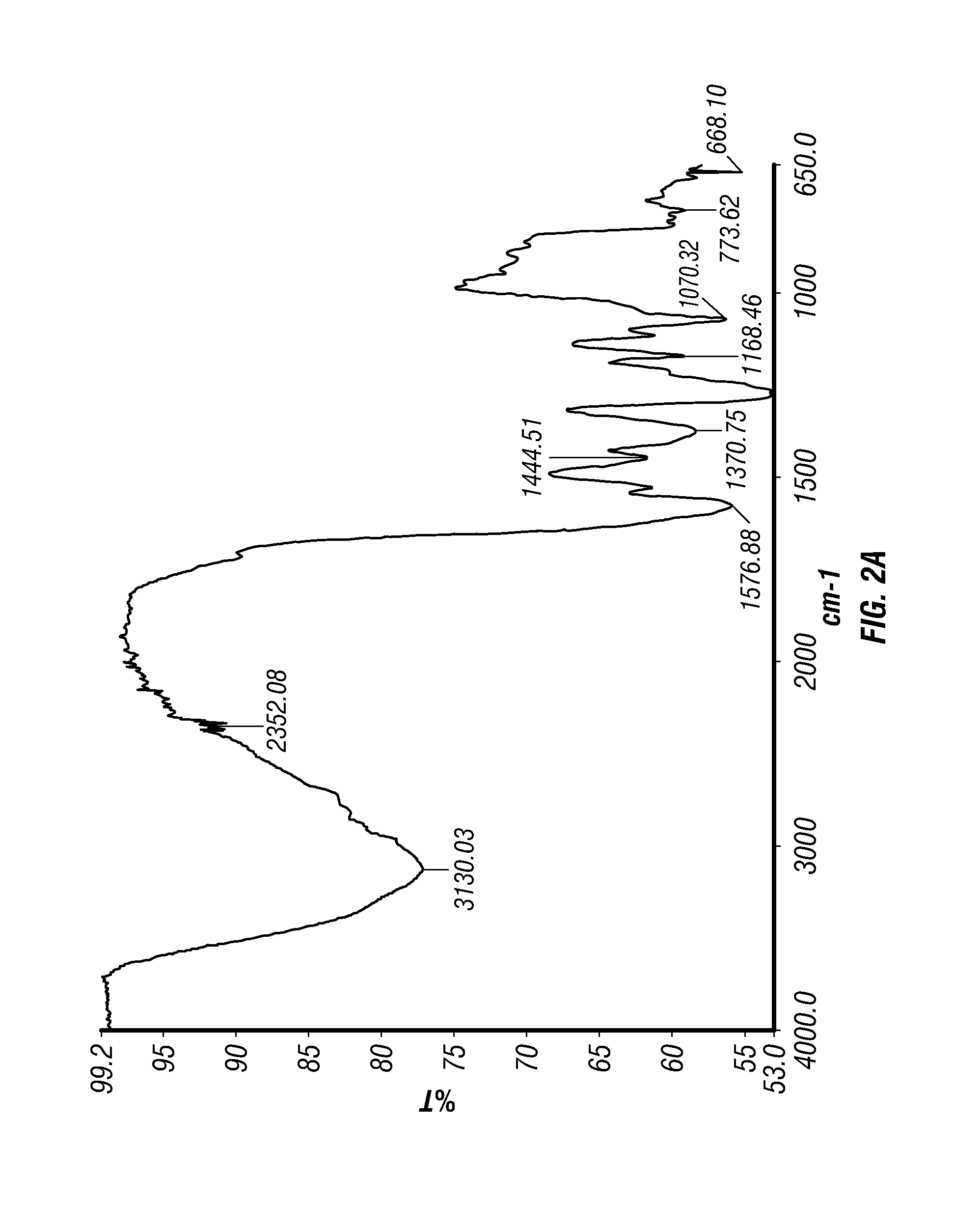
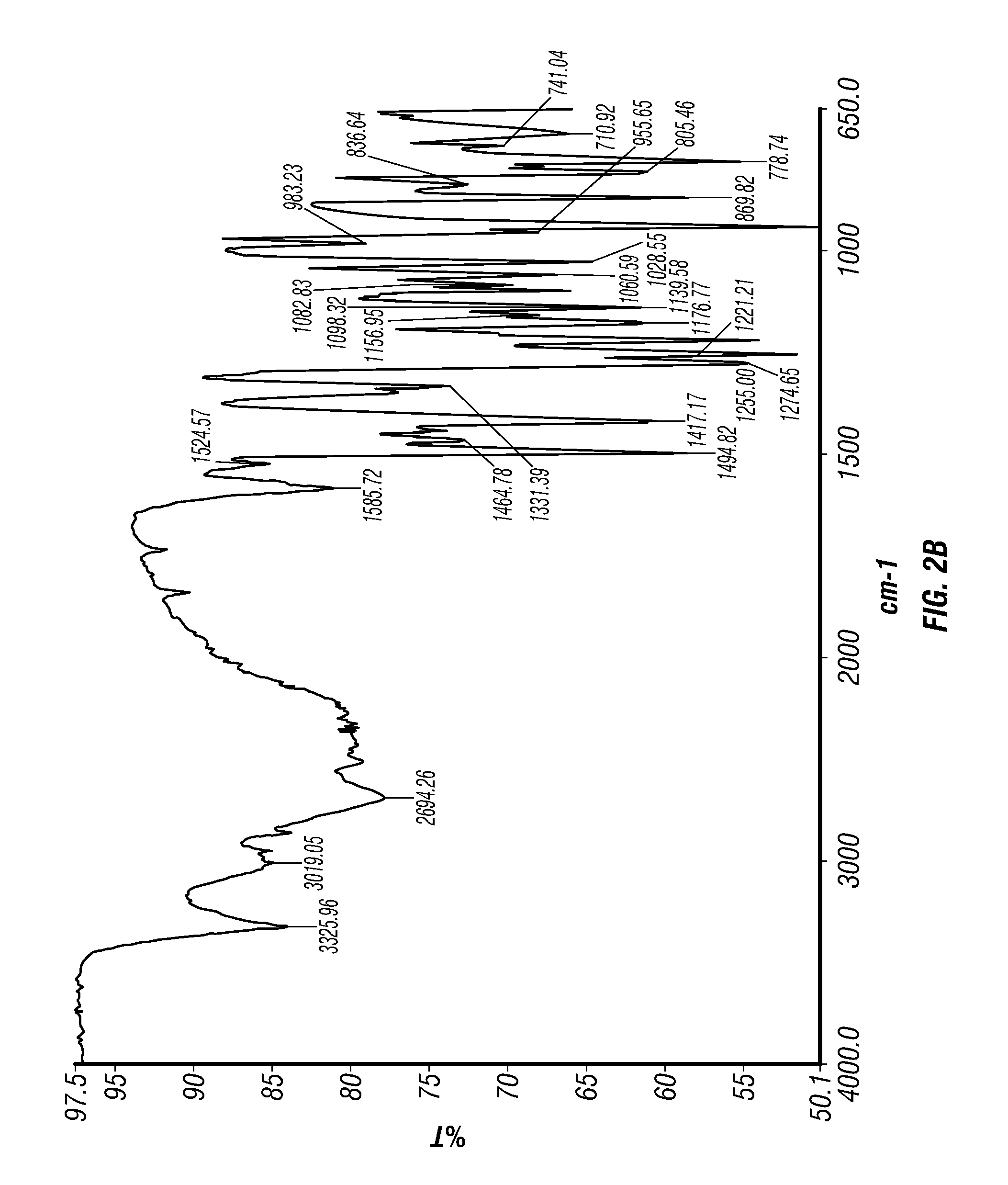
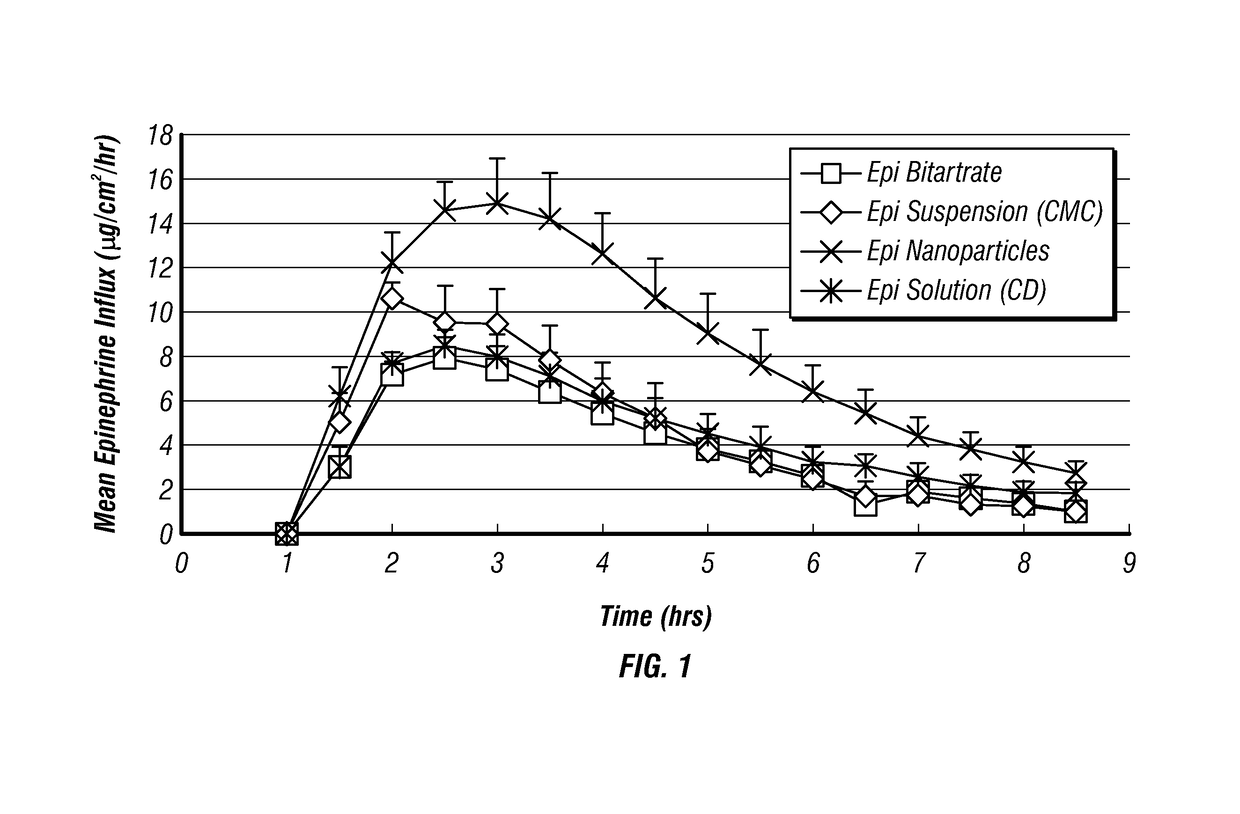
Epinephrine nanoparticles, methods of fabrication thereof, and methods for use thereof for treatment of conditions responsive to epinephrine
InactiveUS20120322884A1Enhancing sublingual bioavailabilityImprove bioavailabilityBiocideOrganic active ingredientsNanoparticleBioavailability
The invention provides a composition including epinephrine nanoparticles and methods for therapeutic use of the composition in the treatment of conditions responsive to epinephrine such as a cardiac event or an allergic reaction, particularly anaphylaxis. The epinephrine nanoparticles can be incorporated into orally-disintegrating and fast-disintegrating tablet pharmaceutical formulations and can significantly increase the sublingual bioavailability of epinephrine, and thereby reduce the epinephrine dose required. Additionally, the invention provides methods for fabrication of stabilized epinephrine nanoparticles for use in the described compositions.
Owner:NOVA SOUTHEASTERN UNIVERSITY
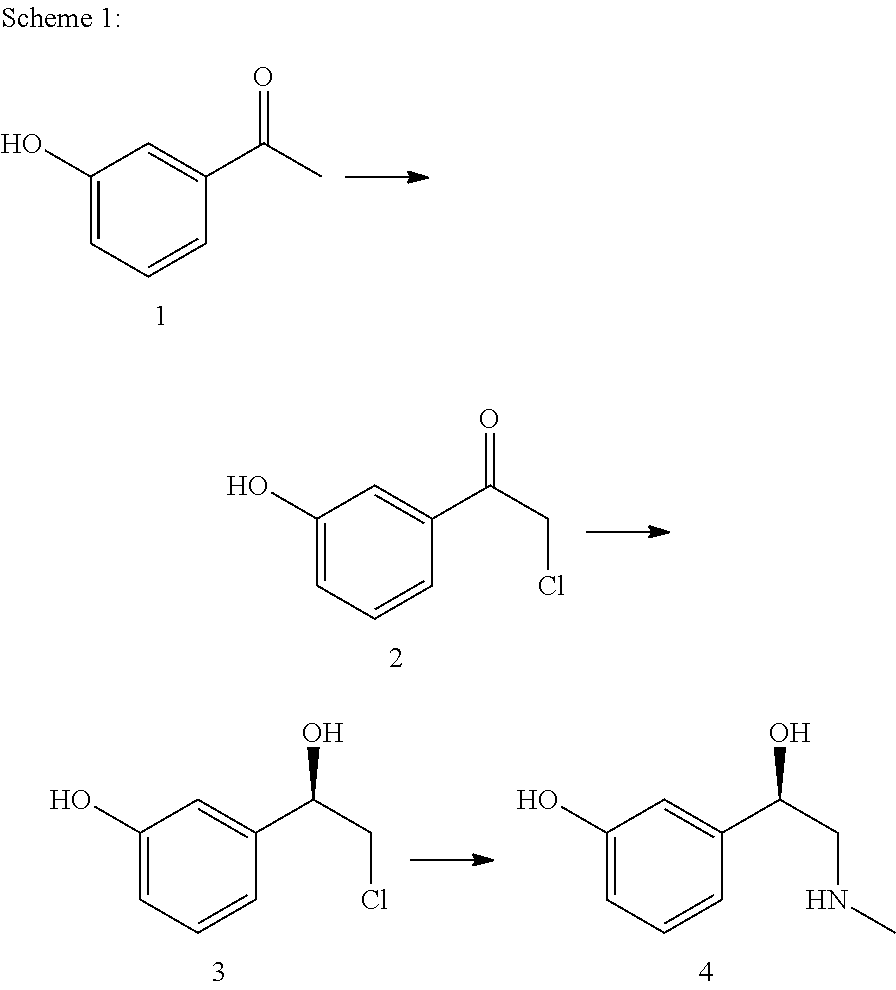
Method for producing l-phenylephrine using an alcohol dehydrogenase of aromatoleum aromaticum ebn1 (azoarcus sp. ebn1)
ActiveUS20110171700A1High stereoselectivityMoreOrganic compound preparationCarbonyl compound preparationAlcoholAzoarcus sp.
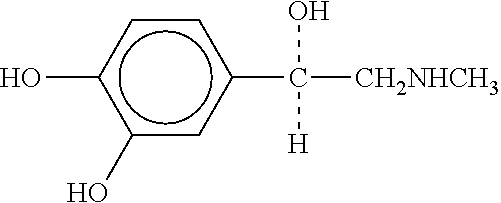
The present invention relates to a multi-stage process for producing substituted, optically active alcohols, comprising an enzyme-catalyzed synthesis step, in particular a synthesis step which is catalyzed by an alcohol dehydrogenase. The inventive method is particularly suitable for producing phenylephrine, i.e. 3-[(1R)-1-hydroxy-2-methylamino-ethyl]-phenol.
