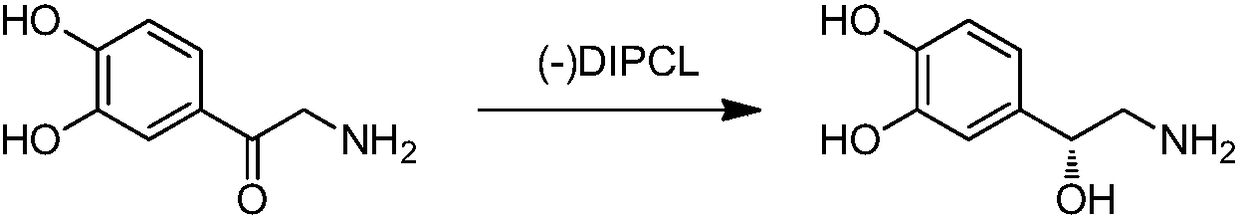
Method for synthesizing noradrenaline
A technology of norepinephrine and epinephrine, which is applied in the field of producing L-norepinephrine by the reaction of reducing agent and norepinephrine, which can solve the problems of flammability, high hidden danger of hydrogenation and high cost, and achieve good safety , reduce costs and potential safety hazards, and improve the effect of chiral purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Add 16.7g (100mmol) of norepinephrine and 200mL of methyl tert-butyl ether to a 1L reaction flask, lower the temperature to minus 25℃ under nitrogen atmosphere, and add 150g (240mmol)(-)-two drops below minus 20℃ The isopine pinyl chloroborane solution, after dropping, control the temperature at minus 25 to minus 20℃ and the reaction is completed for 3 hours, gradually warm to room temperature (25℃), add 6M hydrochloric acid (100mL), stir and reflux for 1h, stir at room temperature for 30min, and divide. Wash the organic layer and the water layer twice with methyl tert-butyl ether (2×50 mL), adjust the pH to 9.5 with ammonia water while stirring, and filter with suction. The filter cake is rinsed with a small amount of absolute ethanol, and it is allowed to dry in the dark Dry white crystalline powder. The reaction time was detected by TLC.
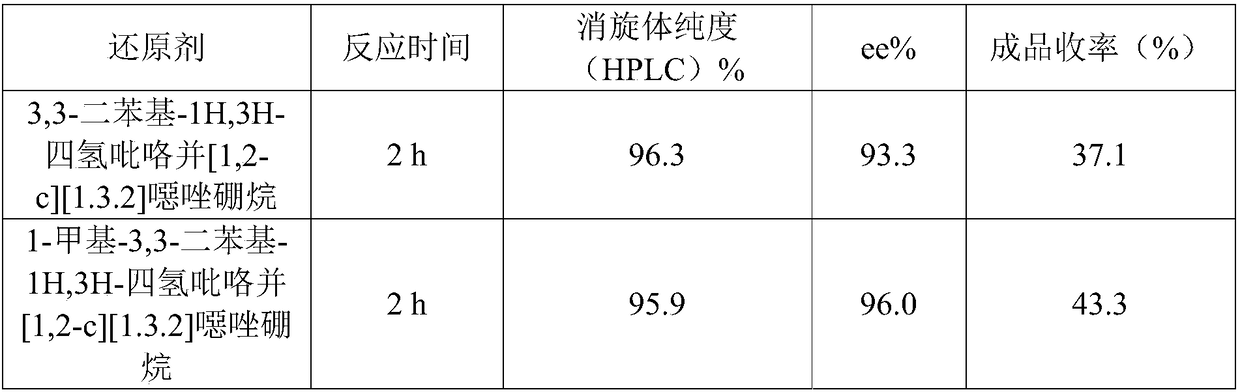
[0047] Replace the above reducing agent with 3,3-diphenyl-1H,3H-tetrahydropyrrolo[1,2-c][1.3.2]oxazoborane and 1-methyl-3,3-dipheny...
Embodiment 2
[0054] Add 16.7g (100mmol) of norepinephrine and 200mL of methyl tert-butyl ether to a 1L reaction flask, lower the temperature to minus 25℃ under nitrogen atmosphere, and add 150g (240mmol)(-)-two drops below minus 20℃ Isopinepinyl chloroborane solution, after dropping, control the temperature at minus 25 to minus 20°C for 3 hours and complete the reaction. Gradually warm to room temperature (30°C) and add 6M hydrochloric acid (100mL), stir and reflux for 1h, stir at room temperature for 30min, and divide. Wash the organic layer and the water layer twice with methyl tert-butyl ether (2×50 mL), adjust the pH to 10 with ammonia water while stirring, and filter with suction. The filter cake is rinsed with a small amount of tert-butanol and allowed to dry in the dark Dry white crystalline powder.
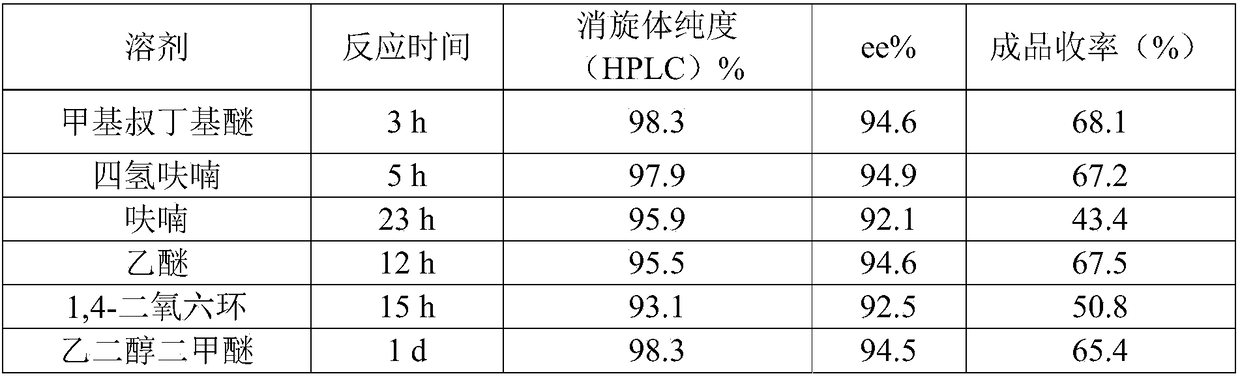
[0055] The above reaction temperature was adjusted to minus 20 to minus 15°C, and minus 30 to minus 25°C for parallel experiments. The results are shown in Table 4.
[0056] Table 4: Effec...
Embodiment 3
[0059] Add 16.7g (100mmol) of norepinephrine and 200mL of methyl tert-butyl ether to a 1L reaction flask, lower the temperature to minus 30℃ under nitrogen atmosphere, and add 150g (240mmol)(-)-two drops below minus 25℃ Isopinepinyl chloroborane solution, after dripping, control the temperature at minus 30 to minus 25℃ and the reaction is completed for 3 hours, gradually warm to room temperature (20℃), add 6M hydrochloric acid (100mL), stir and reflux for 1h, stir at room temperature for 30min, then separate Wash the organic layer and the water layer twice with methyl tert-butyl ether (2×50mL), adjust the pH to 9.5 with ammonia water while stirring, and filter with suction. The filter cake is rinsed with a small amount of isopropanol and allowed to dry in the dark Dry white crystalline powder.
[0060] The feeding amount of (-)-diisopine pinyl chloroborane in the above reaction was adjusted to 1.0eq to 3.0eq, and 9 nodes were selected for parallel experiments. The results are sho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com