Patents
Literature
33 results about "Alpha-phenylethylamine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
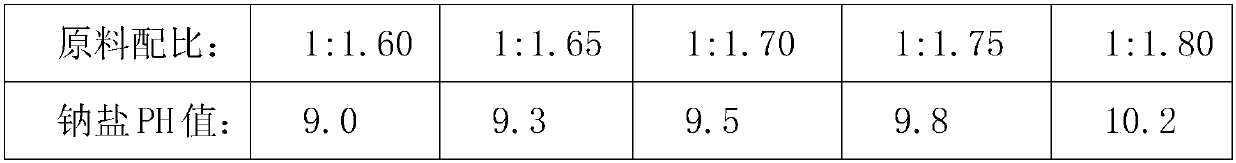
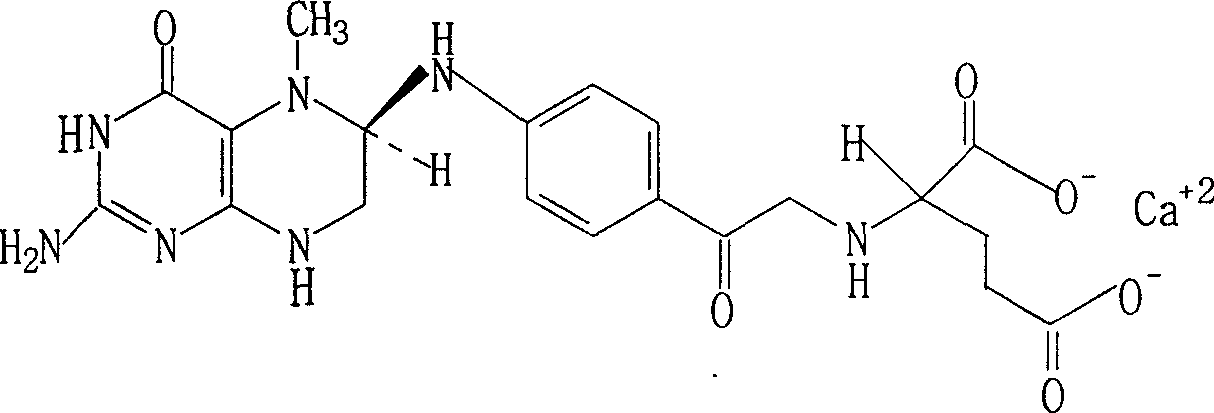
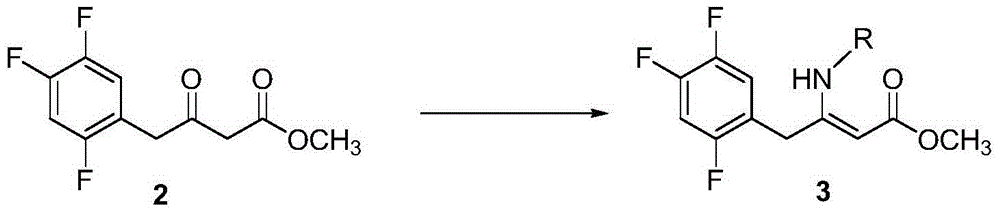
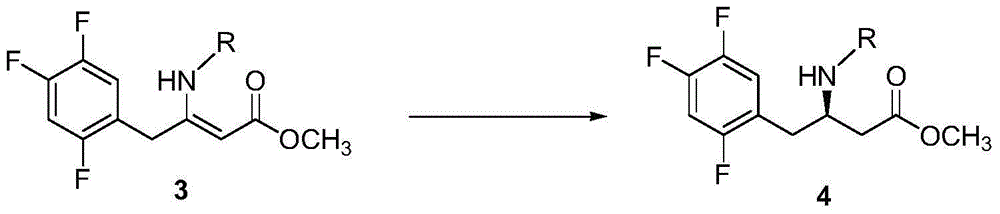
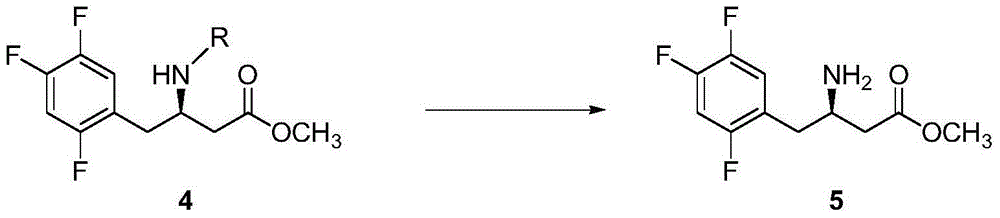
Resolution for 5-methyltetrahydrofolic acid and salifying method thereof
InactiveCN101143863AOptically-active compound separationOrganic racemisationCalcium hydroxideAlkaline earth metal
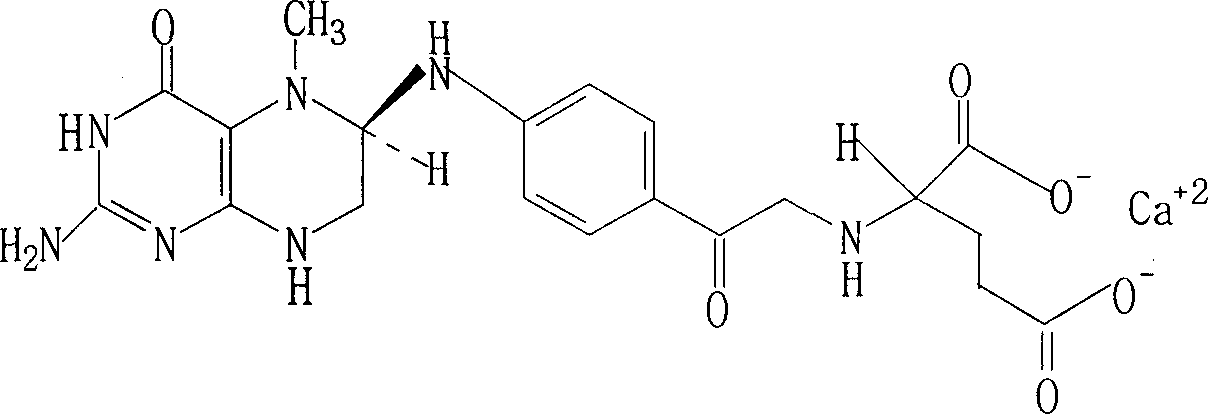
The invention brings forward a preparation method, which utilizes organic alkali Alpha-phenylethylamine to obtain (6S)-5-methyltetrahydrofolate by splitting racemate (6R, S)-5-methyltetrahydrofolate, and utilizes the hydroxide of alkaline earth, particularly calcium hydroxide, to generate (6S)-5-methyltetrahydrofolate calcium salt.
Owner:NAN JING RHINE PHARM TECH
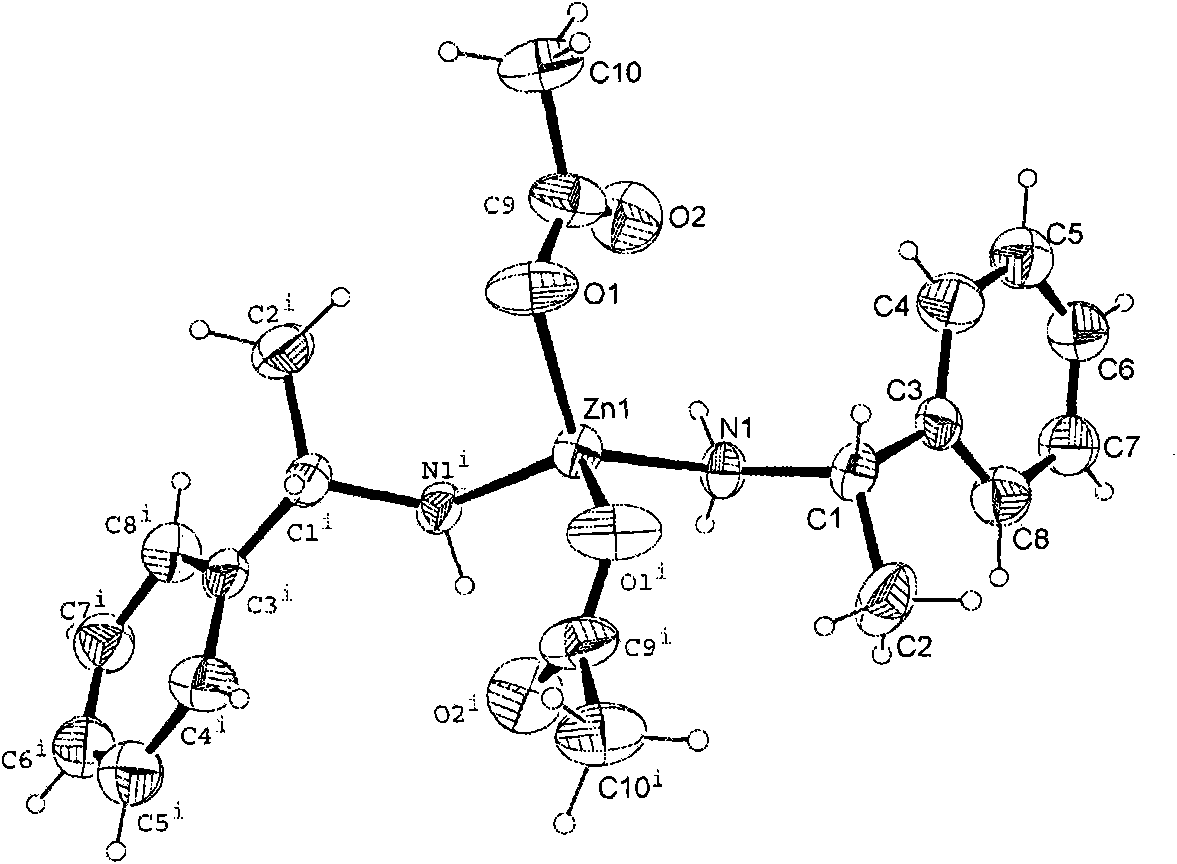
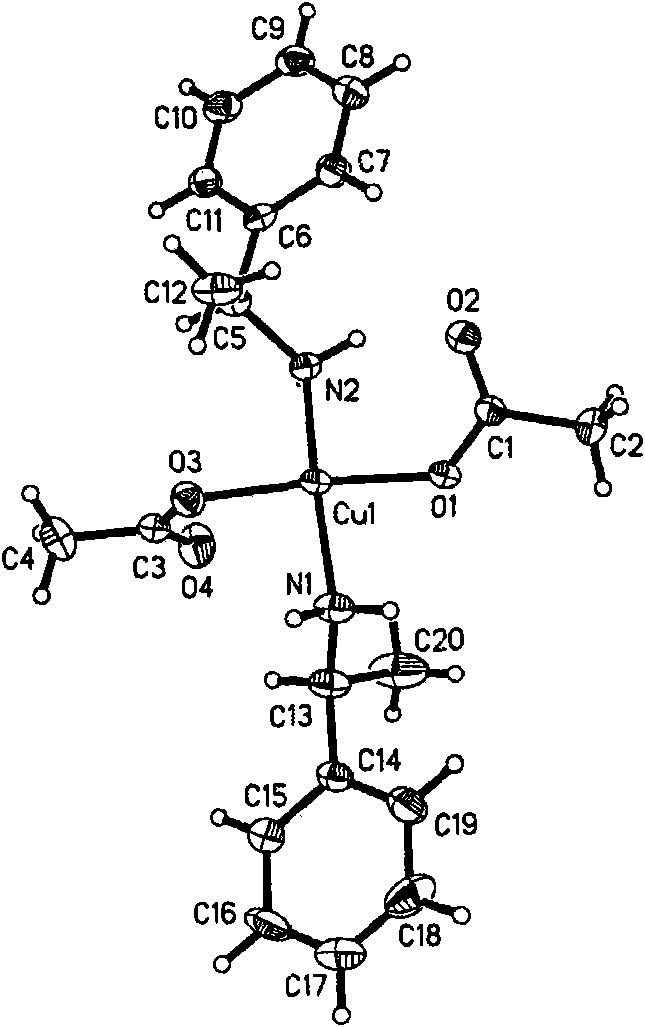
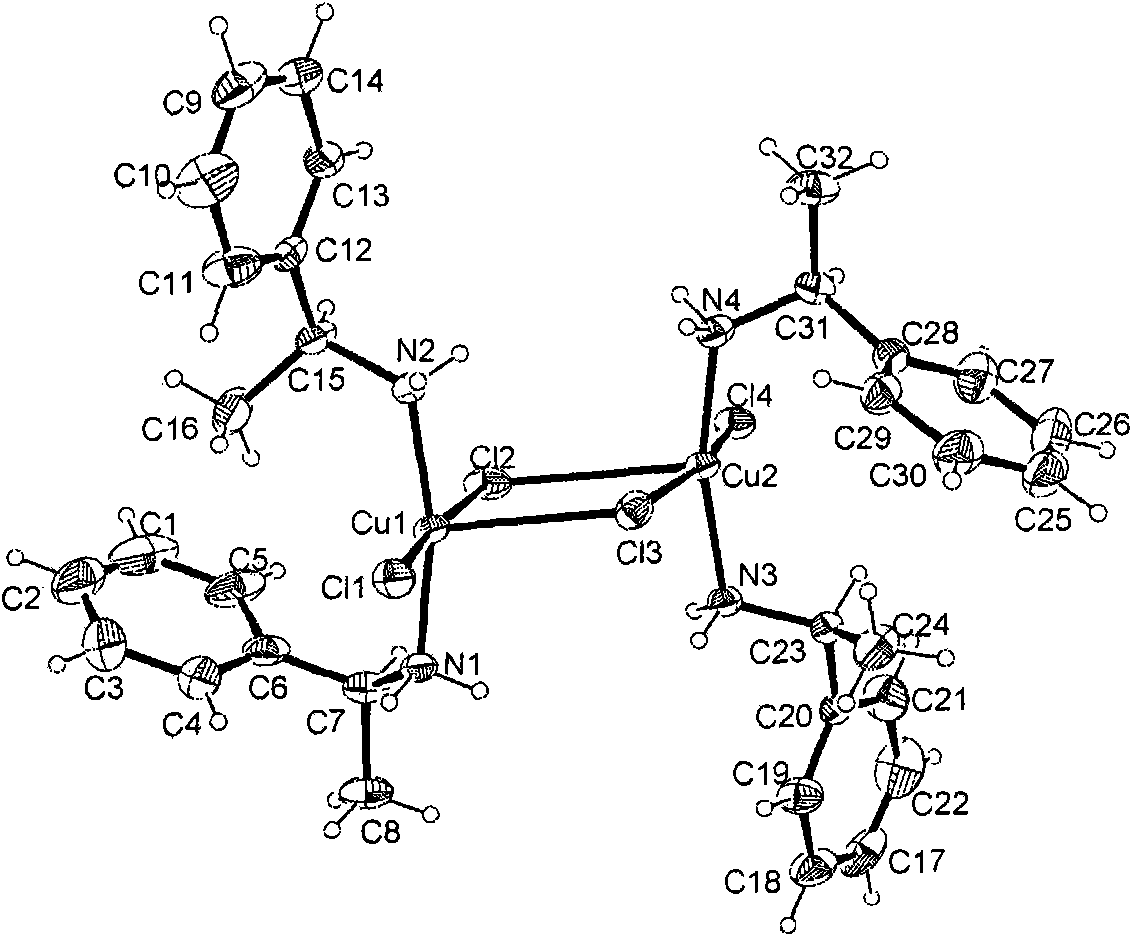
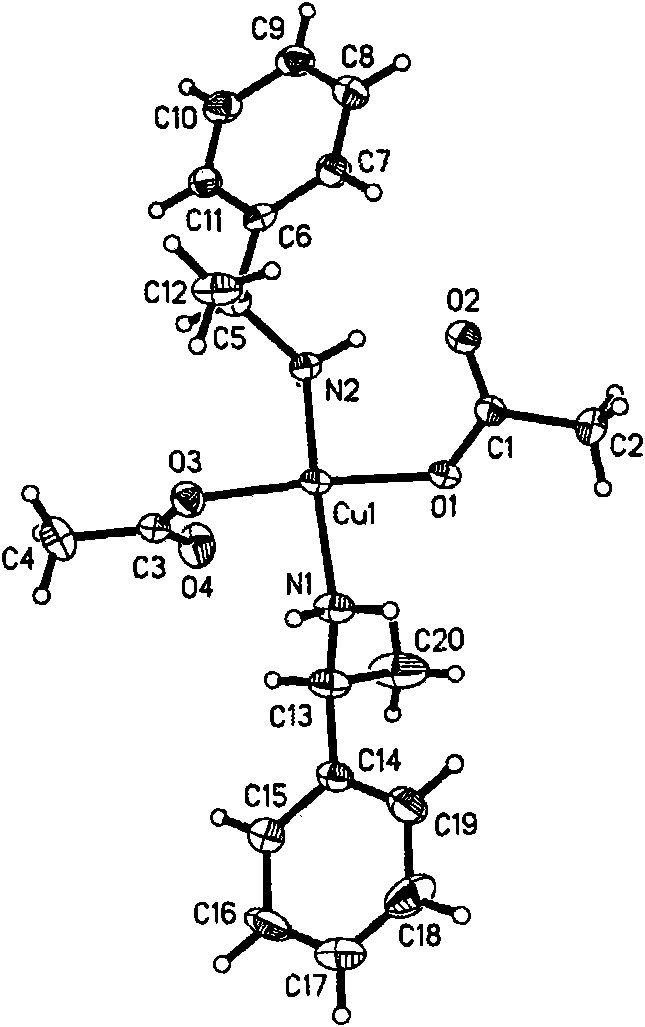
Chiral zinc complex and copper complexes of alpha-phenylethylamine
InactiveCN102069014AGroup 4/14 element organic compoundsCarboxylic acid nitrile preparationBenzaldehyde4-chlorobenzaldehyde
