Preparation method of balosavir intermediate
An intermediate, benzyloxy technology, applied in the field of medicine, can solve the problems of high synthesis cost, long steps, and the need for splitting, etc., and achieve the effect of high yield and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
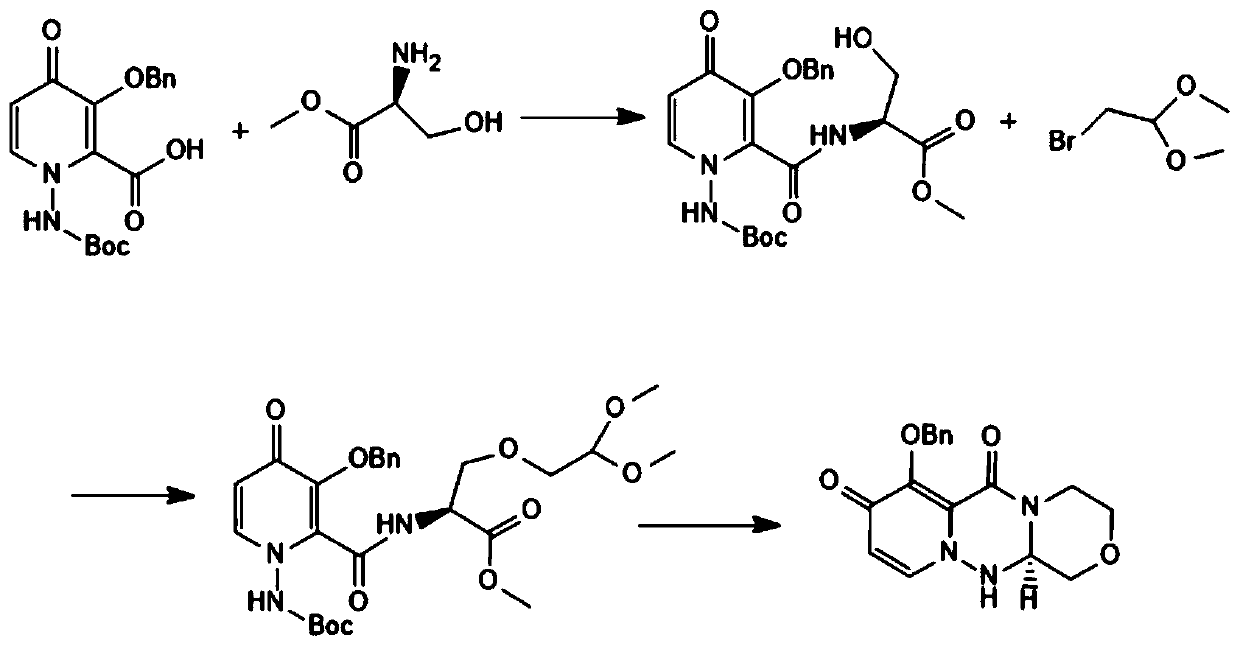
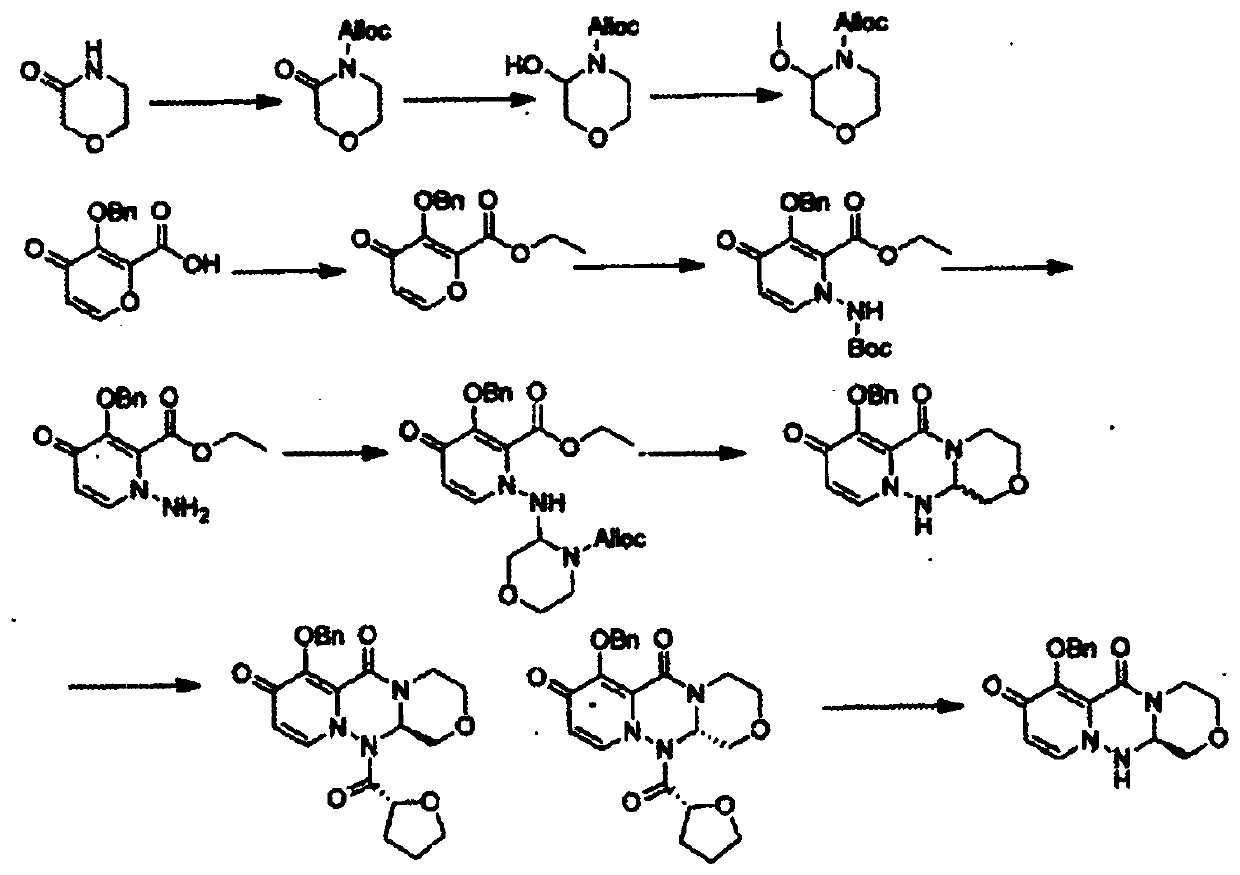
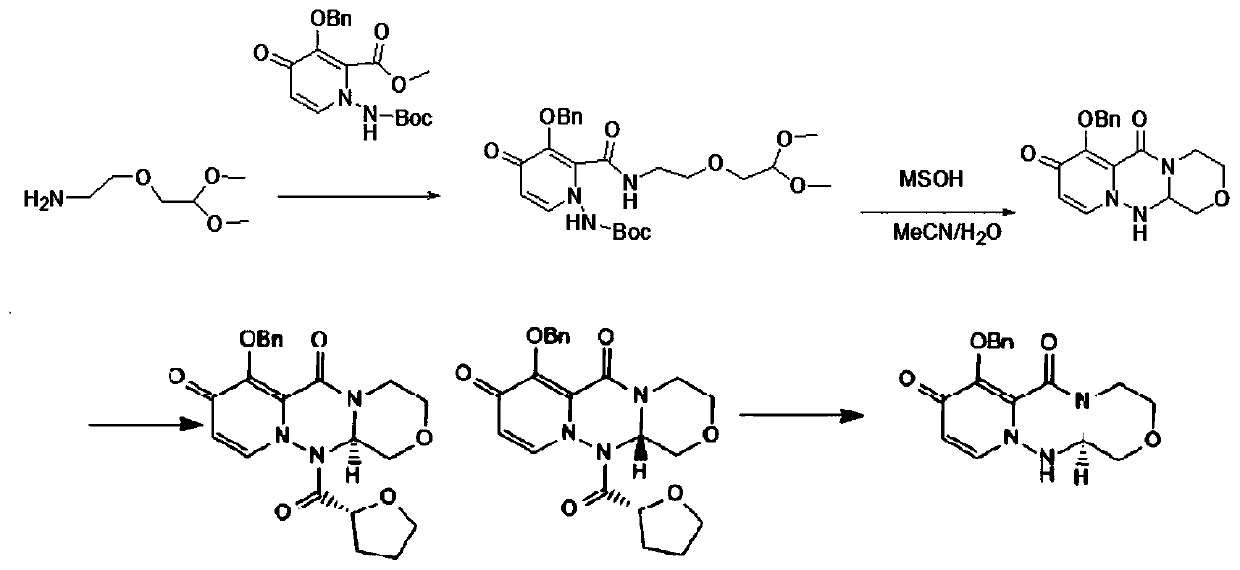
[0026] see figure 1 , a baloxavir intermediate (R)-7-(benzyloxy)-3,4,12,12a-tetrahydro-1H-[1,4]oxazin[3,4-c]pyrido The preparation method of [2,1-f][1,2,4]-triazine-6,8-dione comprises the following steps:
[0027] Step 1: Add 1kg of 3-benzyloxy-1-Bocamino-4-carbonyl-1,4-dihydropyridine-2-carboxylate methyl ester, 400g of L-serine ester, 600g of DCC, 10g of DMAP, and 700g of triethyl in the reaction flask The amine and 10L of dichloromethane were reacted at 30°C. After the reaction, filtered, the filtrate was concentrated, cooled, crystallized and filtered to obtain 935g of intermediate A with a yield of 73%.
