AHU-377alpha-phenethylamine salt polycrystalline type and preparation method and application thereof
A technology of AHU-377, phenethylamine salt, applied in the field of medicine, can solve the problems of difficult storage and weighing, poor quality of AHU-377 products, unfavorable storage and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
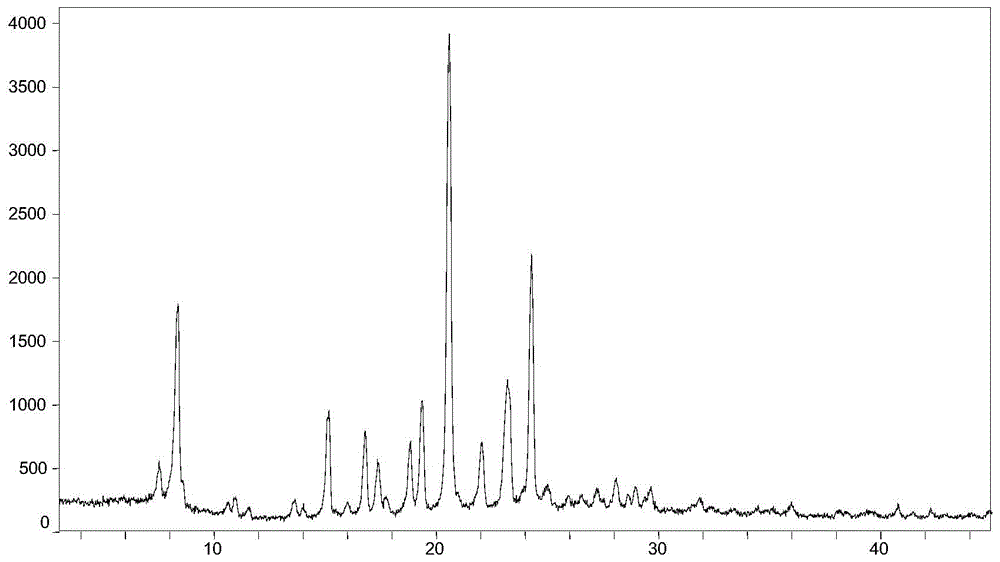
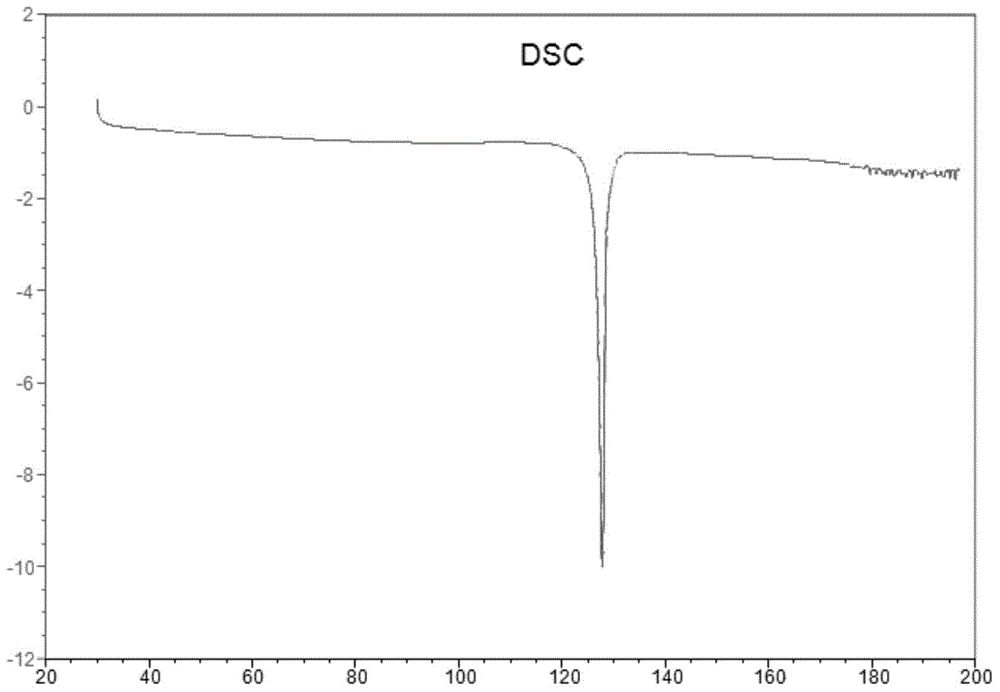
[0068] Weigh 20mg (0.049mmol) of AHU-377 free acid (oil) into a 5.0mL glass bottle, add 1.0mL isopropyl acetate, stir to dissolve, add 5.9mg (0.049mmol) (R)-(+)- α-Phenylethylamine, continue to stir and react for 48 hours, collect the AHU-377(R)-(+)-α-Phenylethylamine salt crystal form obtained by collecting the reaction product, and its powder X-ray diffraction pattern is as follows figure 1 As shown, melting point: 126.6°C (onset point) as figure 2 shown.