Owner:BASF AG
Zilpaterol immune colloidal gold detection card and preparation method thereof
The invention discloses a zilpaterol immune colloidal gold detection card and a preparation method thereof, and relates to the beta-epinephrine receptor agonist detection technical field. A test strip in an outer shell of the detection card is composed of a PVC rubber plate, a sample pad, a colloidal gold combined pad, a coating film and a water-absorbing pad; a colloidal gold film is a glass cellulose film containing a zilpaterol monoclonal antibody, and the coating film is a nitrocellulose film; a T line and a C line are arranged on the nitrocellulose film, the T line is coated with a zilpaterol-protein conjugate, and the C line is coated with a sheep anti-mouse IgG antibody. The detection card is effectively used for rapid detection of zilpaterol, is convenient and fast, and has accurate results.
Owner:JIANGSU WISE SCI & TECH DEV
Methods for Buccal, Lingual or Sublingual Dosing Regimens of Epinephrine for the Treatment of Allergic Emergencies
InactiveUS20070293580A1Patient compliance is goodReduce worriesBiocideOrganic active ingredientsDosing regimenMedicine
The present invention relates to methods of administering dosage forms which comprise epinephrine, including buccal, lingual, sublingual or transmucosal dosage forms comprising epinephrine for treatment of allergic emergencies, including anaphylaxis. Also provided herein are kits and packaging systems useful in these methods.
Owner:SCIELE PHARMA
Preparation method of composite sensitive film electrochemical sensor for detecting epinephrine
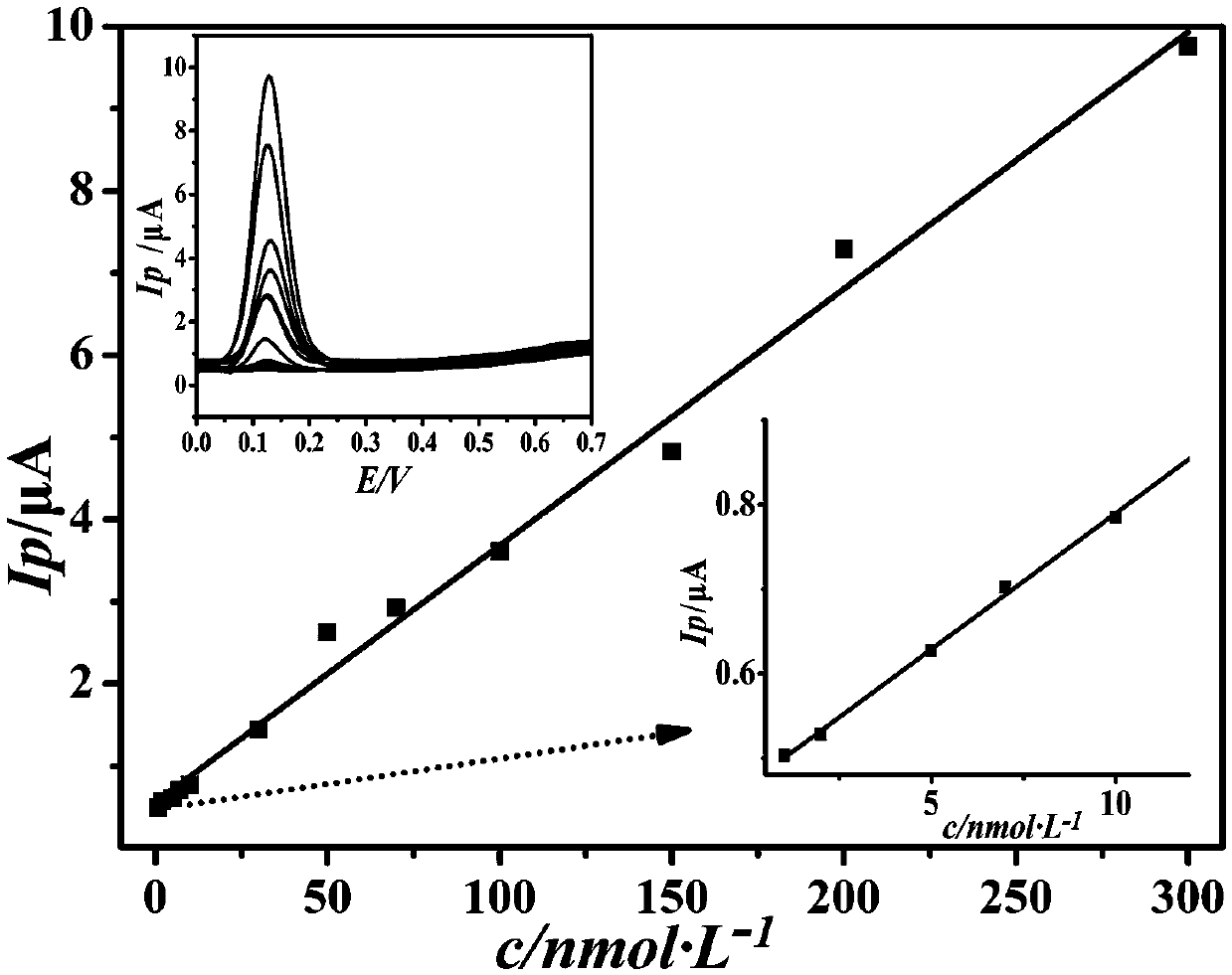
InactiveCN105510419AImprove conductivityHigh detection sensitivityMaterial analysis by electric/magnetic meansCarbon nanotubeElectrochemistry
The invention discloses a preparation method of a composite sensitive film electrochemical sensor for detecting epinephrine. The method takes graphene as a carrier, and an acidic carbon nano tube is covalently combined with the surface of the carrier, so that an electron transferring performance is enhanced; a titanium dioxide nano cluster, which is loaded with nano-gold on the surface, is loaded on the surface of a graphene / carbon nano tube covalent matter, so that a reaction site and reaction activity are improved. The surface of a glassy carbon elctrode is modified with a sensitive film based on the graphene to construct the electrochemical sensor for detecting the epinephrine. The electrochemical sensor constructed by the sensitive film based on the graphene has ultrahigh sensitivity on detection of the epinephrine, a wide detection range and extremely low detection limit, and rapid and sensitive detection on an actual urine sample can be realized.
Owner:UNIV OF JINAN
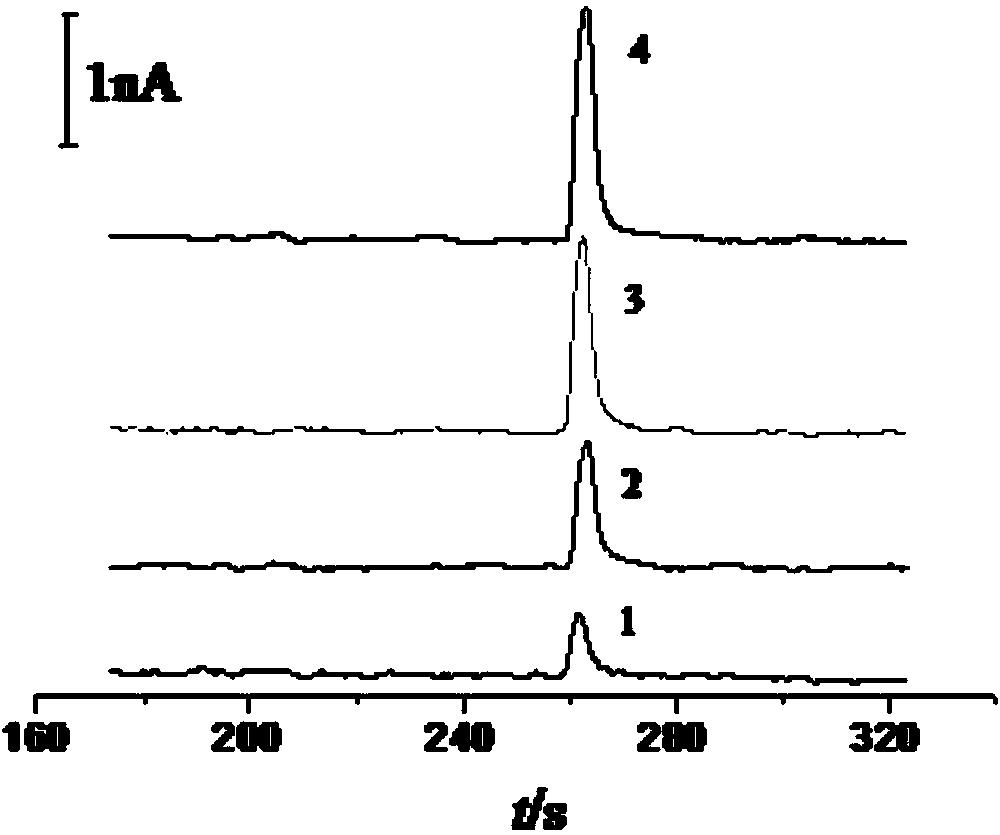
Glutathione sensor, preparation method thereof and application thereof in capillary electrophoresis amperometric detection
InactiveCN103808787AWide linear rangeHigh sensitivityMaterial analysis by electric/magnetic meansFiberCarbon fibers
The invention relates to a glutathione sensor, a preparation method thereof and application thereof in capillary electrophoresis amperometric detection. The preparation method comprises the following steps: polishing the cross section of a carbon fiber micro-disk electrode to be smooth, carrying out ultrasonic cleaning on the polished carbon fiber micro-disk electrode, and immersing the cleaned cross section of the carbon fiber micro-disk electrode into a 0.3gm / mL-0.7mg / mL of graphene oxide solution for 10-20s, and then drying in the air; reducing the graphene oxide on the carbon fiber micro-disk electrode, wherein the electro-deposition potential is at constant potential of -1.2 to -0.7V, the electricity reduction time is 150-370s, and the obtained electrode is the glutathione sensor. The sensor electrode is wide in linear range, high in sensitivity and fast in response speed, electroactive materials possible in actual samples such as dopamine, epinephrine, cysteine and the like have no interference to the determination of the glutathione.
Owner:SHANDONG NORMAL UNIV
Epinephrine nanoparticles, methods of fabrication thereof, and methods for use thereof for treatment of conditions responsive to epinephrine
ActiveUS20170071881A1Improve bioavailabilityPowder deliveryOrganic active ingredientsNanoparticleCombinatorial chemistry
The invention provides a composition including epinephrine nanoparticles and methods for therapeutic use of the composition in the treatment of conditions responsive to epinephrine such as a cardiac event or an allergic reaction, particularly anaphylaxis. The epinephrine nanoparticles can be incorporated into orally-disintegrating and fast-disintegrating tablet pharmaceutical formulations and can significantly increase the sublingual bioavailability of epinephrine, and thereby reduce the epinephrine dose required. Additionally, the invention provides methods for fabrication of stabilized epinephrine nanoparticles for use in the described compositions.
Owner:NOVA SOUTHEASTERN UNIVERSITY
Anti-platelet aggregation polypeptide
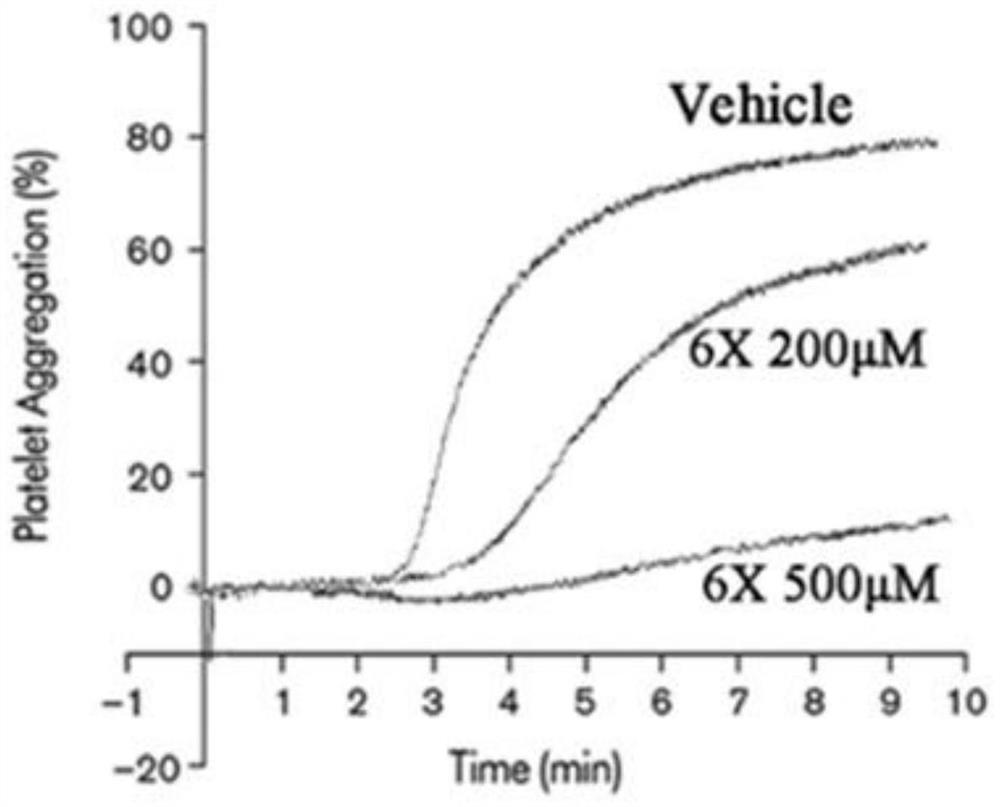
ActiveCN112125953AInhibit aggregationPeptide/protein ingredientsPeptidesAnti plateletInduced platelet aggregation
The invention discloses an anti-platelet aggregation polypeptide 6X. The polypeptide is the polypeptide 6X composed of 10 amino acids, has a sequence of SEQ ID NO:1, and has the molecular weight of 1103.19 Da; the sequence is Thr-Asn-Leu-Thr-Ser-Arg-Asn-Leu-Gly-Gln; the polypeptide is not limited to inhibition of platelet aggregation induced by an Fc[gamma]RIIA specific activator anti-CD9; and thepolypeptide can be used for exploring the influence of the polypeptide and other platelet activators (collagen, arachidonic acid, ADP, epinephrine, ristomycin and the like) on the effects and relatedfunctions of platelets, and can also be used for monitoring existing antiplatelet treatment.