The invention relates to a chiral zinc acetate complex of alpha-phenylethylamine, a chiral copper acetate complex of alpha-phenylethylamine and a chiral copper chloride complex of alpha-phenylethylamine which are used as catalyst. When the complexes are used in the nitrile silicification reactions of aromatic aldehydes such as benzaldehyde, 2-fluorobenzaldehyde, 2-methoxybenzaldehyde, 2-methylbenzaldehyde, 4-methylbenzaldehyde, 4-methoxybenzaldehyde, 4-fluorobenzaldehyde, 4-chlorobenzaldehyde and 4-bromobenzaldehyde to prepare chiral target products, the chiral catalysts have good catalytic activities and high enantioselectivity in the nitrile silicification reactions.
Owner:罗梅
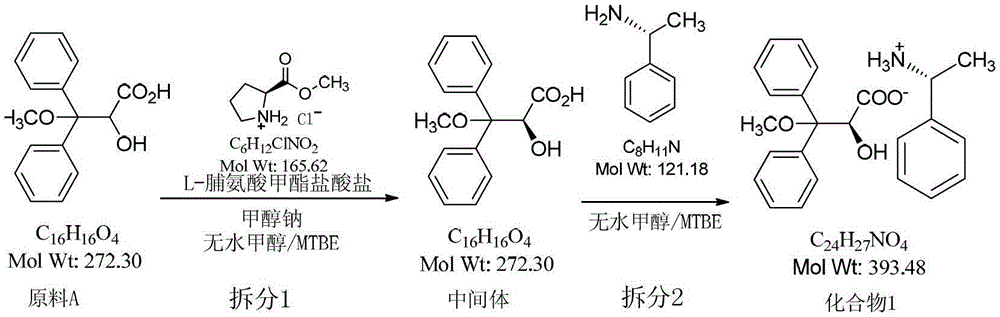
Resolution method of 2-hydroxy-3-methoxy-3,3-dibenzylpropionic acid racemate
ActiveCN104098462AGood reproducibilityEase of industrial implementationOrganic compound preparationOrganic chemistry methodsHydrochlorideReagent
The invention relates to a resolution method of 2-hydroxy-3-methoxy-3,3-dibenzylpropionic acid racemate. The method comprises the following steps: reacting racemic acid with an optical alkali, and separating non-enantiomeric salts of acid and alkali. L-proline methyl ester hydrochloride and R-(+)-alpha-phenylethylamine are used as optically active alkalis. Highly pure target compounds can be obtained in a high yield mode by using an efficient and cheap resolution reagent, and the method has good reappearance in industrial production and has a wide industrial application prospect.