[0028] Step 2: Add 900g of intermediate A, 5LDMF and 56g of sodium hydride into the reaction flask, dropwise add 396g of bromoacetal dimethanol, heat up to 60°C for reaction, after the reaction, pour solvent C into 30L of ice water, filter and wash with water , dried to obtain 860g of intermediate B, yield 80%.
[0029] Step 3: Nitrogen protection, first add 850g of...
Embodiment 2
[0031] A preparation method of baloxavir intermediate, comprising the following steps:
[0032] Step 1: Add 1.5kg of 3-benzyloxy-1-Bocamino-4-carbonyl-1,4-dihydropyridine-2-carboxylate methyl ester, 410g of L-serine ester, 600g of EDCI, 10g of DMAP, 700g of three Ethylamine and 10L dichloroethane were reacted at 40°C. After the reaction was completed, filtered, the filtrate was concentrated, cooled, crystallized and filtered to obtain 945g of intermediate A with a yield of 75%.
[0033] Step 2: Add 900g of intermediate A, 5LTHF and 50g of sodium hydride into the reaction flask, dropwise add 395g of bromoacetal dimethanol, heat up to 60°C for reaction, after the reaction, pour solvent C into 40L of ice water, filter and wash with water , and dried to obtain 850g of intermediate B with a yield of 85%.