Owner:FIRST AFFILIATED HOSPITAL OF KUNMING MEDICAL UNIV
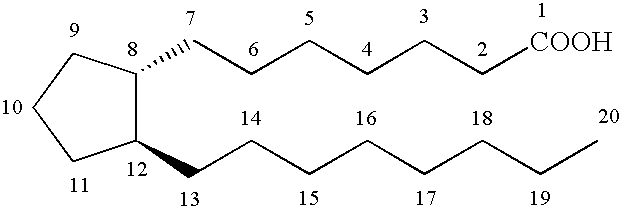
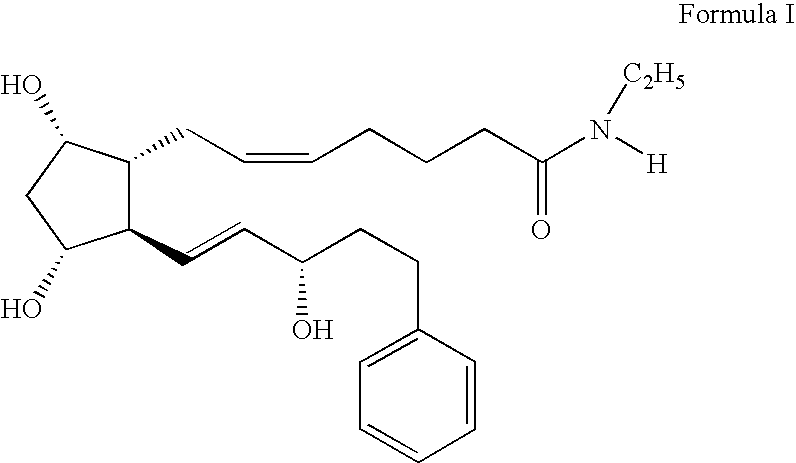
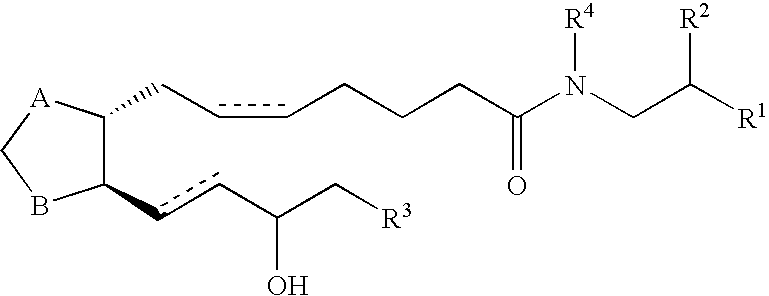
Prostamides for the treatment of glaucoma and related diseases
Disclosed herein are compositions comprising an amide related to a prostaglandin and an amine wherein the amine is selected from the group consisting of epinephrine, dopamine, serotonin, and analogs or prodrugs thereof. Also disclosed are certain chemical compounds, pharmaceutical compositions, and methods of treating glaucoma.
Owner:ALLERGAN INC
Method for producing L-phenylephrine using an alcohol dehydrogenase of Aromatoleum aromaticum EBN1 (Azoarcus sp. EBN1)
ActiveUS8617854B2MoreHigh stereoselectivityOrganic compound preparationCarbonyl compound preparationAlcoholAzoarcus sp.
The present invention relates to a multi-stage process for producing substituted, optically active alcohols, comprising an enzyme-catalyzed synthesis step, in particular a synthesis step which is catalyzed by an alcohol dehydrogenase. The inventive method is particularly suitable for producing phenylephrine, i.e. 3-[(1R)-1-hydroxy-2-methylamino-ethyl]-phenol.
Owner:BASF AG
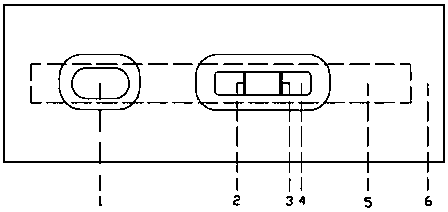
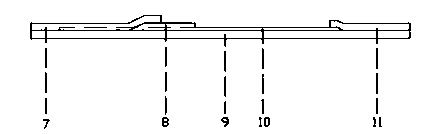
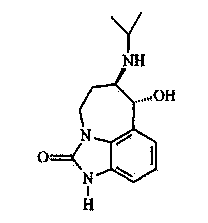
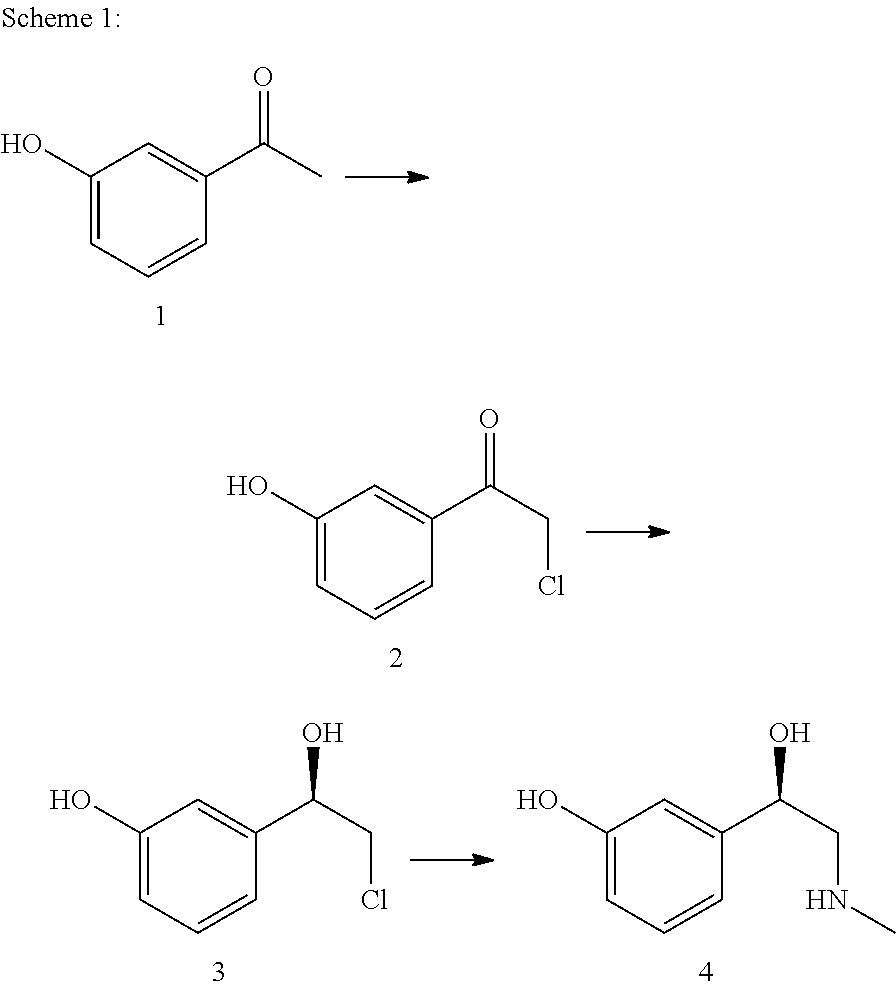
Preparation method of dibenzo [c, e] aza-5, 7 (6H)-dione compound
ActiveCN106699661ABroad market prospectShort synthetic routeOrganic chemistryOrganic synthesisBenzene
The invention belongs to the field of medical and chemical intermediates and related chemical technology, and relates to a preparation method of a dibenzo [c, e] aza-5, 7 (6H)-dione compound. The dibenzo [c, e] aza-5, 7 (6H)-dioneis an important bioactive molecule, has a skeleton structure frequently appearing in a pharmaceutical molecule, has better effects on reducing blood fat, treating obesity, resisting epinephrine and the like, can be significantly applied in the fields such as organic synthesis and pharmaceutical chemistry, and has a wide market prospect. According to the preparation method of the dibenzo [c, e] aza-5, 7 (6H)-dione compound provided by the invention, benzamide is adopted as a raw material, and a series of dibenzo [c, e] aza-5, 7 (6H)-dikone and a derivative thereof are synthesized under the action of a palladium catalyst. The method has the advantages of short synthetic route, simplicity in operation, higher yield and the like. The preparation method of the dibenzo [c, e] aza-5, 7 (6H)-dione compound provided by the invention has a larger use value and social and economic benefits.
Owner:DALIAN UNIV OF TECH
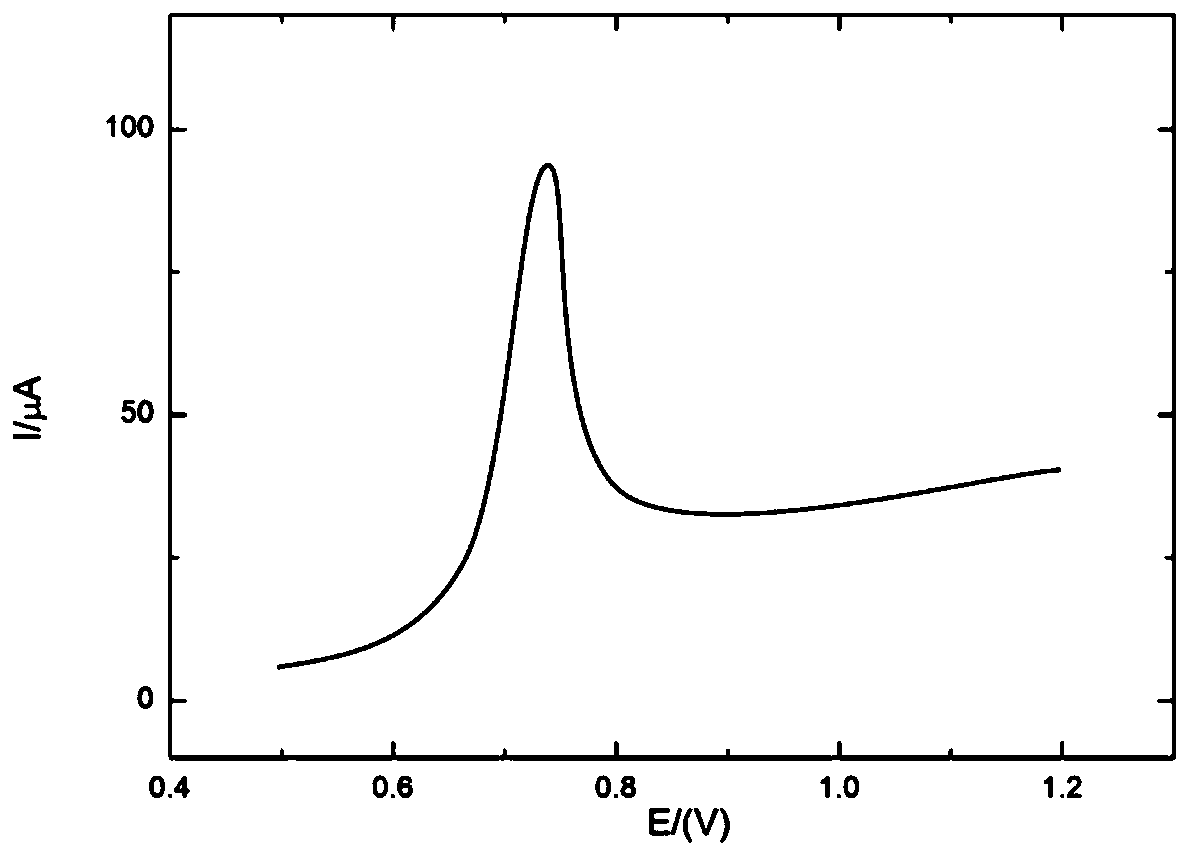
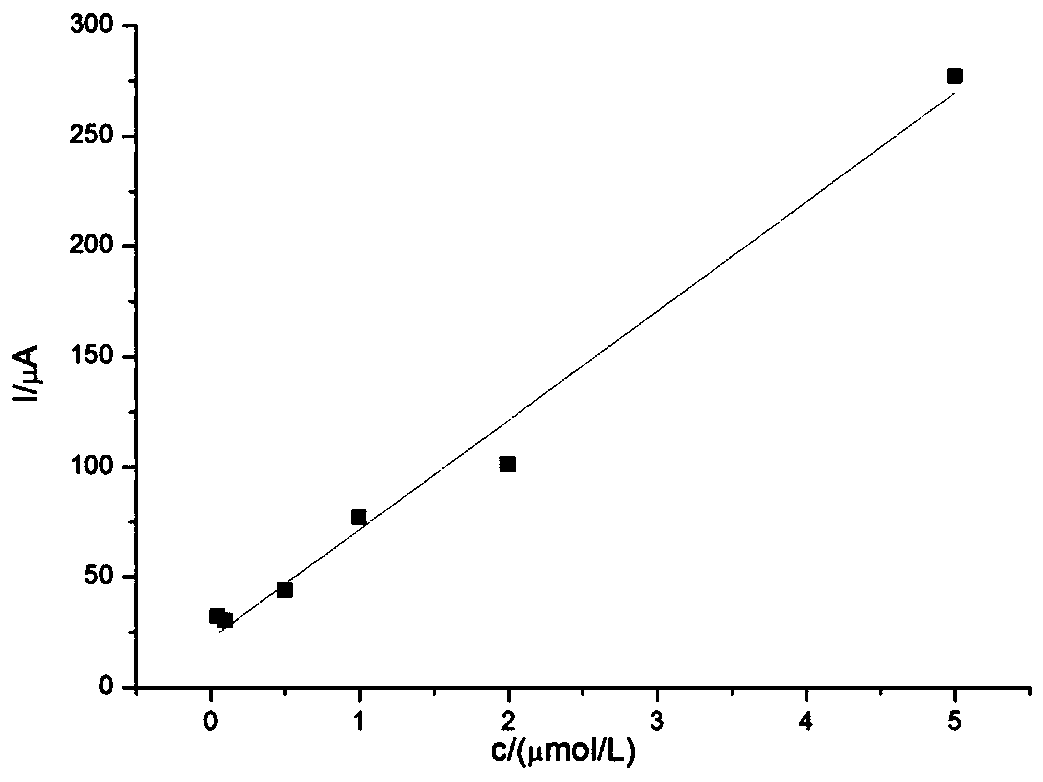
Electrochemical sensor for detecting epinephrine and preparation method and application thereof
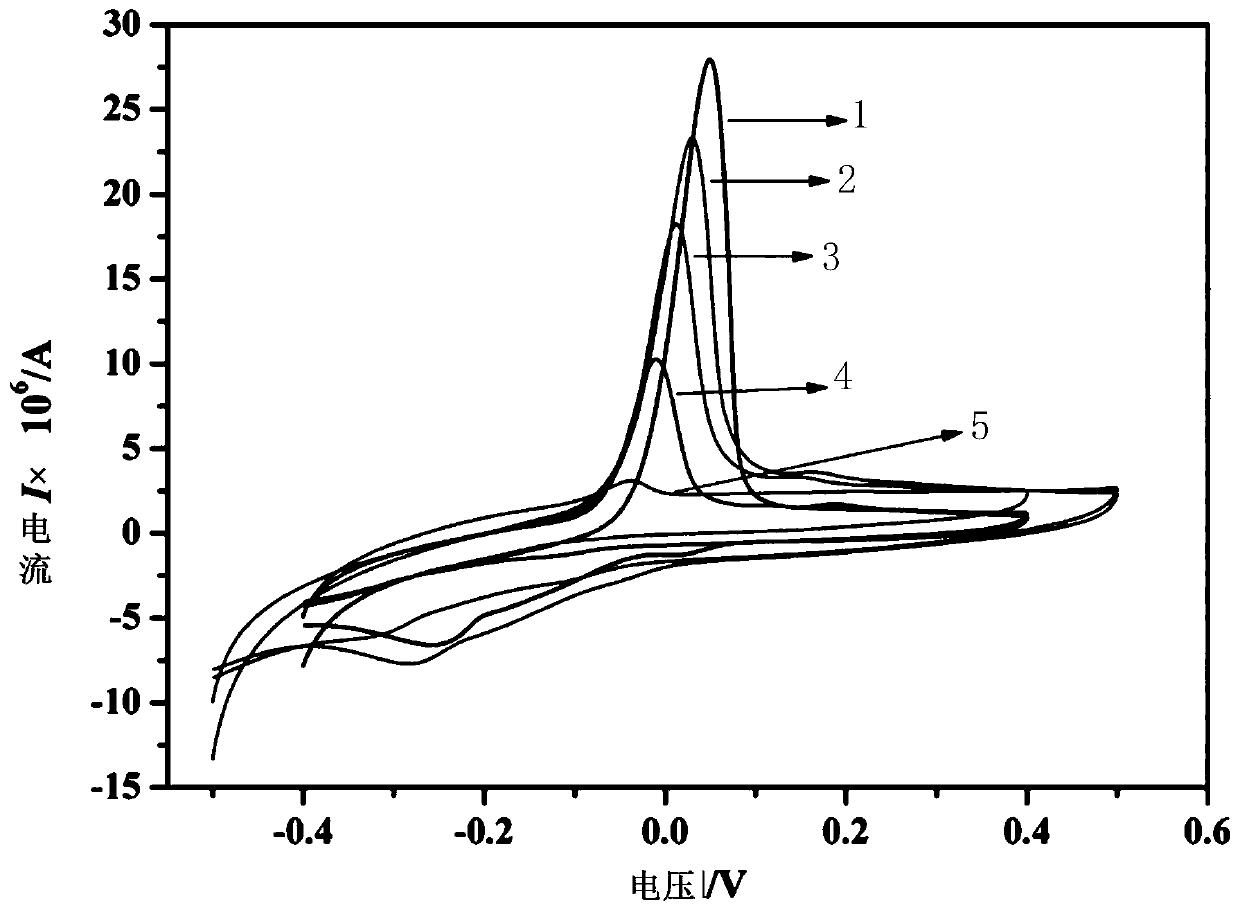
ActiveCN110927233AHigh sensitivityLower activation energyMaterial nanotechnologyNanosensorsEngineeringManganese oxide
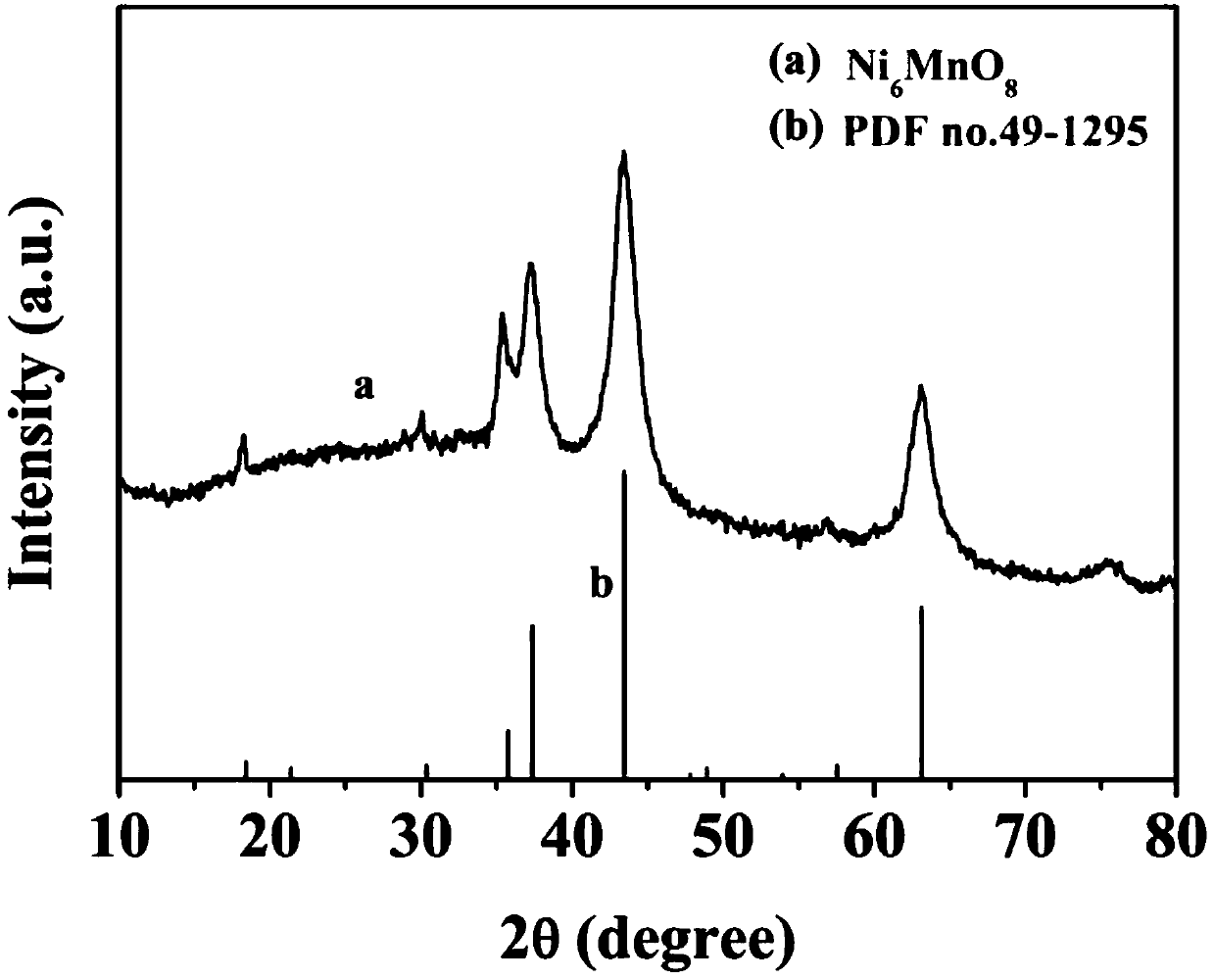
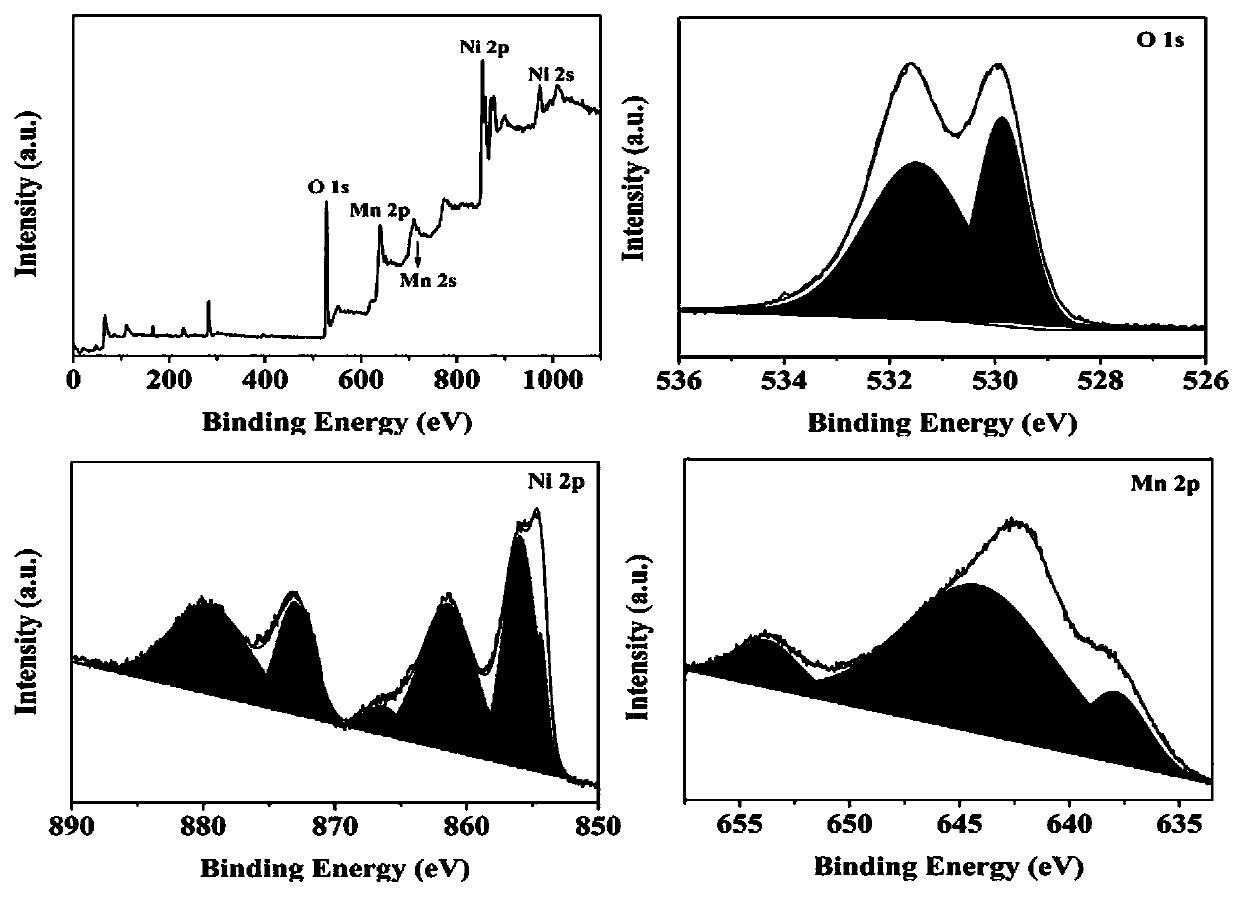