Owner:CHANGZHOU HANSOH PHARM CO LTD +1
Method for recovering pregabalin intermediate resolving agent (R)-(+)-alpha-phenylethylamine
ActiveCN104086439AEmission reductionSimple and fast operationAmino compound purification/separationOrganic solventDistillation
The invention discloses a method for recovering pregabalin intermediate resolving agent (R)-(+)-alpha-phenylethylamine. The method comprises the following steps: a) adding an alkali to free mother liquor at a certain temperature for regulating the pH until the mother liquor is alkaline; b) adding an organic solvent to the system of which the pH is regulated previously for extraction; and c) blending the organic layer, carrying out reduced pressure distillation at a relatively low temperature to remove an extracting agent first, collecting the preceding fraction and then heating to carry out reduced pressure distillation again, thereby obtaining a fraction, namely the resolving agent (R)-(+)-alpha-phenylethylamine. The method has the advantages that the atom utilization rate is increased, the environmental pollution due to direct emission of materials in the mother liquor is avoided, and the production cost is greatly reduced, and the method has the characteristics of green chemistry. In a word, the method for recovering and recycling the (R)-(+)-alpha-phenylethylamine is green and environment-friendly, and low in cost and pollution.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD
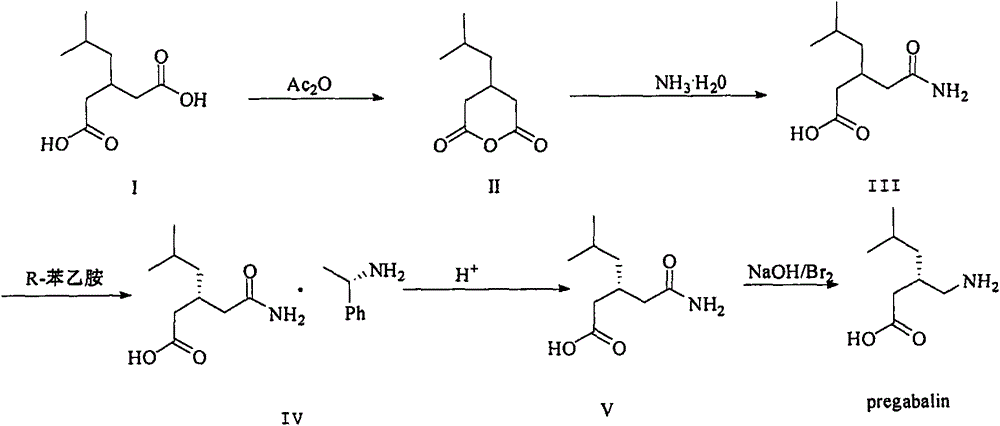
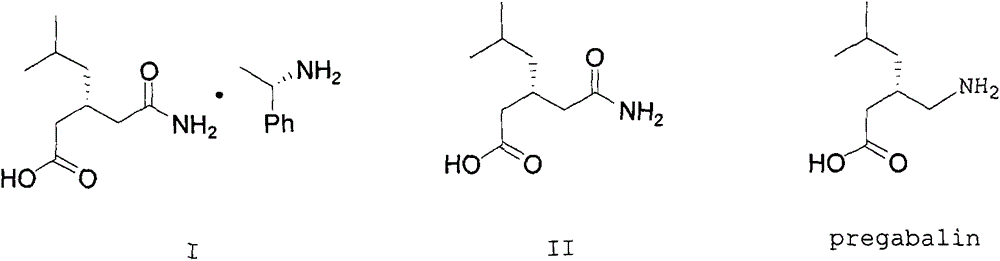
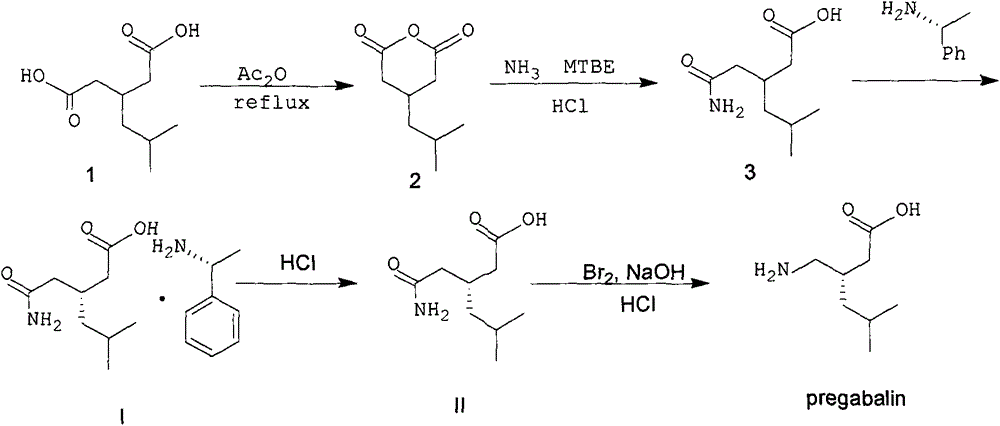
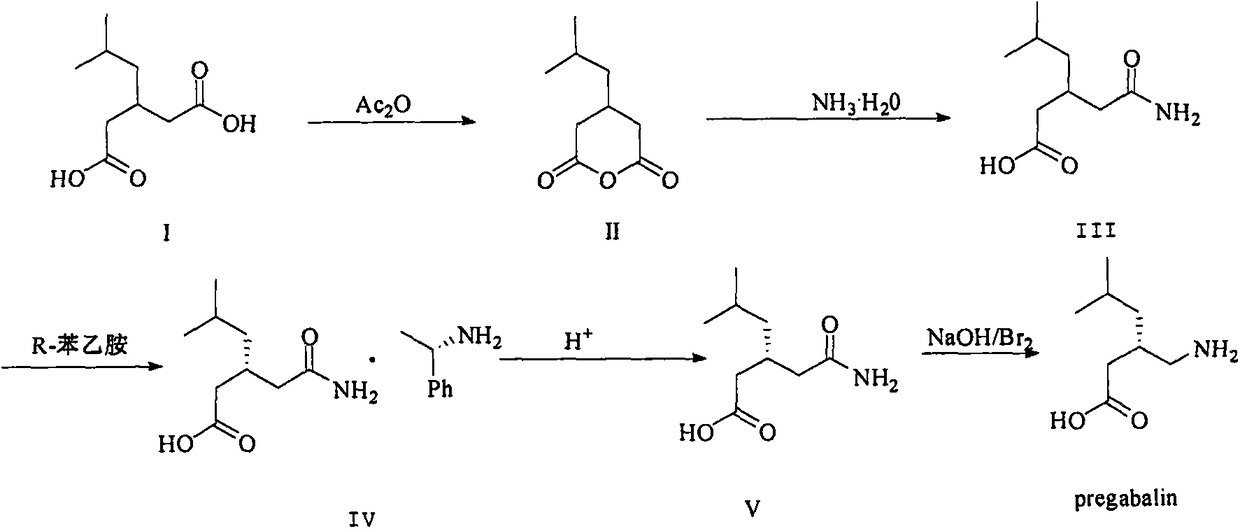
Nesting method for pregabalin intermediate mother liquor
ActiveCN103980144AEmission reductionSimple and fast operationOrganic compound preparationCarboxylic acid amides preparationPregabalinSolvent
The invention discloses a nesting method for a mother liquor of a free pregabalin intermediate (R)-(-)-3-(carbamyl methyl)-5-methylhexanol-(R)-(+)-alpha-phenethylamine salt. The method comprises the following steps: (1) feeding a phenethylamine salt (R)-(-)-3-(carbamyl methyl)-5-methylhexanol-(R)-(+)-alpha-phenethylamine salt to the mother liquor filtered by dissociation in the procedure, and adding a certain amount of solvent to agitate, heat and dissolve; (2) cooling, dropwise adding an acid to adjust the pH; (3) devitrifying at certain temperature, filtering, wherein the filtrate is the mother liquor, and baking the filter cake to obtain (R)-(-)-3-(carbamyl methyl)-5-methylhexanol. The atom utilization rate of the reaction is improved, environmental pollution caused by direct emission of the material in the mother liquor is avoided, the nesting method is mild in reaction condition, has no demands on special equipment and instrument and has green chemistry characteristics, and the production cost is greatly reduced.
Owner:ZHEJIANG HUAHAI PHARMACEUTICAL CO LTD
Method for splitting gamma-dodecalactone chiral molecule
InactiveCN102442981APure fragranceQuality improvementEssential-oils/perfumesOptically-active compound separationGamma-dodecalactoneLactone
The invention discloses a method for splitting a gamma-dodecalactone chiral molecule. The method comprises the following steps of: under the alkaline condition, hydrolyzing gamma-dodecalactone into hydroxy acid; under the action of a catalyst, reacting a chiral splitting agent R-(+)-alpha-phenyl ethylamine with optical activity and the hydroxy acid to form a hydroxyl-acid salt complex; splitting and purifying the racemized hydroxyl-acid salt complex by using a recrystallization method; and under the action of the catalyst, acidifying and cyclizing the purified product to obtain chiral gamma-dodecalactone. According to the method disclosed by the invention, the problems of low half-quantity yield and high splitting cost in the splitting process of the gamma-dodecalactone chiral molecule inthe prior art are solved; and the method for splitting the gamma-dodecalactone chiral molecule with the advantages of high half-quantity yield, chromatogram content as high as over 99.9 percent and higher utilization value in scale production is provided.
Owner:JINGJIANG TAIDA PERFUME CHEM
Zinc nitride and copper nitride compound of chiral alpha-phenylethylamine and use thereof
InactiveCN101830919AAmino preparation from aminesOrganic-compounds/hydrides/coordination-complexes catalystsZinc nitrideCopper chloride
The invention discloses a zinc nitride and copper nitride compound of chiral alpha-phenylethylamine, which comprises a (S) alpha-phenylethylamine zinc nitride and copper nitride compound and a (R) alpha-phenylethylamine zinc nitride and copper nitride compound, which are prepared from the alpha-phenylethylamine, zinc acetate dihydrate, copper acetate monohydrate and copper chloride dihydrate and have the following formulas. In the formulas, ML is Zn(OOCCH3)2, Cu(OOCCH3)2 or CuCl2. The compound serves as a chiral catalyst in a Henry reaction.
Owner:HEFEI UNIV OF TECH
Preparation method for R-(+)-alpha-phenylethylamine salt and R-(+)-alpha-phenylethylamine
ActiveCN104860830ALess waste waterMild reaction conditionsAmino compound purification/separationOrganic compound preparationSolventEthylamine
The invention provides a preparation method for R-(+)-alpha-phenylethylamine salt and R-(+)-alpha-phenylethylamine. The preparation method comprises a step of subjecting DL-alpha-phenylethylamine and a resolving agent to a salt formation reaction in a reaction solvent, wherein the resolving agent is one selected from the group consisting of N-p-nitrobenzoyl-L-glutamic acid, L-glutamic acid and gulonic acid, the reaction solvent is one or more selected from the group consisting of acetone and ethanol, the salt formation reaction is to add the resolving agent into the reaction solvent, then add DL-alpha-phenylethylamine and carry out the reaction, DL-alpha-phenylethylamine is added drop by drop for 20 to 40 min, the ethanol is 95% ethanol, and a ratio of the volume of the reaction solvent to the mass of DL-alpha-phenylethylamine is 2-8: 1, preferably 3-5: 1. The method is finished in only one step, does not need refining, produces little waste water, has high yield, enables high-optical purity R-(+)-alpha-phenylethylamine salt and R-(+)-alpha-phenylethylamine to be obtained, uses commercially available reagents and raw materials and is applicable to industrialization.