[0034] Step 3: Nitrogen protection, first add 850g of intermediate B, 4L of ethylene glycol and 1L of water into the reaction bottle, then add 200g of hydrochloric acid, heat up...
Embodiment 3
[0036] A preparation method of baloxavir intermediate, comprising the following steps:
[0037]Step 1: Add 1kg 3-benzyloxy-1-Bocamino-4-carbonyl-1,4-dihydropyridine-2-carboxylate methyl ester, 400g L-serine ester, 600g DIC, 15g HOBT, 700g diiso Propylethylamine and 10L of dichloromethane were reacted at 30°C. After the reaction, filtered, the filtrate was concentrated, cooled, crystallized and filtered to obtain 930g of intermediate A with a yield of 78%.
[0038] Step 2: Add 1000g of intermediate A, 5LDMSO and 56g of sodium hydride into the reaction flask, dropwise add 400g of bromoacetal dimethanol, and heat up to 60°C for reaction. After the reaction, pour solvent C into 30L of ice water, filter and wash with water , dried to obtain 860g of intermediate B, yield 82%.
[0039] Step 3: Nitrogen protection, first add 850g of intermediate B, 4L of tetrahydrofuran and 1L of water into the reaction bottle, then add 220g of sulfuric acid, heat up for 2 hours, then add 20gPt / C, he...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com