The invention belongs to the technical field of electrochemical electrode material preparation, and provides an electrochemical sensor for detecting epinephrine and a preparation method and application thereof. The electrochemical sensor is a nano material of nickel-manganese oxide Ni6MnO8 prepared by a hydrothermal method and calcination, and the electrochemical sensor is prepared by modifying the surface of a glassy carbon electrode with the obtained nano material of nickel-manganese oxide Ni6MnO8. The electrochemical sensor has high sensitivity and selectivity, the electrode modification process is simpler and more convenient, and good stability and reproducibility are achieved. The electrode modification process is simpler and more convenient, and the electrochemical sensor has high sensitivity and selectivity and good stability and reproducibility. The nickel and manganese oxide (Ni6MnO8) is prepared through a hydrothermal method and calcination, and then the electrode is prepared. The sensitivity of the electrode is improved; and the electrode modification process is simpler. The electrochemical sensor is used for constructing a sensing system for detecting epinephrine in saliva, and the selectivity of the electrode is remarkably improved.
Owner:SHANXI UNIV
Ketoreductase mutant for preparing R-type phenylephrine
The invention relates to a ketoreductase mutant for preparing R-type phenylephrine, and belongs to the technical field of protein engineering. Through the protein engineering, ketoreductase is subjected to saturation mutation to build a mutant library, the library is screened, and the ketoreductase mutant with chiral selectivity which is improved in the process of catalyzing alpha-chloro-3-hydroxyacetophenone into an (R)-2-Cl-1-(3-hydroxyphenyl) ethanol intermediate is obtained. The ee value of the intermediate is increased from 98.5% of a mutation template enzyme to 99.8%, and the ketoreductase mutant can be directly used for subsequent chemical reactions without being purified to directly prepare the R-type phenylephrine; and a process is further simplified, the production cost is lowered, and the ketoreductase mutant is suitable for industrial production.
Owner:SYNCOZYMES SHANGHAI
Phenylephrine hydrochloride injection and preparation process thereof
ActiveCN102525893ALow costOrganic active ingredientsInorganic non-active ingredientsFormularyPhenylephrine Injection
The invention discloses a formula of phenylephrine hydrochloride injection and a preparation process thereof. The phenylephrine hydrochloride injection contains, per 10000 mL, 100 g of phenylephrine hydrochloride, 40 g to 80 g of sodium chloride, 0.8 g to 1.2 g of disodium ethylenediaminetetraacetate, and water for injection added up to 10000 mL. The invention provides a low-cost prescription of phenylephrine hydrochloride injection and a preparation process thereof. The quality of the phenylephrine hydrochloride injection conforms to the regulations of notice on issue of chemical injection and multi-component biochemical injection basic technical requirements ([2008] No.7 file) issued by State Food and Drug Administration in 2008.
Owner:上海禾丰制药有限公司
Epinephrine compositions and containers
ActiveUS10653646B2Organic active ingredientsPharmaceutical delivery mechanismBiochemical engineeringBiomedical engineering
The inventive subject matter provides ready-to-administer, preferably anti-oxidant free, epinephrine compositions with improved stability, and methods for preparing the same. Contemplated compositions can be packaged using blow-fill-seal technology or packaged into flexible IV bags and maintain degradation of the epinephrine at a level of less than 5 wt % when stored over at least one months at between 2-40° C.
