Ketoreductase mutant for preparing R-type phenylephrine
A technology of phenylephrine and reductase, which is applied in the field of protein engineering, can solve problems such as unfavorable methylation reactions and increase operating costs, and achieve high atom utilization, simplified process costs, and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 Construction of site-directed saturation mutant library of ketoreductase
[0023] The ketoreductase of (SEQ ID No.2, corresponding nucleotide sequence is SEQ ID No.1) is docked with the substrate through computer simulation structure, it is speculated that the 199 position is closely related to the catalysis, and the The saturation mutant library was constructed, and the specific sequence information is shown in Table 1.
[0024] Table 1 Primer sequences for saturation mutation
[0025]
[0026] The underlined sequence in Table 1 is the mutation site, and the vector with the mutant gene was amplified by PCR amplification reaction of the whole plasmid. Then utilize the DpnI restriction endonuclease to digest the recombinant plasmid template of the PCR product, and after purification, transform into Escherichia coli BL21 (DE3) competent, then spread it on the LB plate containing 50ug / L Amp, in Single clones were grown by upside-down culture in an incubator ...
Embodiment 2
[0027] High-throughput screening of embodiment 2 positive clones
[0028] Randomly select 188 single clones for 96-well plate shaking culture, add 400uL LB medium to a sterile 96-well plate, culture at 37°C for about 12 hours, and transfer to the second 96-well plate according to the inoculum size of 10%. Medium culture, continue to culture for about 3h, add IPTG with a final concentration of 0.1mM to induce expression for 16h, and the induction temperature is 25°C. After the incubation, the supernatant was discarded by centrifugation and stored in a -20°C refrigerator until use.
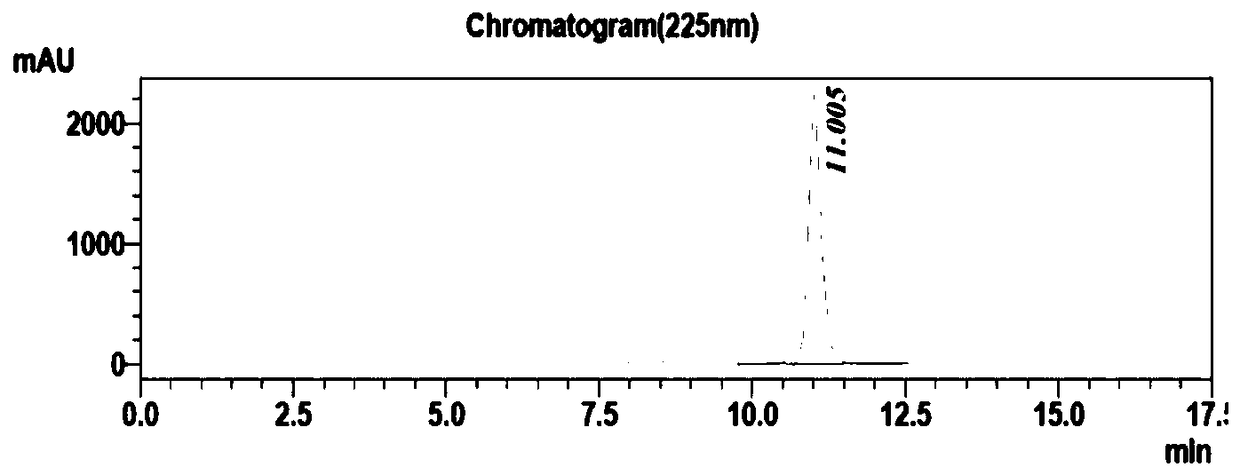
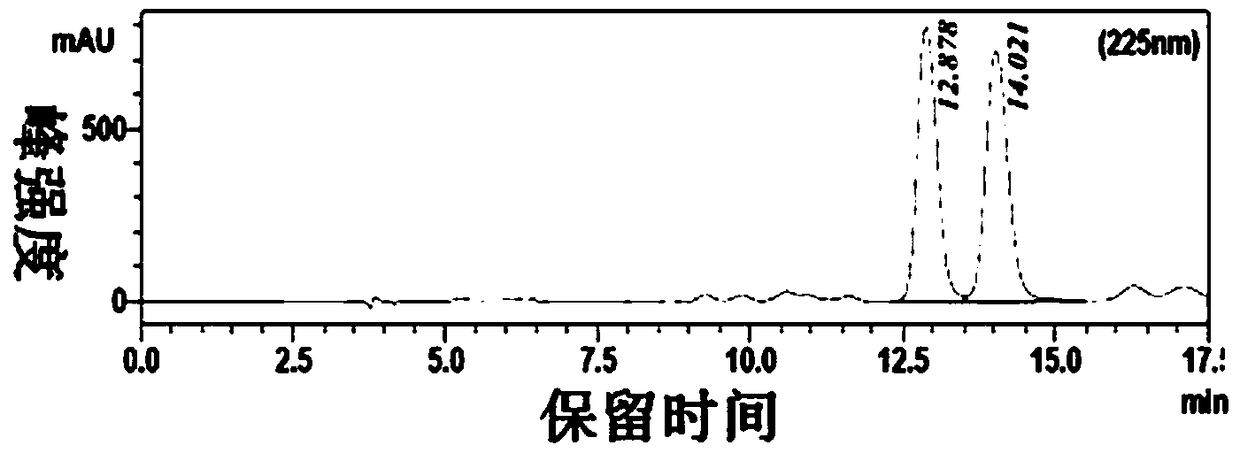
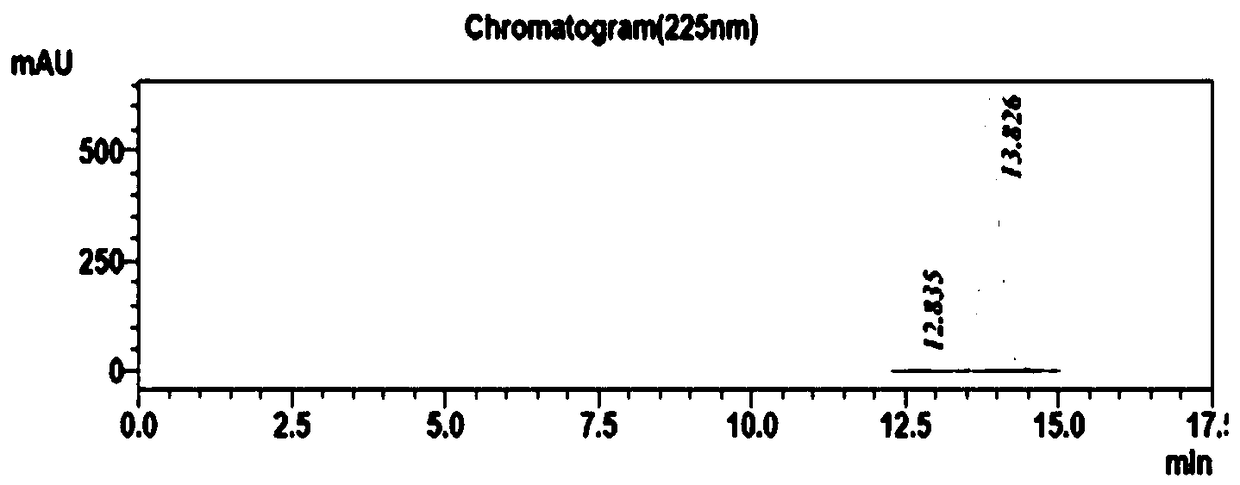
[0029] The transformation system was prepared according to Table 2, and 400uL was drawn into the centrifuged 96-well plate with a row gun, and reacted at a constant temperature of 30°C and a rotation speed of 400rpm for 24h. Finally, TLC plate detection was performed on all conversion reaction solutions, and candidate reaction solutions that could almost completely convert the substrate were select...
Embodiment 3
[0032] The sequencing of embodiment 3 positive candidate mutants
[0033] From the results of TLC and HPLC, find the number of mutants with conversion rate>95% and chiral purity>99.9% (ee>99.8%), a total of 3, select the corresponding strains for sequencing, and find the mutation according to the sequence comparison The amino acids were all changed to Leu, and 1C7 was selected as the mutant strain for species preservation. Table 3 is the sequence mutation information obtained by sequencing.
[0034] Table 3 Codon and amino acid mutation information after sequencing
[0035] Sample
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com