Embodiment 2
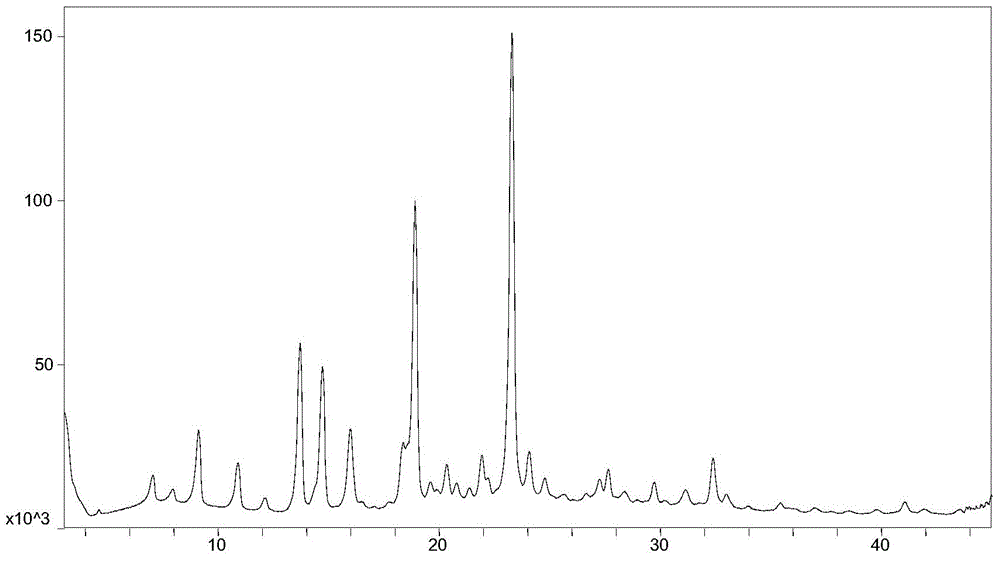
[0070] Weigh 20mg (0.049mmol) of AHU-377 free acid (oil) into a 5.0mL glass bottle, add 0.4mL of isopropanol, stir to dissolve, add 6.5mg (0.053mmol) of (R)-(+)-α -Phenylethylamine, continue to stir and react for 48 hours, collect the AHU-377 (R)-(+)-alpha-phenethylamine crystal form obtained by the reaction product, its powder X-ray diffraction pattern is basically the same as figure 1 unanimous.
Embodiment 3
[0072] Weigh 20mg (0.049mmol) of AHU-377 free acid (oil) into a 5.0mL glass bottle, add 0.2mL methyl tert-butyl ether, heat to 50°C and stir to dissolve, add 5.9mg (0.049mmol) (R )-(+)-α-phenylethylamine, lower the temperature to room temperature (20-25°C) and continue stirring for 48 hours, and collect the AHU-377(R)-(+)-α-phenylethylamine salt crystals obtained from the reaction product type, its powder X-ray diffraction pattern is basically the same as figure 1 unanimous.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com