Owner:NEVAKAR INJECTABLES INC
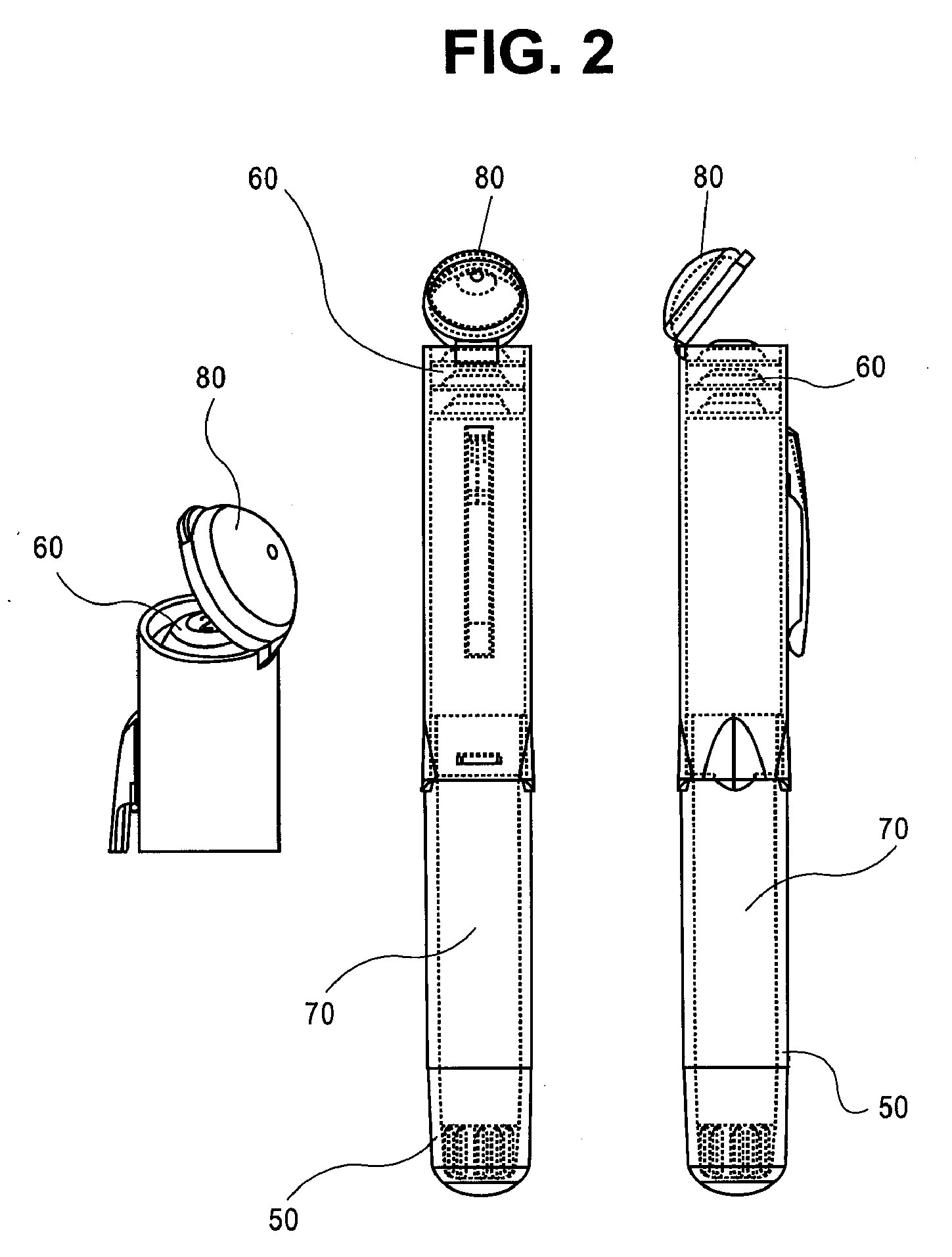
Mobile phone case with epinephrine injection pen carrying feature
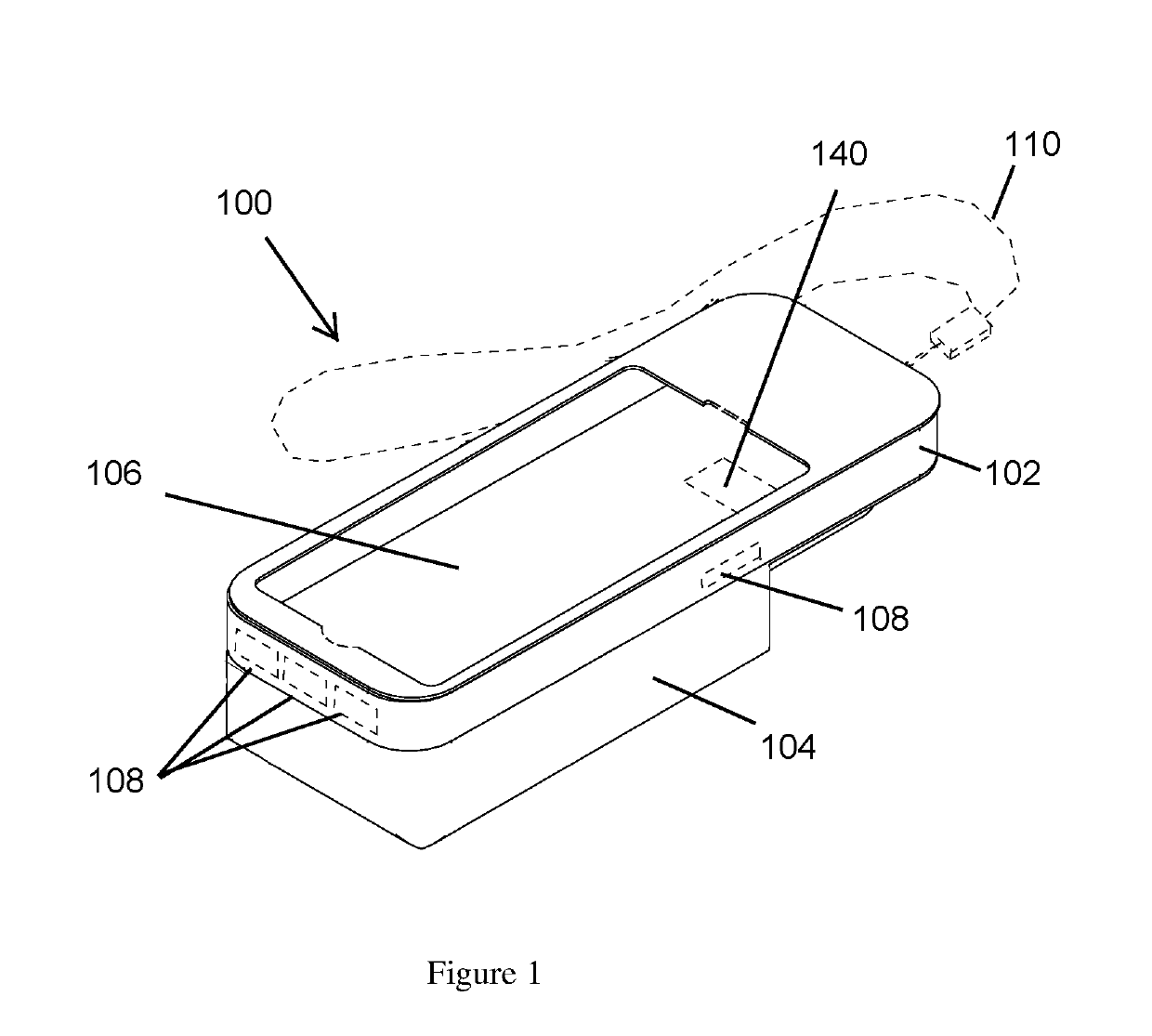
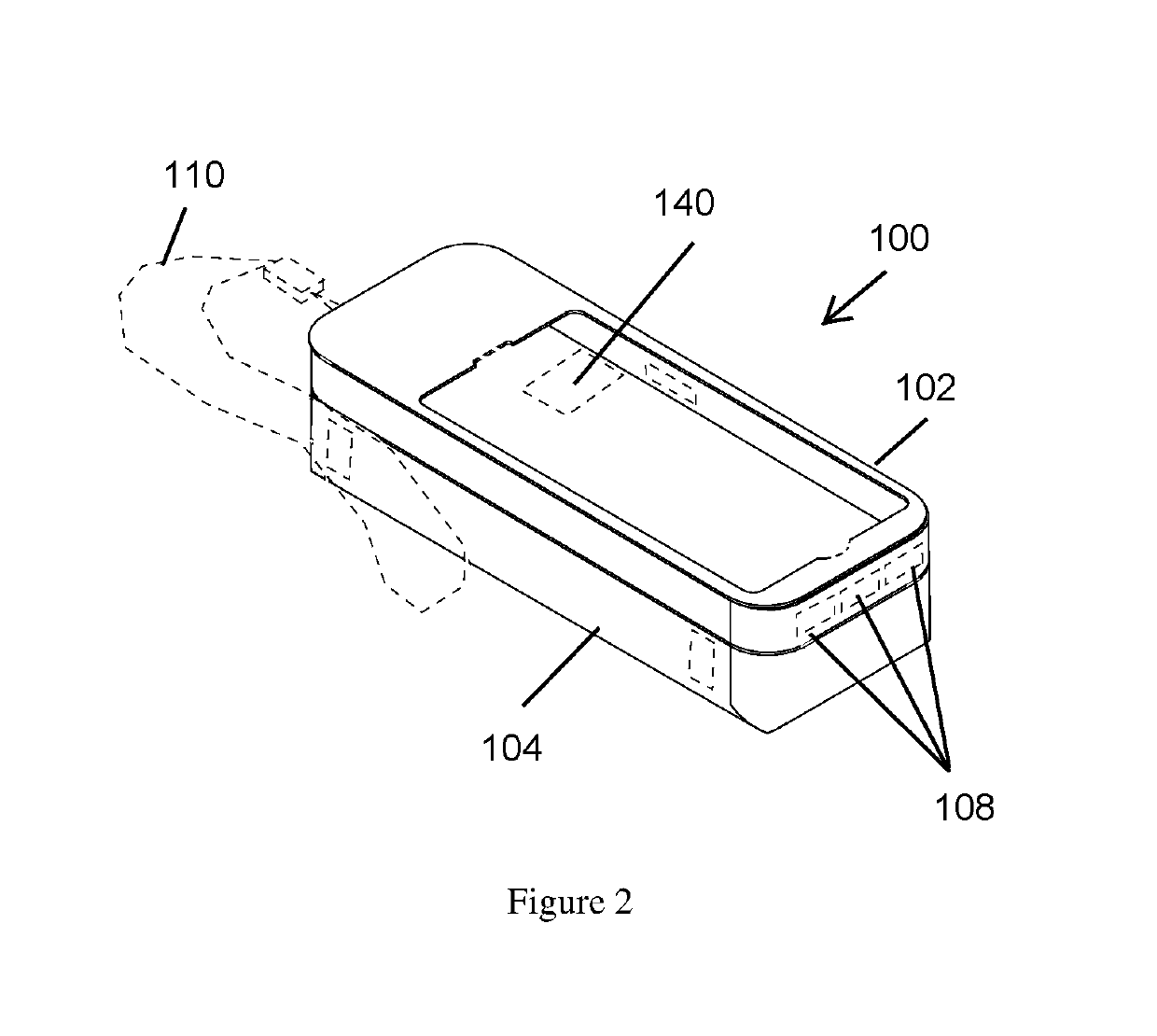
ActiveUS20190260411A1Improve securityAmpoule syringesIntravenous devicesComputer hardwareComputer network
A case assembly is provided having a mobile phone case and storage case that is designed to securely hold a mobile phone and an epinephrine injection pen concurrently. The case assembly includes the mobile phone case which is configured to securely contain a mobile phone therein. The mobile phone case includes a viewing region which allows an operator to view a screen of the mobile phone. The storage case is coupled to the mobile phone case and is configured to securely contain and allow access to an epinephrine injector pen. The mobile phone case is selectively moveable relative to the storage case between an open position and a closed position. The opening in the storage case is covered when the mobile phone case is in the closed position, and the opening in the storage case is open when the mobile phone case is in the open position.
Owner:LANGHANS VENTURES LLC
Methods of stabilizing epinephrine
ActiveUS20190209494A1Organic active ingredientsInorganic non-active ingredientsEngineeringEpinephrine
The present invention is directed to methods of stabilizing a pharmaceutical composition comprising epinephrine containing the steps filling a container, capping the container, assembling the container and placing the assembled capped container (assembled device) in a secondary packaging system.
Owner:HIKMA PHARMA USA INC
Iontophoresis drug delivery formulation providing acceptable sensation and dermal anesthesia
InactiveUS20090312688A1Great confidenceFewer returnsElectrotherapyAnaesthesiaVasoconstrictor AgentsBlood vessel
A shelf-stable electrically assisted transdermal drug delivery system for highly effective electrotransport of an anesthetic and a vasoconstrictor producing clinically acceptable dermal anesthesia and sensation is provided. In certain embodiments the anesthetic includes lidocaine and the vasoconstrictor includes epinephrine. Medicament delivery is affected to provide dermal anesthesia with little or no sensation during delivery, as measured by a variety of indicator tests. Methods of producing dermal anesthesia in patients are also provided.
Owner:VYTERIS
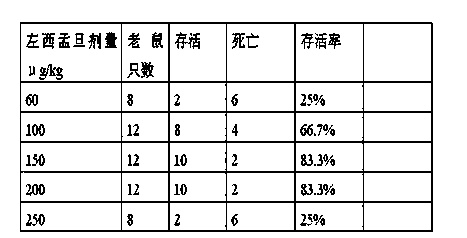
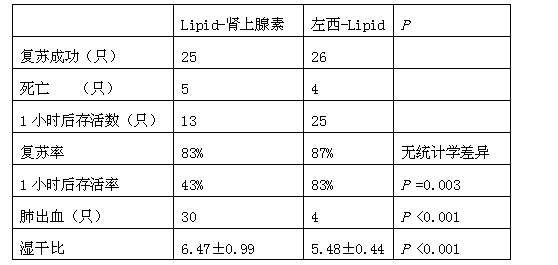
Application of levosimendan in preparation of drug for cardio-pulmonary resuscitation after cardiac arrest caused by amide-type local anesthetics and cardio-pulmonary resuscitation therapy
ActiveCN103520161AAntinoxious agentsRespiratory disorderLUNG HEMORRHAGECardio-pulmonary resuscitation
The invention relates to a cardio-pulmonary resuscitation method for cardiac arrest caused by a poisonous dose of a long-acting amide-type local anesthetic, specifically to application of levosimendan in preparation of a drug for cardio-pulmonary resuscitation after cardiac arrest caused by poisoning of the long-acting amide-type local anesthetic and a cardio-pulmonary resuscitation therapy. Through application of levosimendan in preparation of the drug for cardio-pulmonary resuscitation after cardiac arrest caused by poisoning of the long-acting amide-type local anesthetic and the cardio-pulmonary resuscitation therapy provided by the invention, a cardio-pulmonary resuscitation effect on cardiac arrest caused by clinical anaesthesia of the long-acting amide-type local anesthetic is substantially improved, and the problems of rapid exacerbation of blood-oxygen exchange in the lung and generation of the side effects of severe pneumorrhagia and acidosis in conventional treatment of bupivacaine clinical anaesthesia-induced cardiac arrest by using the scheme of combined usage of a lipid emulsion and epinephrine are overcome; moreover, the cardio-pulmonary resuscitation therapy employs combination of the lipid emulsion and levosimendan, and the incidence rate of arrhythmia occurred after combined usage of the lipid emulsion and levosimendan is substantially lower than the incidence rate of arrhythmia occurred after combined usage of the lipid emulsion and epinephrine.