Owner:NORTHEAST PHARMA GRP
AHU-377alpha-phenethylamine salt polycrystalline type and preparation method and application thereof
InactiveCN105367438AEasy to separateHigh chiral purityOrganic active ingredientsSenses disorderOperabilityCrystallinity
The invention discloses AHU-377alpha-phenethylamine salt polycrystalline type and a preparation method and application thereof and particularly discloses the AHU-377alpha-phenethylamine salt polycrystalline type. An X-radial powder diffraction pattern comprises peaks located at the diffraction angles 2 theta, namely 20.58 + / -0.2 degrees, 24.28 + / -0.2 degrees, 8.38 + / - 0.2 degrees and 23.20 + / -0.2 degrees or peaks located at the diffraction angles 2 theta, namely 23.28+ / -0.2 degrees, 18.9+ / -0.2 degrees, 13.7+ / -0.2 degrees and 14.72+ / -0.2 degrees. The AHU-377 is prepared into the phenethylamine salt to change the physicochemical properties of the AHU-377, such as crystallinity, solubleness and hygroscopicity. The AHU-377alpha-phenethylamine salt polycrystalline type and the preparation method and application thereof are mature in process and high in operability, the obtained product is high in quality, homogeneous and stable, chemical stability is achieved, storage is facilitated, and wide application prospect is achieved.
Owner:SHANGHAI HANSOH BIOMEDICAL +1
Resolution for 5-methyltetrahydrofolic acid and salifying method thereof
InactiveCN101143863BOptically-active compound separationOrganic racemisationCalcium hydroxideAlkaline earth metal
The invention brings forward a preparation method, which utilizes organic alkali Alpha-phenylethylamine to obtain (6S)-5-methyltetrahydrofolate by splitting racemate (6R, S)-5-methyltetrahydrofolate, and utilizes the hydroxide of alkaline earth, particularly calcium hydroxide, to generate (6S)-5-methyltetrahydrofolate calcium salt.
Owner:NAN JING RHINE PHARM TECH
Method for synthesizing fosfomycin phenylethylamine calt
InactiveCN101759719AEliminate potential safety hazardsIncrease the number of applicationsGroup 5/15 element organic compoundsSodium bicarbonateMass content
The invention discloses a method for synthesizing fosfomycin phenylethylamine calt. The invention relates to the fine chemical field. The method of the invention comprises the following steps: under the condition of stirring, after the hydrogenation reaction is finished, adding 95% mass-content ethanol into the hydrogenation solution in which palladium-carbon catalyst is filtrated; adding sodium bicarbonate saturated solution while stirring; controlling temperature within the range of 30-40 DEG C, then adding DL-alpha-phenylethylamine or D-alpha-phenylethylamine; stirring continuously and adding the aqueous solution of sodium tungstate and EDTA disodium salt; heating the reaction system to 40-50 DEG C and adding hydrogen peroxide, and then heating again till the temperature reaches 50-55 DEG C and maintaining the temperature; cooling to 5 below-10 below DEG C and maintaining the temperature; and washing filter cake with ethanol after filtration and separation, thus obtaining the levorotary-dextrorotatory mixed salts or the crude levorotary salts. The obtained levorotary-dextrorotatory mixed salts are separated to yield crude levorotary salts, and further the crude levorotary salts are refined to form fine levorotary salts. The method has the advantages of simple process, convenient operation, high safety, low material consumption, less pollution discharge, low cost, and short production cycle.
Owner:NORTHEAST PHARMA GRP
Method for catalytically synthesizing L-cis-1,2-epoxypropylphosphonic acid-D-alpha-phenylethylamine by using phosphotungstic heteropoly acid phase transfer catalyst
InactiveCN105315307AAchieve recyclingHigh recovery rateAmino preparation from aminesGroup 5/15 element organic compoundsHeteropoly acidPhenethylamines
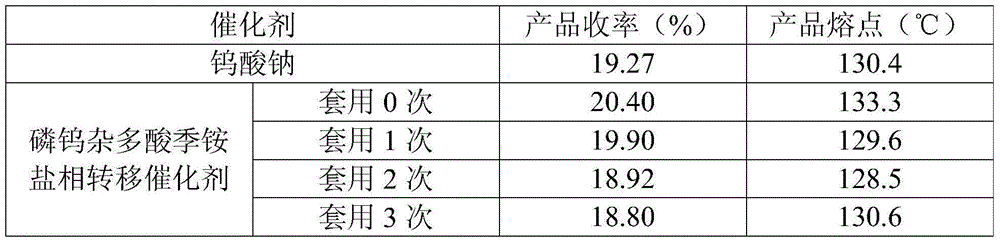
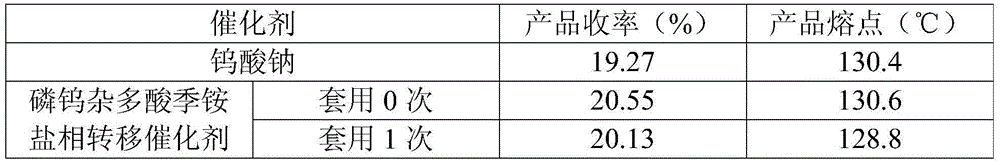
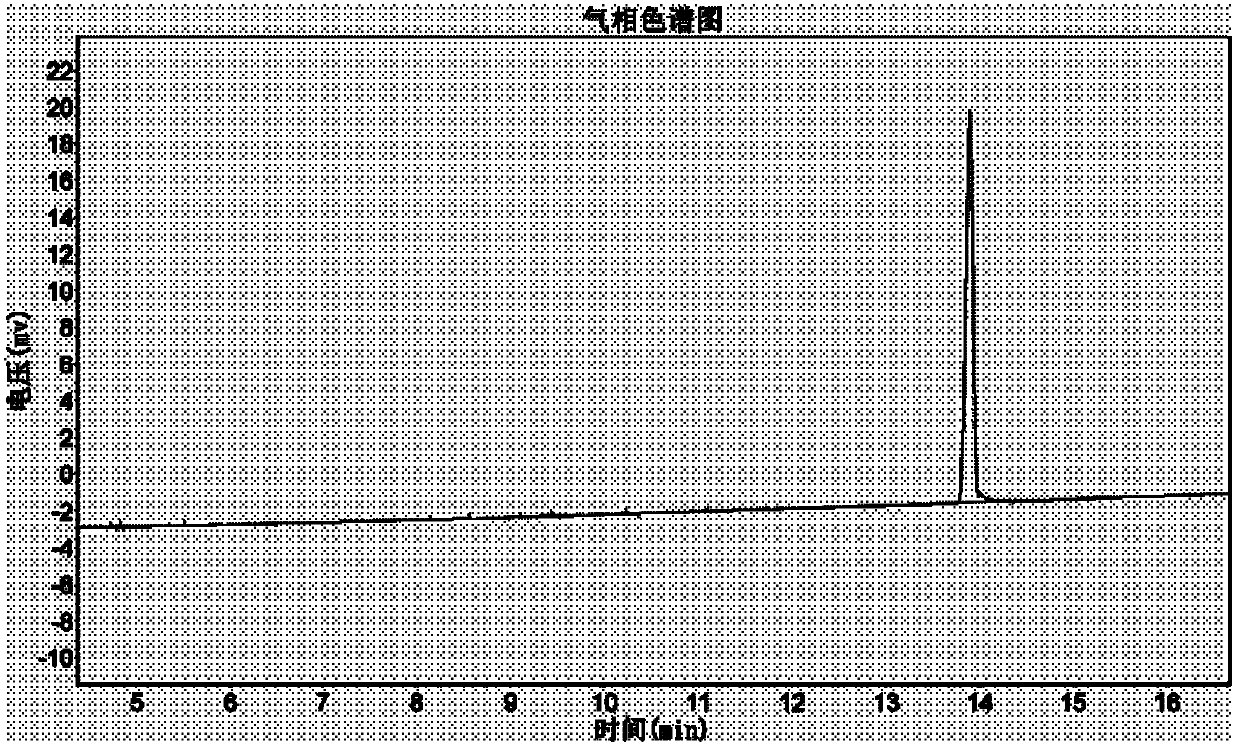
The invention relates to a method for catalytically synthesizing L-cis-1,2-epoxypropylphosphonic acid-D-alpha-phenylethylamine by using a phosphotungstic heteropoly acid phase transfer catalyst. By taking cis-1-propenylphosphonic acid, alpha-phenylethylamine and a hydrogen dioxide solution as raw materials, L-cis-1,2-epoxypropylphosphonic acid-D-alpha-phenylethylamine is catalytically synthesized by using the phosphotungstic heteropoly acid phase transfer catalyst in the presence of industrial ethanol and EDTA. After a product is separated, the pH of the filtrate is adjusted by using a solution A with certain concentration, so that the catalyst is fully separated out, and the catalyst is recycled and can be repeatedly used for three times; the recovery rate of the catalyst per time is relatively high. The method provided by the invention is low in production cost and less in emission, and meets the demand of current green chemistry and atom economy.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