Owner:THE FIRST AFFILIATED HOSPITAL OF WENZHOU MEDICAL UNIV
Stabilization of epinephrine formulations
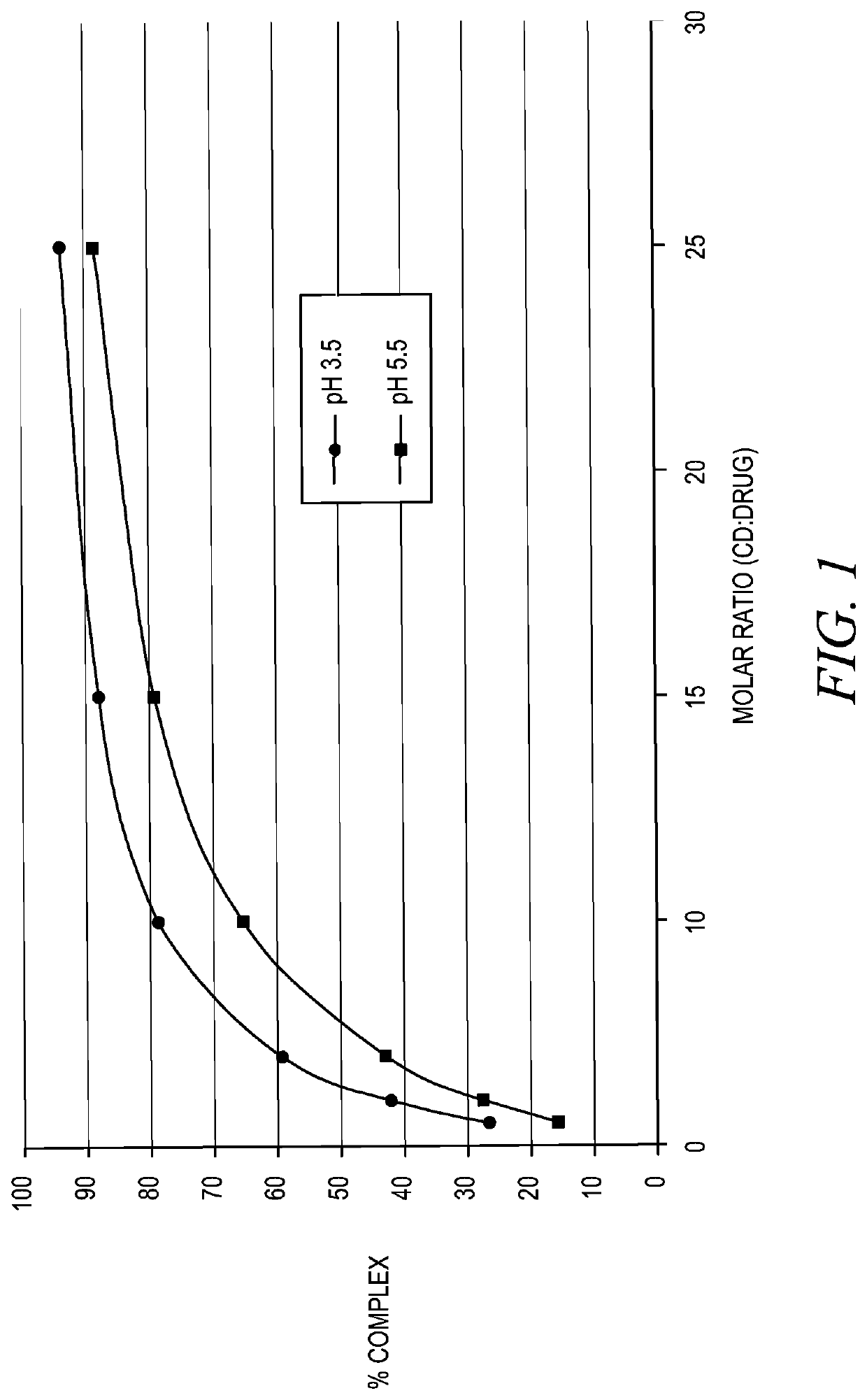
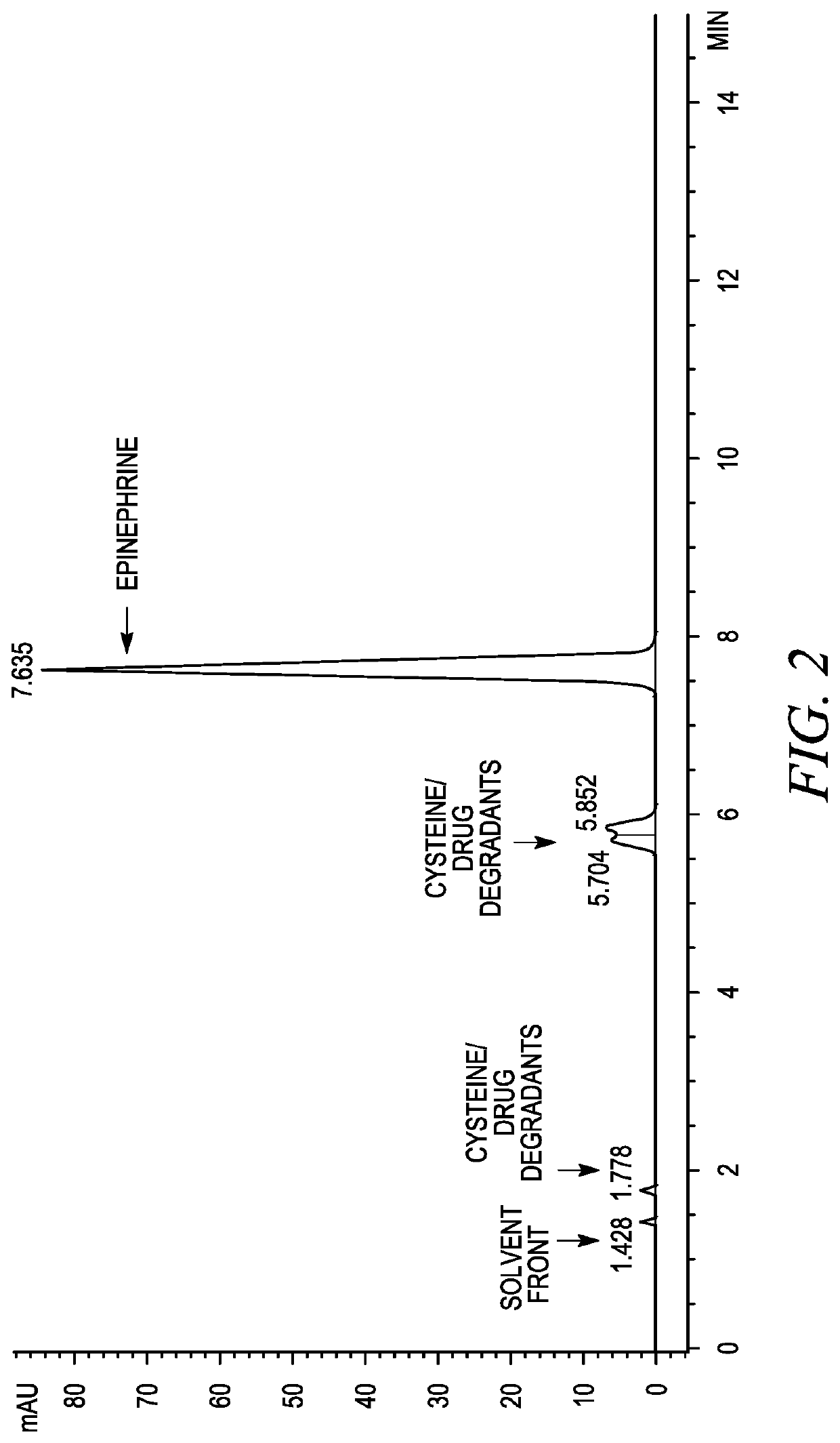
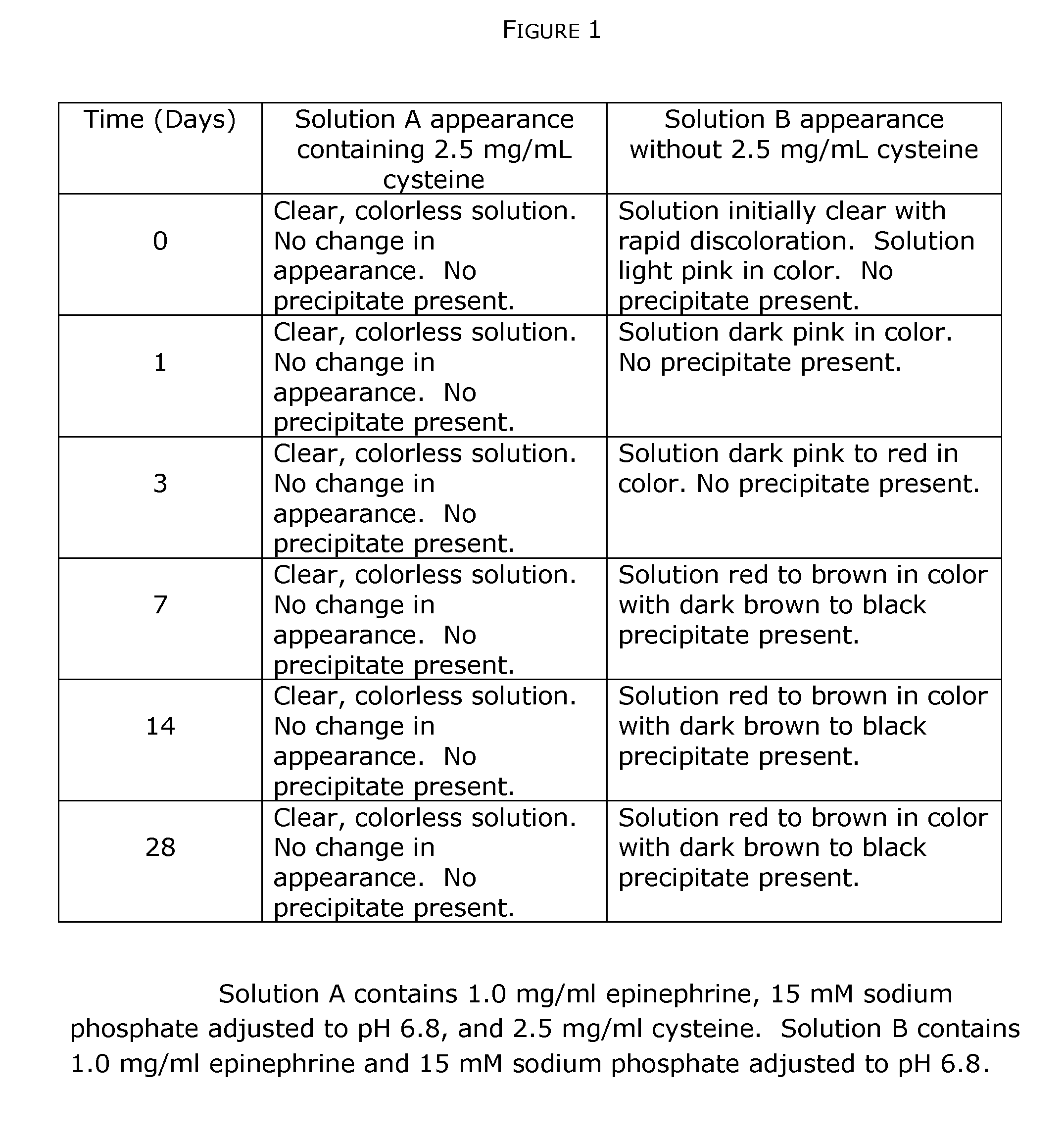
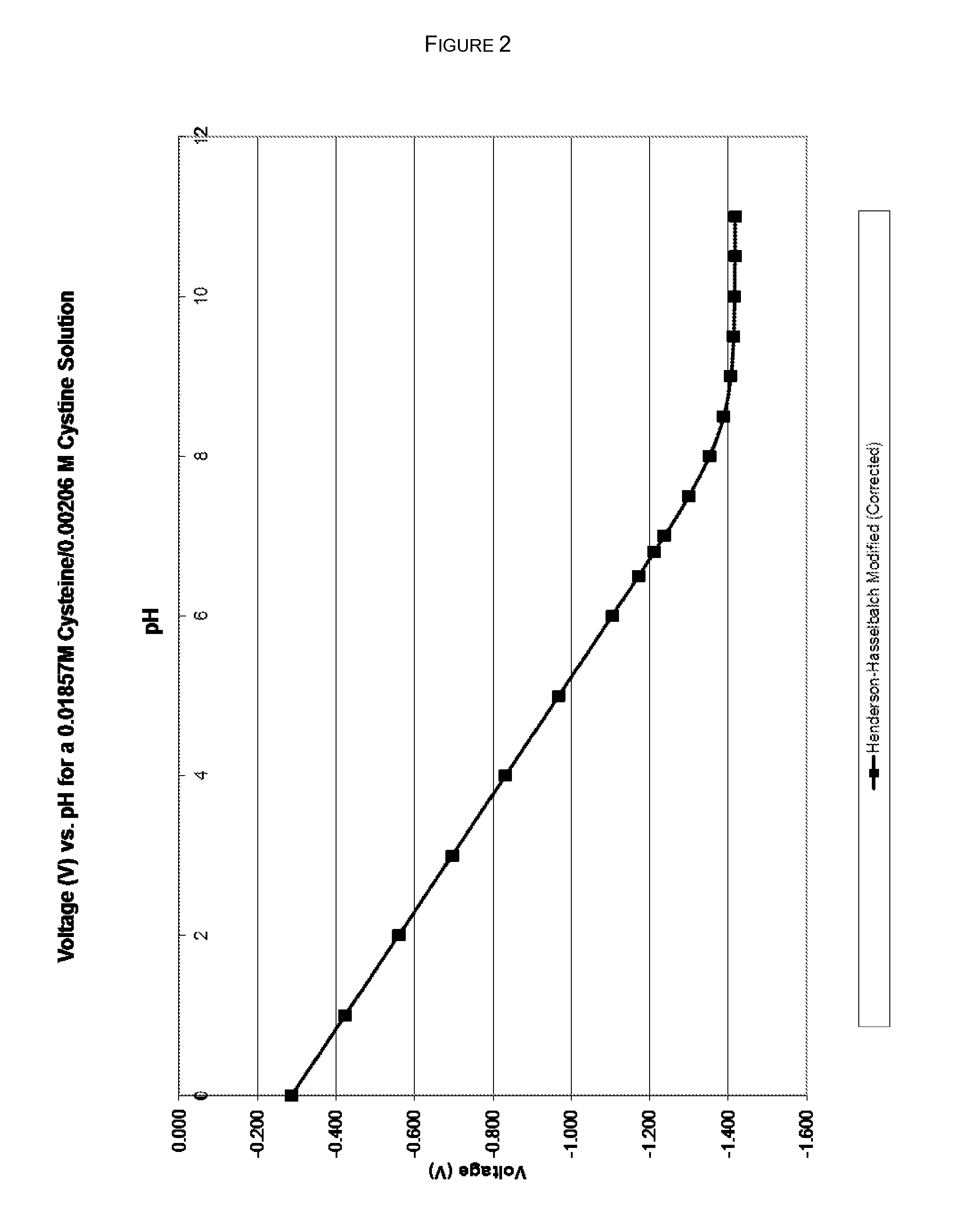
InactiveUS20190350881A1Good physical and chemical stabilityReduce the amount requiredSulfur/selenium/tellurium active ingredientsPharmaceutical delivery mechanismAntioxidantSulfite salt
The disclosure herein relates to the innovative epinephrine formulations in aqueous solution of medicinal products that enhance the physicochemical stabilities of epinephrine and extend the product shelf life. In some instances, the formulations comprise epinephrine or a salt thereof, a complexing agent, and a “non-sulfite” antioxidant. The epinephrine formulations substantially demonstrated the superior physicochemical stabilities to conventional sulfite formulation of commercial medications currently available. In some instances, sulfite-free formulations further provide further benefit (e.g., safety benefits) to sulfite-sensitive patients. The compositions, methods for preparing the formulations, and methods of using the same (e.g., in the treatment of anaphylaxis) are also provided.