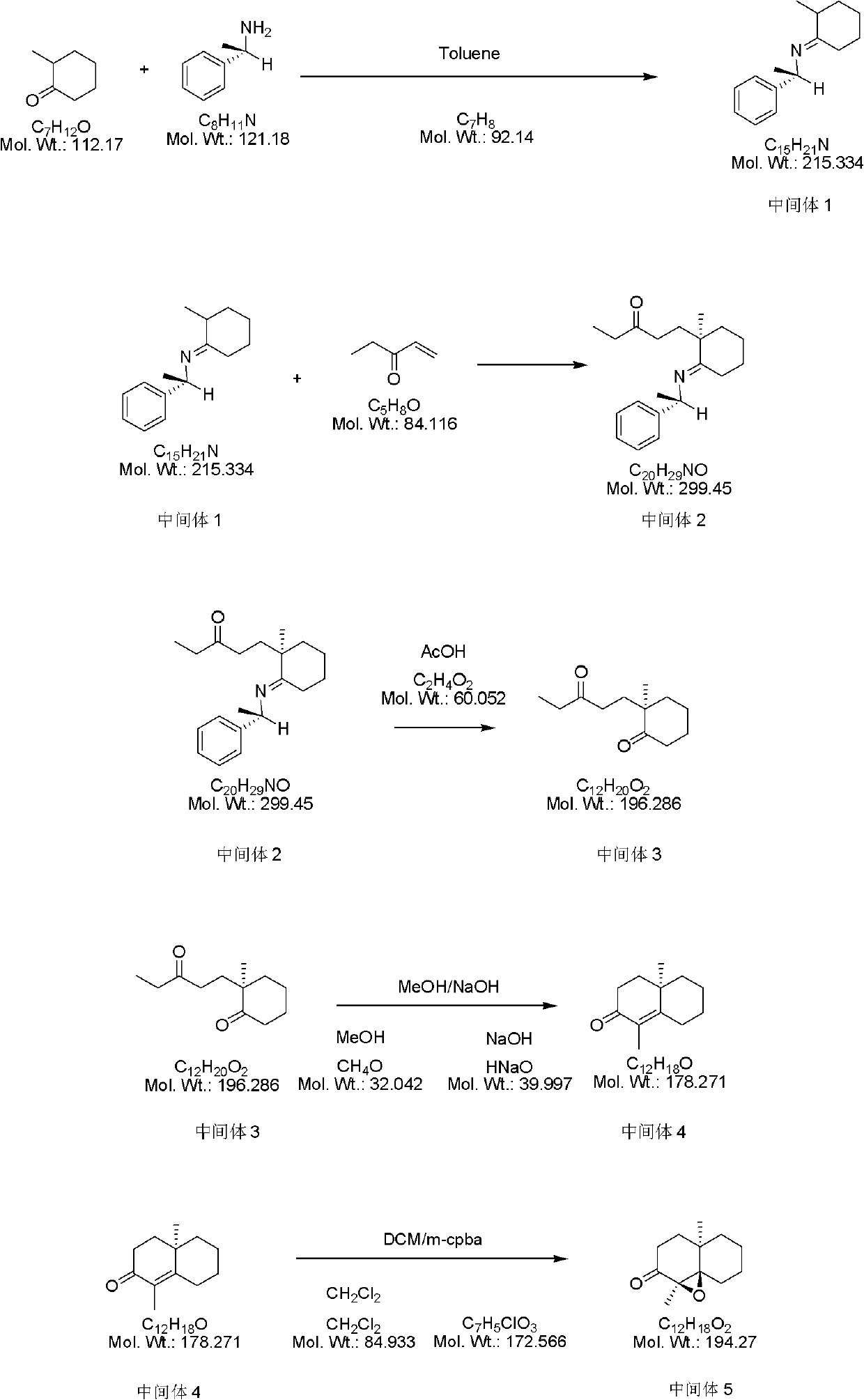
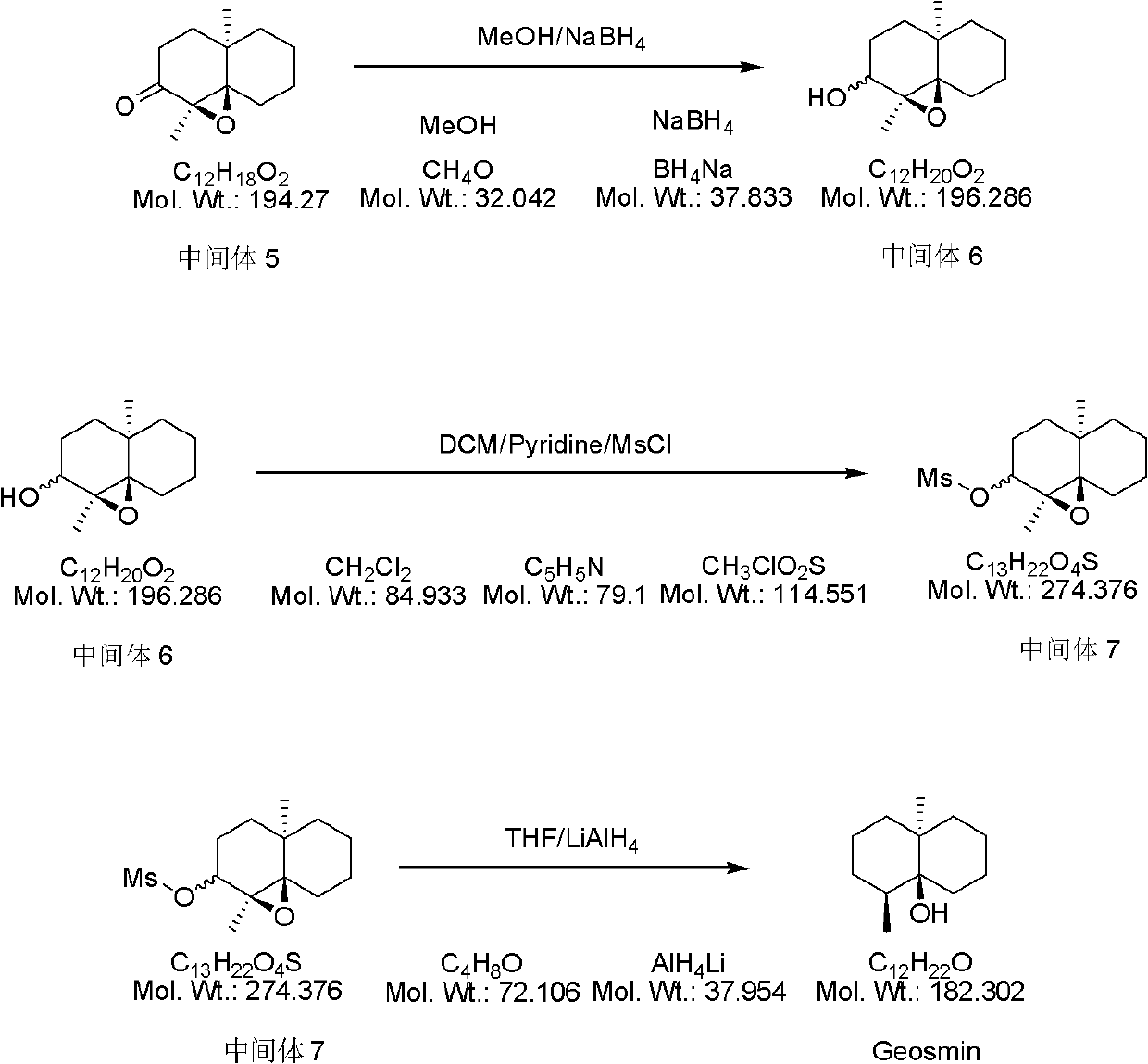
Synthesis method of geosmin
InactiveCN102557882AHigh yieldLow pricePreparation by oxygen reductionCyclohexanoneSynthesis methods
The invention provides a synthesis method of geosmin. The method is characterized in that 2-methyl cyclohexanone used as a raw material for an initial reaction firstly is in ammoniation reaction with (S)-Alpha-phenylethylamine to produce an enimic compound; the enimic compound is in substitution reactio with olefine ketone to produce an enimic compound substituted by pentyl ketone; afterwards, the enimic compound is converted to a cyclohexanone derivative under an acidic condition; Alpha, Beta unsaturated derivatives are produced through intramolecular cyclization under an alkaline condition; and the enimic compound is subject to reactions of epoxidation reaction, reduction, dehydroxylation, and the like to produce the final target compound, namely the geosmin. The synthesis method of the geosmin, which is provided by the invention, has the advantages that the efficiency is high, the operation is simple, convenient and safe, the costs of raw materials used for the reactions are low, the use amount of solvents is small, and the preparation cost can be reduced on the premise that the product purity and the yield are guaranteed, so that the synthesis method is very suitable for hundred-milligram scale batch production.
Owner:天津市佰斯康科技有限公司 +1
Preparation method of chiral gamma-decalactone
InactiveCN108299346ALow enantiomeric excessLow costOrganic chemistry methodsOrganic solventEnantiomer
The invention discloses a preparation method of chiral gamma-decalactone. The method comprises the following steps that (1) racemic gamma-decalactone is subjected to loop opening by an inorganic alkali solution to obtain a gamma-hydroxy acid and alkali metal saline solution; then, an organic solvent is added; the PH value is regulated to a weak acid state by inorganic acid, so that generated gamma-hydroxy acid enters an organic phase; the separated organic phase is dried; (2) (S)-(-)-alpha-phenylethylamine is added into the organic phase; crystallization is performed to separate out gamma-hydroxy acid (S)-(-)-alpha-phenylethylamine salt with optical activity; (3) obtained amine salt is added into water; stirring, dissolution, crystallization and filtering are performed; after filter cake is added with water to be dissolved, inorganic acid is added; after acidification cyclization, an organic solvent is added for extraction to obtain (R)-(+)-gamma-decalactone; (4) inorganic acid is added into crystallization mother liquid in the steps (2) and (3) for further acidizing treatment; the organic solvent is used for extraction to obtain (S)-(-)-gamma-decalactone. When the preparation method is used, the operation is easy; two kinds of configuration of chiral gamma-decalactone can be obtained; the fragrance is pure, the advantages of high-half-quantity yield, high enantiomer excess values and low cost are realized.
Owner:博润生物科技南通有限公司
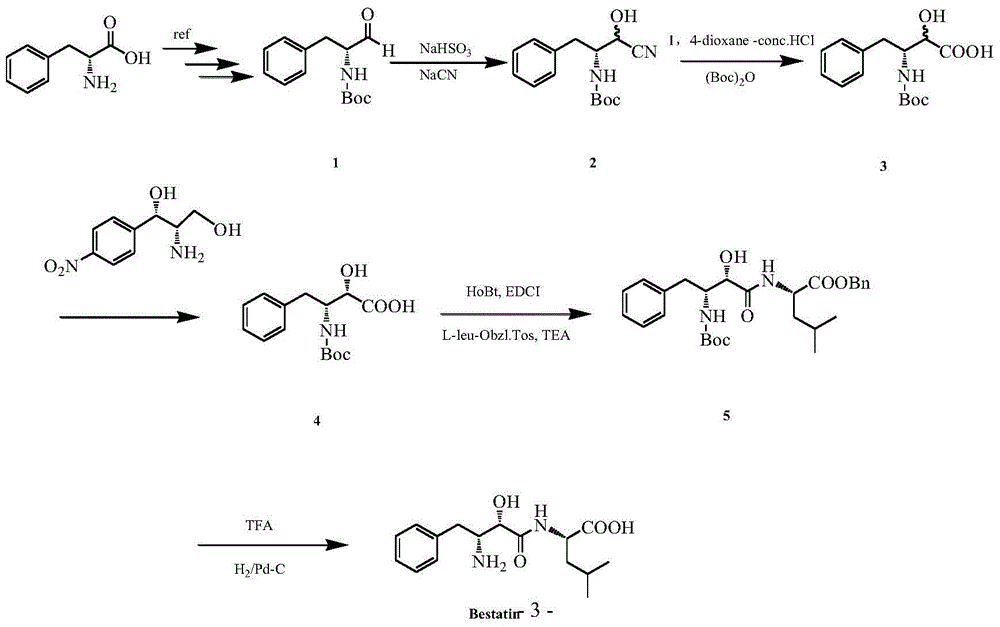
Method for synthesizing ubenimex
ActiveCN104496843ASimple stepsReduce usageOrganic compound preparationCarboxylic acid amides preparationPhenylbutyramideHydrolysis
The invention discloses a method for synthesizing ubenimex. The method comprises the following steps: by taking D-Boc-phenyl alaninal as a raw material, sequentially preparing hydroxynitrile, carrying out a nitrile hydrolysis reaction, performing amino protection, performing chiral resolution on (2RS,3R)-3-tert-butoxyacylamino-2-hydroxy-4-phenylbutyric acid, and synthesizing (S)-phenyl-2-((2S,3R)-3-(tert-butoxyacylamino)-2-hydroxy-4-phenylbutyramide)-4-mevalonate, thereby synthesizing the ubenimex. According to the synthetic method disclosed by the invention, the defect that the resolving agent used in the conventional synthetic method is high in toxicity (such as strychnine) or high in price (such as alpha-phenylethylamine) is overcome, the synthetic method is simple in steps and low in cost, and use of reagents such as strychnine is avoided; and moreover, a dextro-amino compound is taken as the chiral resolution agent, the price is low, the operation is simple, the stirring and recrystallization time is shortened, and the synthetic efficiency is greatly improved.
Owner:山东益康药业股份有限公司
Synthesis method of chiral gamma-dodecalactone
InactiveCN102391215AIncrease productionPure fragranceAsymmetric synthesesChemical synthesisSynthesis methods
The invention discloses a synthesis method of chiral gamma-dodecalactone. The method comprises the following steps of: synthesizing a lactone molecule with optical activity from a reactant under the environment and induction of a chiral molecule; proportionally mixing nonanol, acrylic acid and peroxide, and adding a certain amount of chiral alpha-phenylethylamine zinc acetate ruthenium or copper chromium organic complex into the reaction backing material; and synthesizing the gamma-dodecalactone with optical cavity under the action of silica-alumina mixed catalyst and the induction of D or L tartaric acid. Through the invention, the problems that the gamma-dodecalactone spice is mainly made by chemical synthesis in the prior art, the lactone spices synthesized from a substance without optical activity under general conditions are all racemic mixtures with turbid aroma, lots of foreign gases and relatively obvious artificial traces; and the synthesis method of chiral gamma-dodecalactone provided by the invention is easy to operate, has a relatively good use effect; and the synthesized product has a relatively high enantiomeric excess value and the aroma of the spice is more fresh.
Owner:JINGJIANG TAIDA PERFUME CHEM
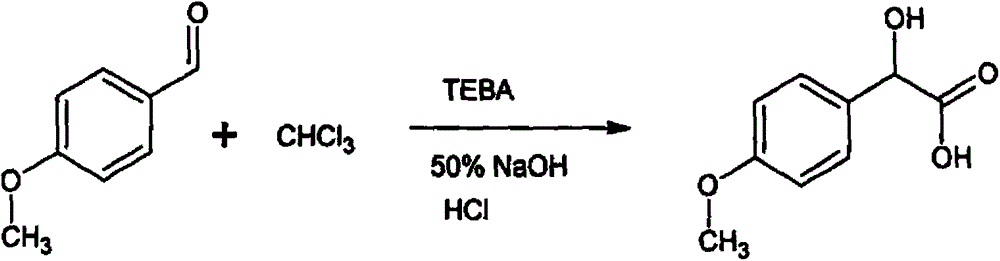
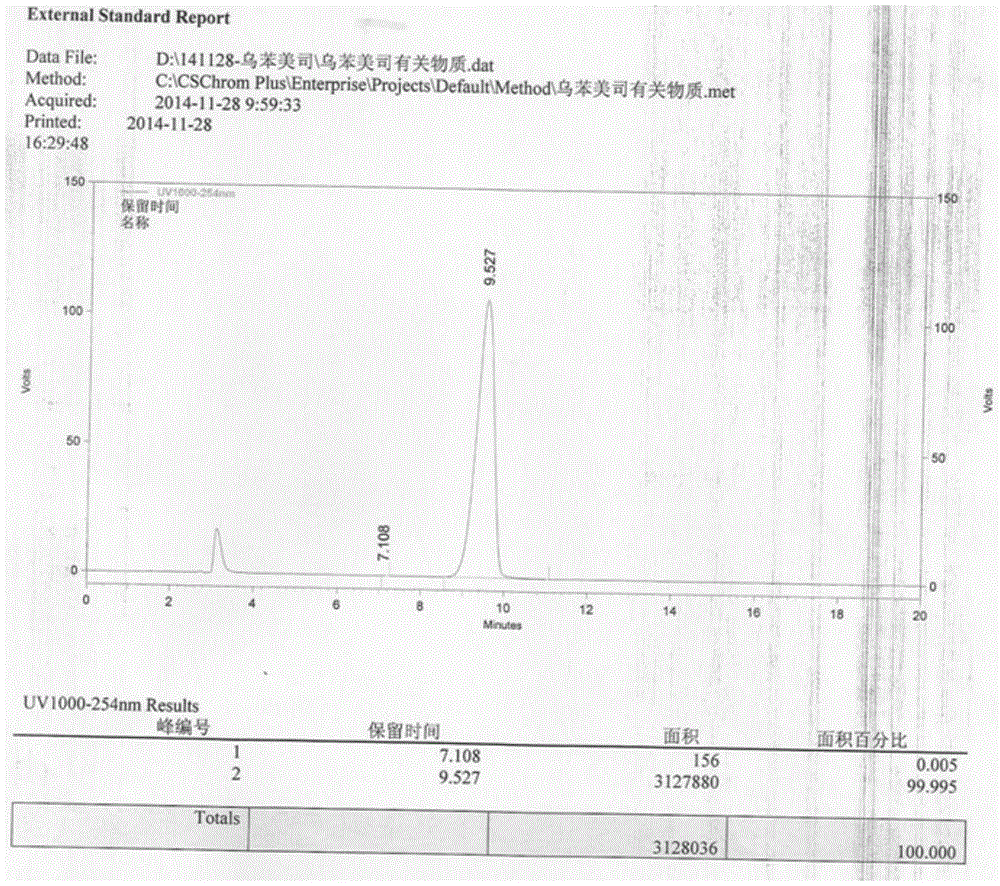
Resolving synthetic method for paramethoxymandelic acid
InactiveCN105294427AChiral pureEasy to separateOrganic compound preparationOptically-active compound separationReaction temperatureSolvent
The present invention relates to a resolving synthetic method for paramethoxymandelic acid. The method comprises the following steps of: by taking chiral alpha-phenylethylamine acid as a resolving agent, carrying out a salt forming reaction on the paramethoxymandelic acid and the chiral alpha-phenylethylamine acid in a molar charge ratio of 1: (1.0-1.2) in a mixed solvent, wherein the reaction temperature is 40-70 DEG C; and effectively resolving L-paramethoxymandelic acid and D-paramethoxymandelic acid. Compared with the prior art, the method provided by the invention is reasonable in process and simple in operation. Raw materials which are low in cost and are easily available are synthesized and resolved to obtain a product with high optical purity and chemical purity, so that the method is suitable for industrial production of chiral paramethoxymandelic acid on a large scale.
Owner:上海予利生物科技股份有限公司
Method for synthesizing (S)(-)-gamma-lactone
ActiveCN1872849BUnique aromaImprove aroma qualityOrganic chemistryGamma-dodecalactoneGamma-decalactone
This invention discloses a method for synthesizing (S)-(-)-gamma-lactone. The method comprises: (1) using (R)-(+)-alpha-phenylethylamine as the chiral resolving agent, and reacting with racemic gamma-hydroxy acid to obtain a pair of diastereotopic salts; (2) separating by crystallization; (3) acidifying and cyclizing to obtain chiral (S)-(-)-gamma-lactone. (S)-(-)-gamma-lactone is (S)-(-)-gamma-decalactone, (S)-(-)-gamma-undercalactone or (S)-(-)-gamma-dodecalactone. (S)- (-)-gamma-lactone has special fragrance, and can be used to manufacture high-grade essence.
Owner:APPLE FLAVOR & FRAGRANCE GRP +1
Application of silver catalyst in preparation of antibacterial drug intermediate
InactiveCN112409410AHigh yieldHigh catalytic activityAmino preparation from aminesGroup 5/15 element organic compoundsPhosphonomycinPtru catalyst
The invention provides an application of a silver catalyst in preparation of an antibacterial drug intermediate fosfomycin levoforight amine salt. The application is characterized by comprising the following steps: at room temperature, dissolving cis-propenylphosphonic acid in an alcohol solvent, slowly dropwise adding (+) alpha phenylethylamine, regulating the pH value of the system to 5.5-6 after dropwise adding, continuing stirring for 1-3 minutes, and adding the silver catalyst, continuously and slowly dropwise adding hydrogen peroxide, then continuously stirring for 10-30 minutes, quicklyheating the system to 50-55 DEG C, filtering while the system is hot, and cooling, crystallizing and washing the filtrate to obtain the fosfomycin levoforight amine salt. Silver carbonate is used asthe catalyst, hydrogen peroxide is used as an oxidizing agent, heating is not needed in the oxidative cyclization process, and the reaction can be performed at normal temperature. Silver carbonate hasvery high catalytic activity in the invention, and compared with the prior art, the application has the advantages of small dosage, mild reaction, effective shortening of the reaction time, simple post-treatment, and realization of separation of the catalyst from the system only through filtration of the system while the system is hot.
Owner:商河探荣新技术开发中心
A kind of synthetic method of ubenimex
ActiveCN104496843BSimple stepsReduce usageOrganic compound preparationCarboxylic acid amides preparationPhenylbutyramideHydrolysis
The invention discloses a method for synthesizing ubenimex. The method comprises the following steps: by taking D-Boc-phenyl alaninal as a raw material, sequentially preparing hydroxynitrile, carrying out a nitrile hydrolysis reaction, performing amino protection, performing chiral resolution on (2RS,3R)-3-tert-butoxyacylamino-2-hydroxy-4-phenylbutyric acid, and synthesizing (S)-phenyl-2-((2S,3R)-3-(tert-butoxyacylamino)-2-hydroxy-4-phenylbutyramide)-4-mevalonate, thereby synthesizing the ubenimex. According to the synthetic method disclosed by the invention, the defect that the resolving agent used in the conventional synthetic method is high in toxicity (such as strychnine) or high in price (such as alpha-phenylethylamine) is overcome, the synthetic method is simple in steps and low in cost, and use of reagents such as strychnine is avoided; and moreover, a dextro-amino compound is taken as the chiral resolution agent, the price is low, the operation is simple, the stirring and recrystallization time is shortened, and the synthetic efficiency is greatly improved.