Owner:YS PHARMTECH
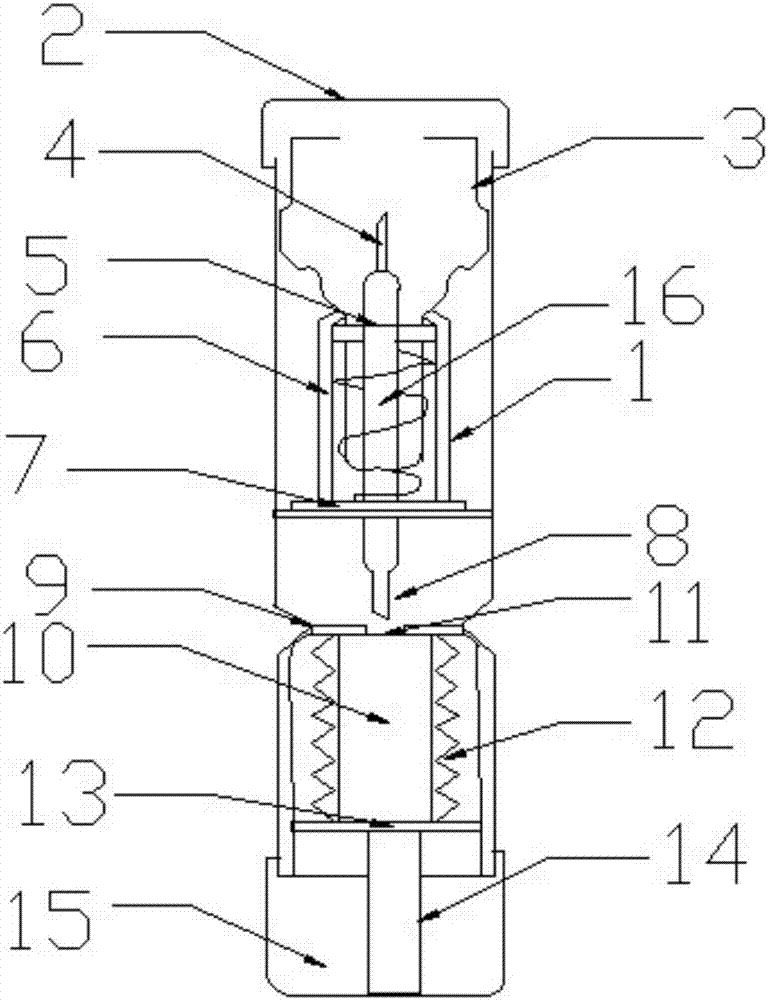
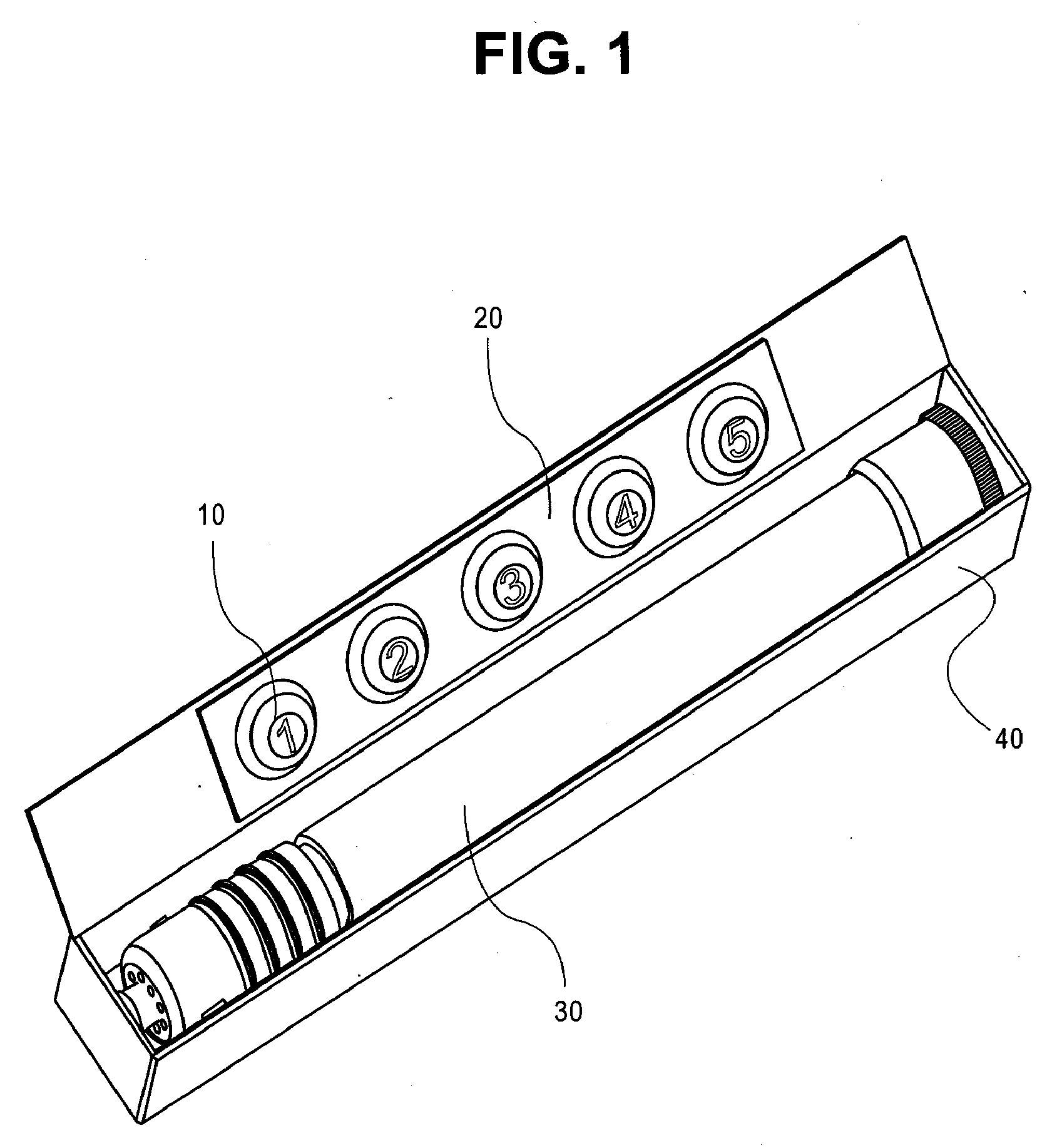
Epinephrine pen
InactiveCN106964028AConvenient self-injectionEasy to carryAmpoule syringesInfusion needlesPhysical therapySurgery
The invention discloses an epinephrine pen. The epinephrine pen comprises a pen body. A medicine storage device is arranged in the pen body, the bottom end of the medicine storage device is connected with a piston rod through a medicine sealing film, medicine storage device springs are symmetrically installed on the two sides of the medicine storage device, a medicine outlet of the medicine storage device is located below a lower needle, the lower needle is connected with an upper needle through a medicine tube, the medicine tube is sleeved with a spring and an ejection base, the spring is installed on the ejection base, and a spring chuck is arranged at the bottom end of a wedge-shaped device. The epinephrine pen is simple in structure, low in manufacturing cost and high in practicality, can be used by allergic constitution patients, more convenience is brought for the patients to inject medicine by themselves, the epinephrine pen is exactly like a pen, can be put in a pocket or a wallet, and is convenient to carry, injection is rapid and convenient, the epinephrine liquid medicine is stored in a refill, the refill and the pen body are connected, the specially-designed needles are ultrafine and ultrashort, needle points are sharp, needle tubes are smooth, no pain is generated in the injection process, the agent application amount is accurate, and production and use are promoted.
Owner:李燕 +1
Preparation method of phenylephrine hydrochloride impurity
ActiveCN103553942AOrganic compound preparationAmino-hyroxy compound preparationPhenylephrine HydrochlorideNMR - Nuclear magnetic resonance
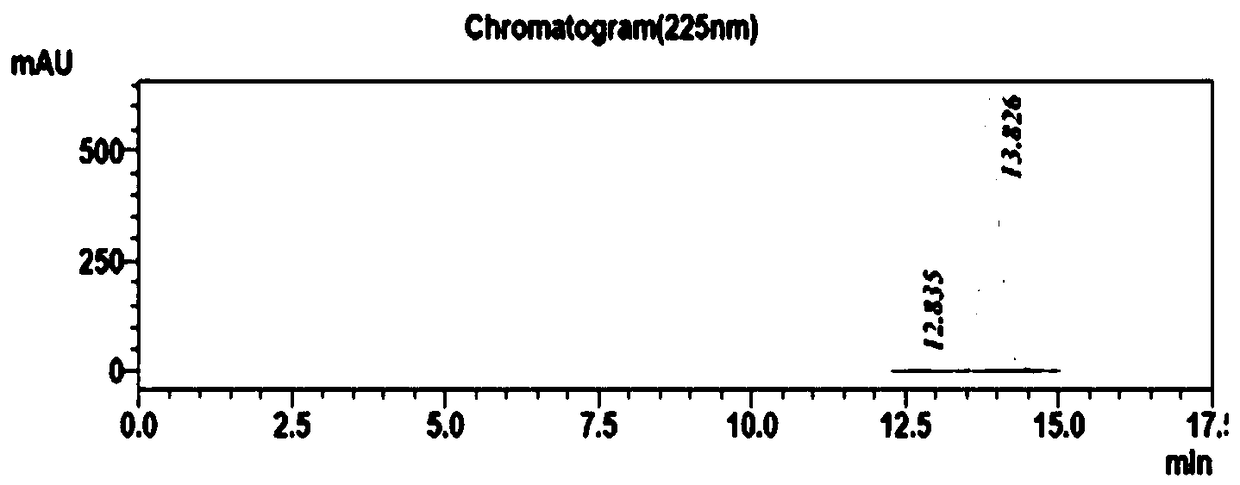
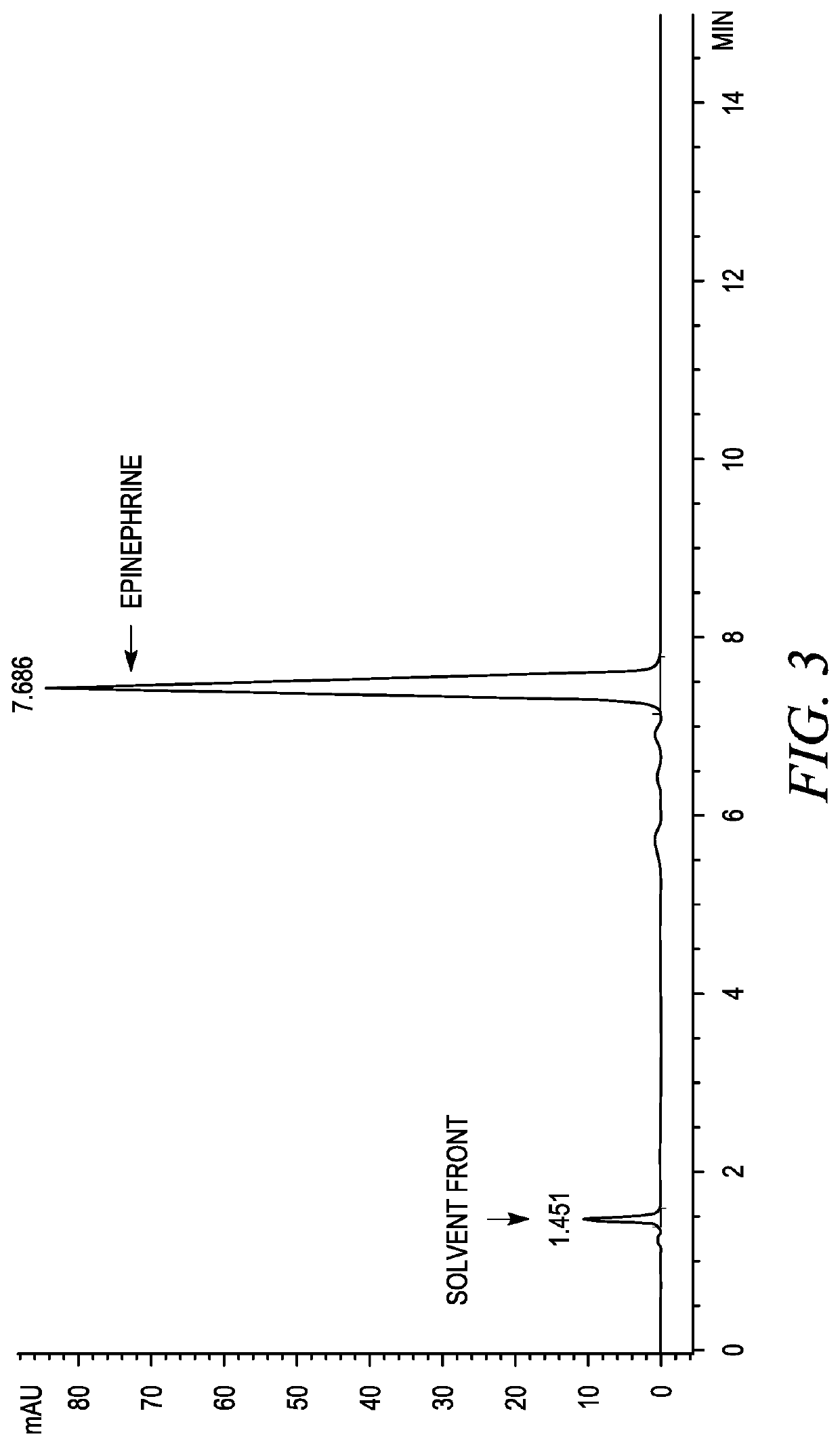
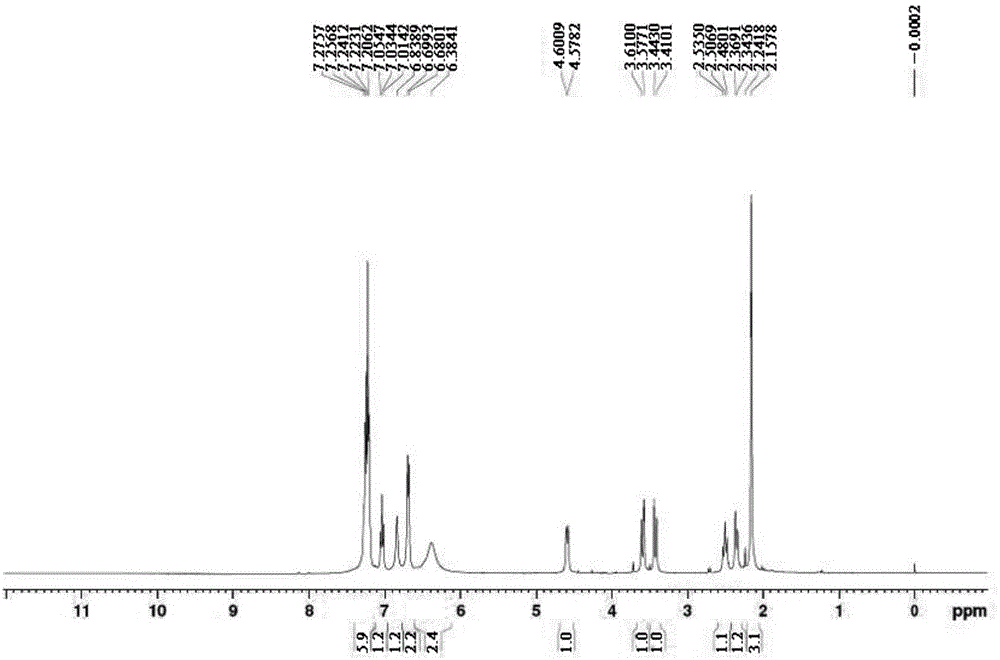
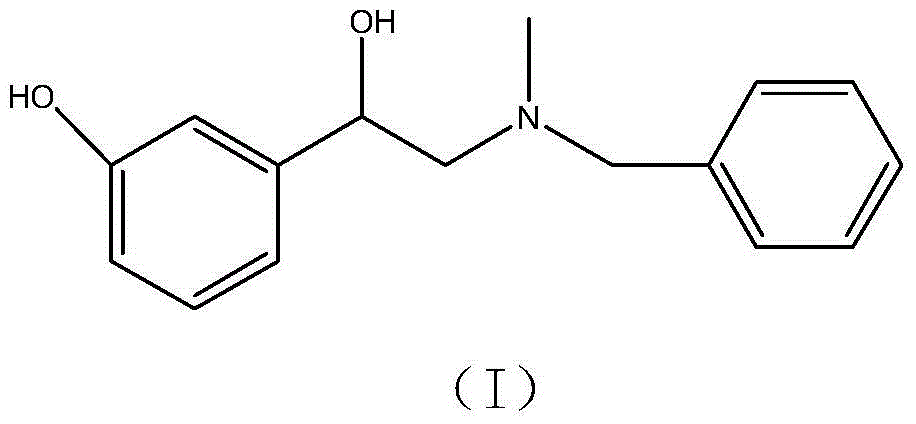
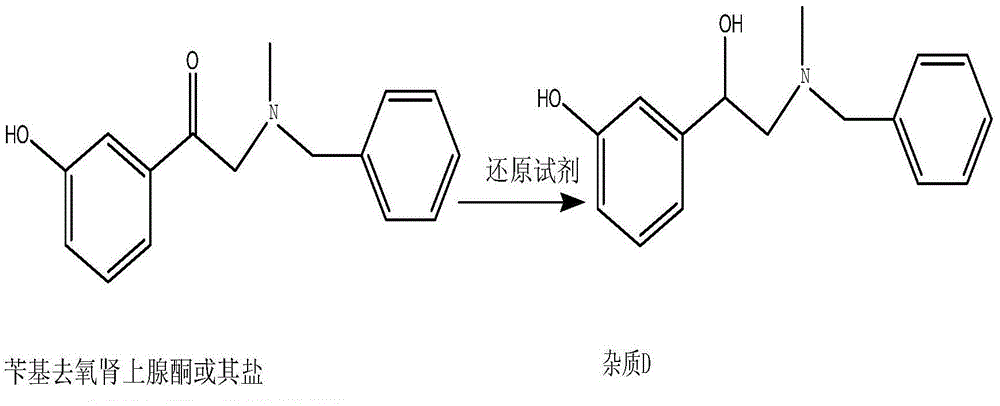
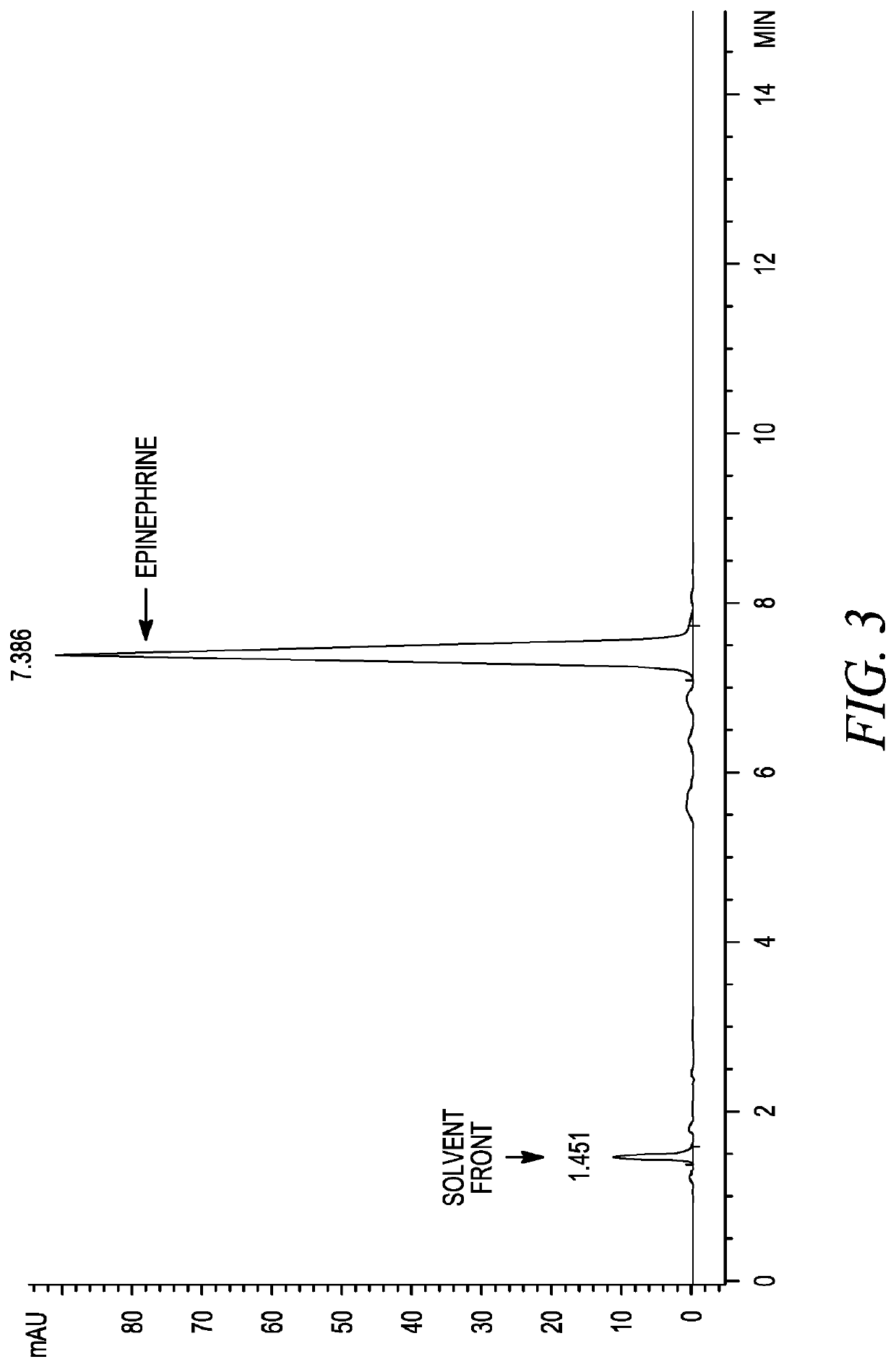
The invention belongs to the field of pharmaceutical chemistry and discloses a phenylephrine hydrochloride impurity D, namely 2-(N-benzyl methylamino)-1-(3-hydroxyl phenyl) alcohol and a preparation method thereof. An impurity D is one of main impurities of crude drugs of phenylephrine hydrochloride; the phenylephrine hydrochloride impurity D is synthesized, a reference substance is provided for examination and quantitative and qualitative analysis of the phenylephrine hydrochloride impurity, so that the quality standards of phenylephrine hydrochloride are improved, and guidance is provided for safe medication of phenylephrine hydrochloride; meanwhile, an effective test basis is provided for obtaining phenylephrine hydrochloride which satisfies EP (European Pharmacopeia) quality standards; the figure 1 is an impurity D H-nuclear magnetic resonance spectrogram.
Owner:WUHAN WUYAO SCI & TECH
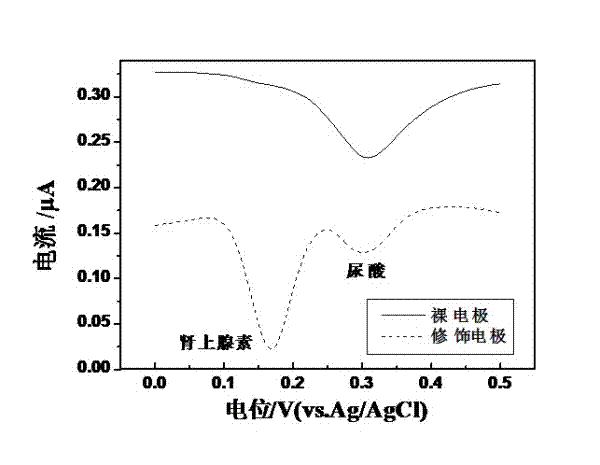
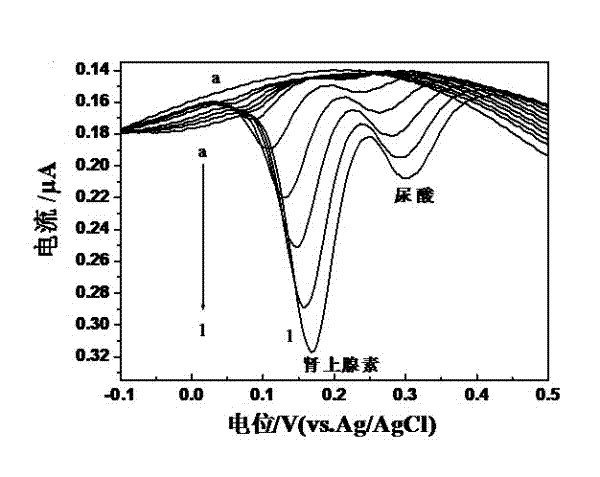
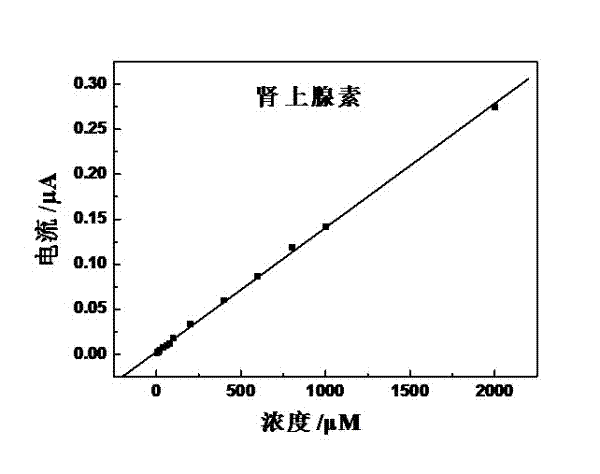
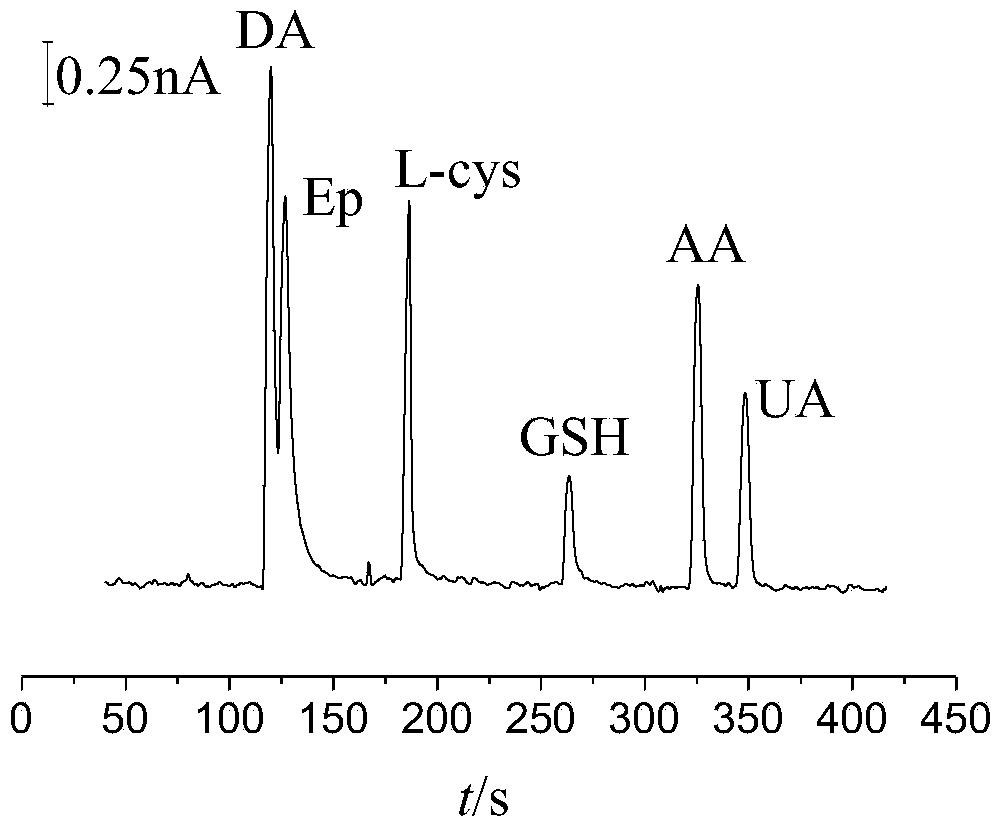
Preparation method of quasi-microelectrode and application of quasi-microelectrode to simultaneous detection of epinephrine and uric acid
InactiveCN102401810AReduce manufacturing costQuick and easy to makeMaterial electrochemical variablesModified carbonCatalytic oxidation
The invention relates to a reparation method of a sodium dodecyl benzene sulfonate / naphthol modified quasi-microelectrode and application of the quasi-microelectrode as an electrochemical sensor to rapid determination of epinephrine and uric acid, and belongs to the technical field of chemical analysis and detection. The invention utilizes catalytic oxidation effect of the electrochemical sensor, prepared from the sodium dodecyl benzene sulfonate / naphthol modified carbon paste quasi-microelectrode, on epinephrine and uric acid to carry out sensitive quantitative analysis on epinephrine and uric acid by a concentration-current curve method. The invention has main points that a working electrode has a small diameter of 0.5mm, and a three-electrode system comprising the quasi-microelectrode, a platinum filament and an Ag / AgCl reference electrode can carry out detection on microscale solution. After drying under a normal temperature, the sodium dodecyl benzene sulfonate / naphthol modified quasi-microelectrode forms a self-assembling modification layer to realize stable catalysis on oxidation of epinephrine and uric acid.