Owner:山东益康药业股份有限公司
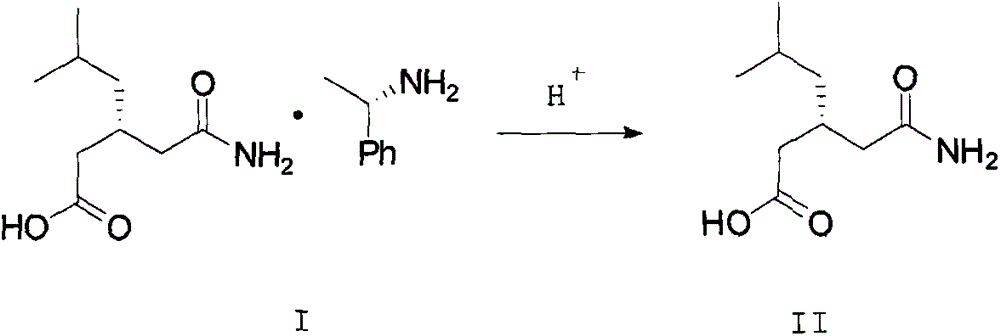
A kind of recovery method of pregabalin intermediate resolving agent (r)-(+)-alpha-phenylethylamine
ActiveCN104086439BEmission reductionSimple and fast operationAmino compound purification/separationOrganic solventPregabalin
The invention discloses a method for recovering pregabalin intermediate resolving agent (R)-(+)-alpha-phenylethylamine. The method comprises the following steps: a) adding an alkali to free mother liquor at a certain temperature for regulating the pH until the mother liquor is alkaline; b) adding an organic solvent to the system of which the pH is regulated previously for extraction; and c) blending the organic layer, carrying out reduced pressure distillation at a relatively low temperature to remove an extracting agent first, collecting the preceding fraction and then heating to carry out reduced pressure distillation again, thereby obtaining a fraction, namely the resolving agent (R)-(+)-alpha-phenylethylamine. The method has the advantages that the atom utilization rate is increased, the environmental pollution due to direct emission of materials in the mother liquor is avoided, and the production cost is greatly reduced, and the method has the characteristics of green chemistry. In a word, the method for recovering and recycling the (R)-(+)-alpha-phenylethylamine is green and environment-friendly, and low in cost and pollution.
Owner:ZHEJIANG HUAHAI PHARMA CO LTD
Synthesis method of chiral compound R-(+)-2-methyl-3-phenyl-1-propyl alcohol
ActiveCN109503319AWith industrial realizabilityGuaranteed achievabilityOrganic compound preparationOrganic chemistry methodsSynthesis methods1-Propanol
The invention discloses a synthesis method of a chiral compound R-(+)-2-methyl-3-phenyl-1-propyl alcohol. The synthesis method comprises the following steps: taking alpha-methyl cinnamaldehyde as a raw material and performing a catalytic hydrogenation reaction on the alpha-methyl cinnamaldehyde to obtain (plus or minus)-2-methyl-3-phenyl-1-propyl alcohol; performing an esterification reaction on the (plus or minus)-2-methyl-3-phenyl-1-propyl alcohol and phthalic anhydride to form monoester; performing an acid-base reaction on the monoester and S-(-)-alpha-phenylethylamine to form salt; separating and purifying a single chiral isomer in the salt through crystallization and recrystallization; performing a hydrolysis reaction to obtain R-(+)-2-methyl-3-phenyl-1-propyl alcohol and a hydrolysisby-product; and finally, removing the hydrolysis by-product through separation and purification to obtain the R-(+)-2-methyl-3-phenyl-1-propyl alcohol.
Owner:江苏广域化学有限公司
A kind of preparation method of sitagliptin intermediate
ActiveCN103755596BChiral raw materials are cheap and readily availableShort reaction pathCarbamic acid derivatives preparationOrganic compound preparationEnamineChiral amine
Owner:ZHEJIANG UNIV OF TECH
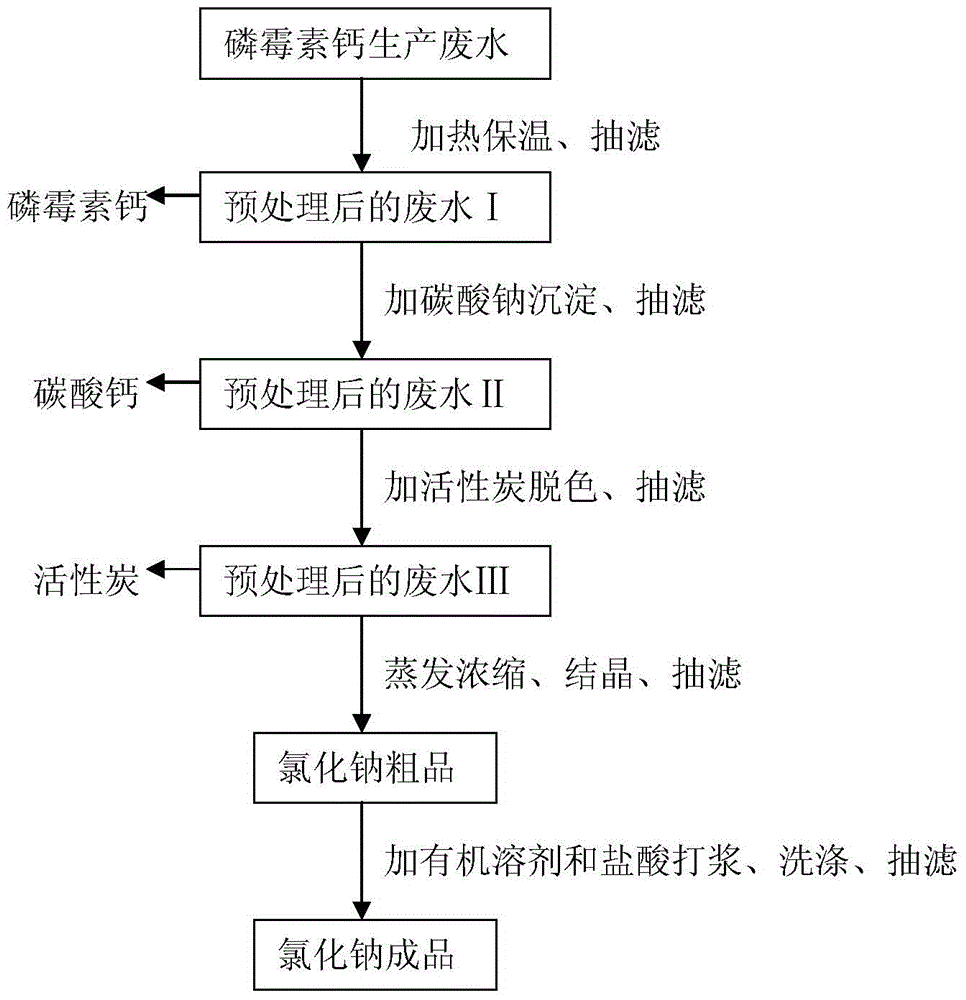
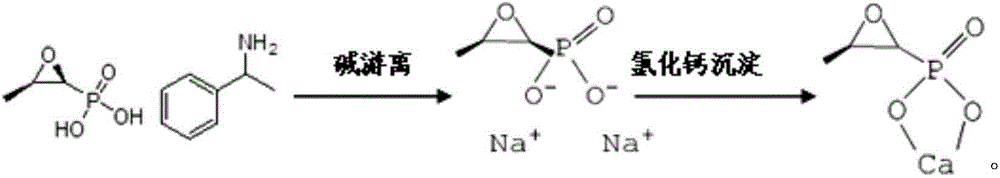
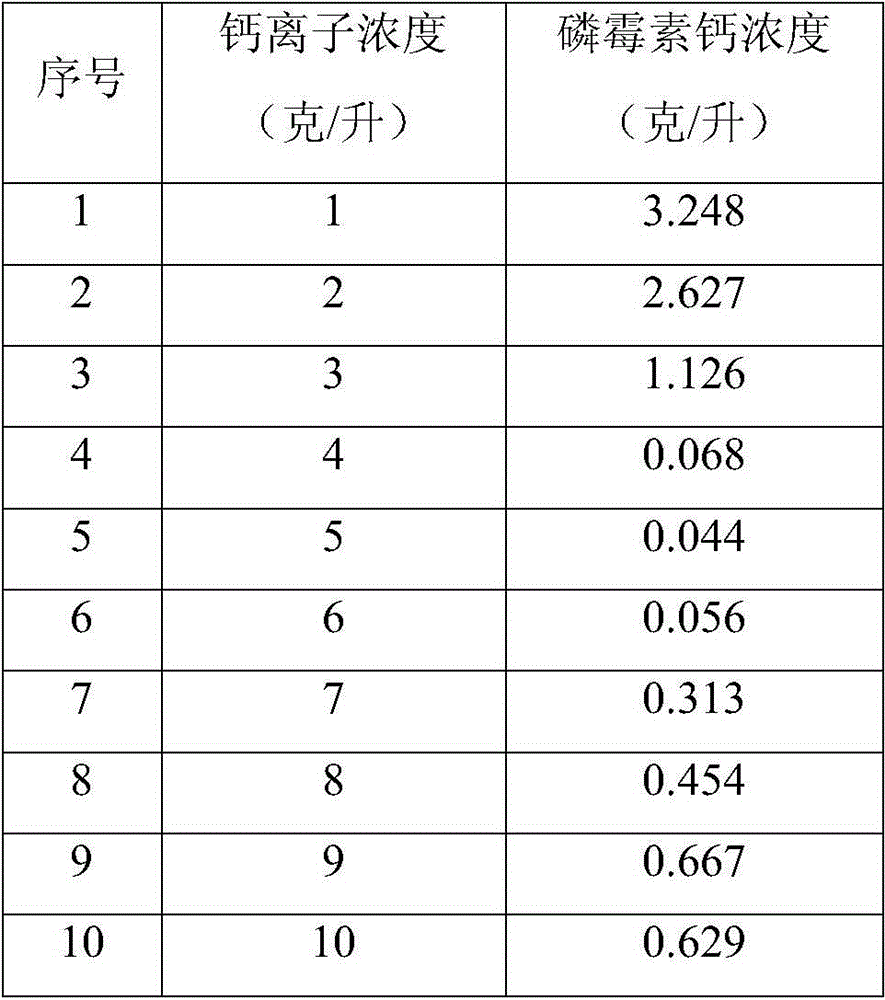
Treatment method of fosfomycin calcium salt-containing high-concentration organic process wastewater
ActiveCN104843924BCalcium/strontium/barium carbonatesGroup 5/15 element organic compoundsHigh concentrationFiltration
The invention discloses a treatment method for saliferous high-concentration organic process waste water during fosfomycin calcium production. The treatment method comprises the following steps of adding a proper amount of calcium chloride into waste water, controlling concentration of calcium ions to reach 1-10g / L, heating and keeping the temperature, and performing suction filtration to obtain a fosfomycin calcium crude product and waste water I after pretreatment; adding a proper amount of sodium carbonate solution into the waste water I after pretreatment, depositing the calcium ions in a form of calcium carbonate and then removing the calcium ions; absorbing the obtained waste water II after pretreatment by active carbon to remove alpha-phenylethylamine dissolved in the waste water, performing suction filtration to obtain waste water III after pretreatment, then evaporating, condensing and crystallizing to obtain a sodium chloride crude product, then pulping and refining by methyl alcohol, performing suction filtration and drying to obtain a sodium chloride finished product. All indicators are higher than first-class product standard of national industrial salt; the absorbed active carbon can be used as boiler fuel; the evaporated and condensed water obtained from the evaporation and condensation returns to the production process of fosfomycin calcium to be recycled; the methyl alcohol mother liquor is rectified to recover methyl alcohol to be reused.