Owner:SHANGHAI UNIV
Preparation method of graphene modified glassy carbon electrode and application thereof
InactiveCN111103341AIncreased electron transport capacityHigh sensitivityMaterial electrochemical variablesMicrosphereHydrothermal synthesis
The invention discloses a preparation method of a graphene modified glassy carbon electrode, and the method is applied to electrochemical detection of epinephrine. The preparation method comprises thefollowing steps: carrying out hydrothermal synthesis on Bi(NO3)3.5H2O and Na2MoO4 to obtain BiMoO6 hollow microspheres by adopting the detection method; and modifying the surface of the graphene modified glassy carbon electrode with the BiMoO6 hollow microspheres to obtain the BiMoO6 hollow microsphere / graphene modified glassy carbon electrode. The adrenaline is detected by adopting the BiMoO6 hollow microsphere / graphene modified electrode differential pulse voltammetry, the linear range is wide, the sensitivity is high, the electrode can be used for multiple times, and the operation is convenient.
Owner:广州百兴网络科技有限公司
Stabilization of epinephrine formulations
PendingUS20200268689A1Reduce the amount requiredLow formationOrganic active ingredientsAmpoule syringesAntioxidantSulfite salt
The disclosure herein relates to the innovative epinephrine formulations in aqueous solution of medicinal products that enhance the physicochemical stabilities of epinephrine and extend the product shelf life. In some instances, the formulations comprise epinephrine or a salt thereof, a complexing agent, and a “non-sulfite” antioxidant. The epinephrine formulations substantially demonstrated the superior physicochemical stabilities to conventional sulfite formulation of commercial medications currently available. In some instances, sulfite-free formulations further provide further benefit (e.g., safety benefits) to sulfite-sensitive patients. The compositions, methods for preparing the formulations, and methods of using the same (e.g., in the treatment of anaphylaxis) are also provided.
Owner:YS PHARMTECH
Preparation method and application of functionalized graphene modified glassy carbon electrode
ActiveCN110672691AResolve detectionSolution rangeMaterial electrochemical variablesTriethoxysilaneEpinephrine Hydrochloride
The invention discloses a preparation method and application of a functionalized graphene modified glassy carbon electrode. The preparation method comprises the following steps of modifying a surfaceof the polished glassy carbon electrode by adopting a modifier containing metal-doped gamma-ureidopropyltriethoxysilane functionalized graphene oxide with effective concentration after electrochemically activating the polished glassy carbon electrode, thereby obtaining the functionalized graphene modified glassy carbon electrode. The invention further discloses application of the functionalized graphene modified glassy carbon electrode in detecting the concentration of epinephrine in an electrochemical method. The problems that the traditional electrode detection is slow in response, narrow indetection range and low in sensitivity due to the defects that the epinephrine is slow in electron transfer rate on a bare electrode and the oxidation products are easy to adsorb are solved by utilizing the unique electrocatalytic property of graphene oxide. The testing method disclosed by the invention is good in reproducibility and stability, and can be used for measuring the concentration of the epinephrine hydrochloride, and has the features of being fast, sensitive and accurate.
Owner:SICHUAN UNIVERSITY OF SCIENCE AND ENGINEERING
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com




![Preparation method of dibenzo [c, e] aza-5, 7 (6H)-dione compound Preparation method of dibenzo [c, e] aza-5, 7 (6H)-dione compound](https://images-eureka.patsnap.com/patent_img/3725bf39-ee8d-416f-ab0f-36ca737d7e31/HDA0001158315700000011.png)
![Preparation method of dibenzo [c, e] aza-5, 7 (6H)-dione compound Preparation method of dibenzo [c, e] aza-5, 7 (6H)-dione compound](https://images-eureka.patsnap.com/patent_img/3725bf39-ee8d-416f-ab0f-36ca737d7e31/HDA0001158315700000021.png)
![Preparation method of dibenzo [c, e] aza-5, 7 (6H)-dione compound Preparation method of dibenzo [c, e] aza-5, 7 (6H)-dione compound](https://images-eureka.patsnap.com/patent_img/3725bf39-ee8d-416f-ab0f-36ca737d7e31/HDA0001158315700000031.png)