Owner:ZHEJIANG DAYANG BIOTECH GROUP
Method for splitting gamma-dodecalactone chiral molecule
InactiveCN102442981BPure fragranceQuality improvementEssential-oils/perfumesOptically-active compound separationGamma-dodecalactonePhenethylamines
The invention discloses a method for splitting a gamma-dodecalactone chiral molecule. The method comprises the following steps of: under the alkaline condition, hydrolyzing gamma-dodecalactone into hydroxy acid; under the action of a catalyst, reacting a chiral splitting agent R-(+)-alpha-phenyl ethylamine with optical activity and the hydroxy acid to form a hydroxyl-acid salt complex; splitting and purifying the racemized hydroxyl-acid salt complex by using a recrystallization method; and under the action of the catalyst, acidifying and cyclizing the purified product to obtain chiral gamma-dodecalactone. According to the method disclosed by the invention, the problems of low half-quantity yield and high splitting cost in the splitting process of the gamma-dodecalactone chiral molecule inthe prior art are solved; and the method for splitting the gamma-dodecalactone chiral molecule with the advantages of high half-quantity yield, chromatogram content as high as over 99.9 percent and higher utilization value in scale production is provided.
Owner:JINGJIANG TAIDA PERFUME CHEM
Preparation method of optically pure 2-oxotricyclo[2.2.1.0]-heptane-7-carboxylic acid
InactiveCN107573228AEase of industrial productionOrganic compound preparationCarboxylic preparation by oxidationAcetic acidCarboxylic acid
The invention provides a preparation method of optically pure 2-oxotricyclo[2.2.1.0]-heptane-7-carboxylic acid, and relates to the technical field of Corey lactone intermediate synthesis. The preparation method comprises the following steps: (1) preparing racemate 2-oxotricyclo[2.2.1.0]-heptane-7-carboxylic acid from norbornadiene HR through two steps: Prinee reaction and Jones oxidation; (2) mixing the product with isopropyl acetate to obtain white crystals namely optically pure (+)-2-oxotricyclo[2.2.1.0]-heptane-7-carboxylic acid (S)-(-)-N-benzylbenzene-alpha-phenylethylamine salt; and (3) extracting the product obtained in the step (2) by ethyl acetate to obtain the final product namely optically pure 2-oxotricyclo[2.2.1.0]-heptane-7-carboxylic acid. The method is simple and convenient,is easy to operate, and is beneficial for the industrial production.
Owner:沈阳欧利康化学科技有限公司
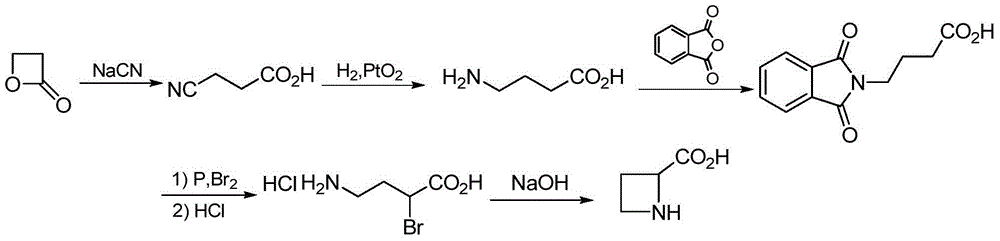
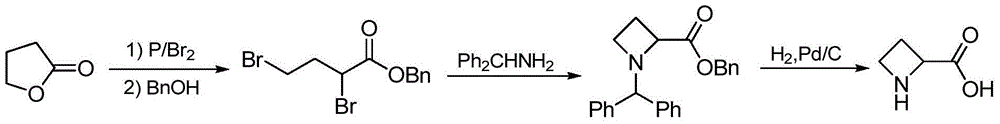
The preparation method of (s)-azetidine-2-carboxylic acid
Owner:中国科学院嘉兴应用化学工程中心
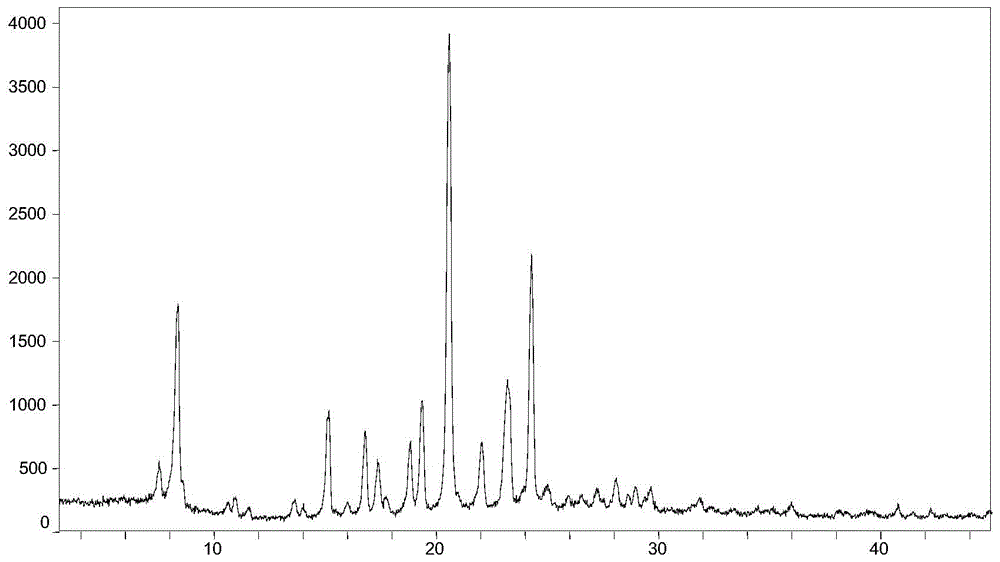
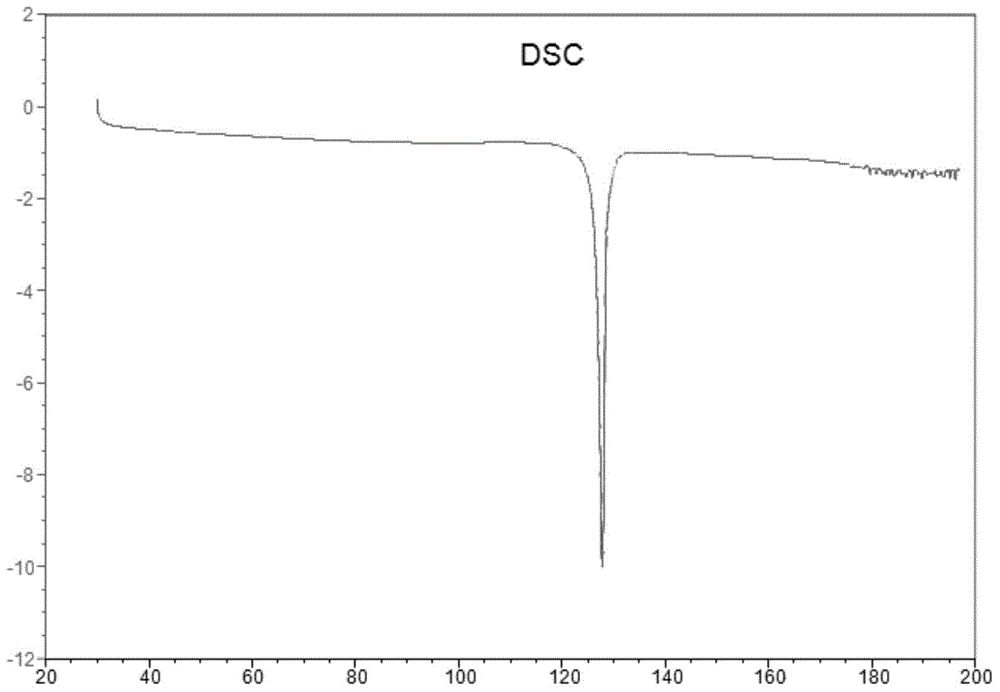
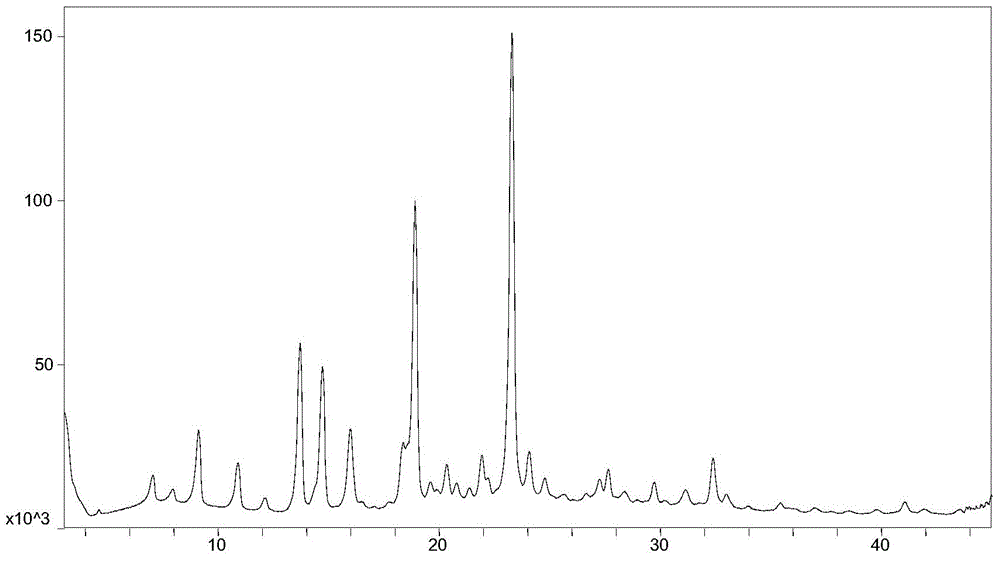
Resolution method and intermediate of cis-1-hydroxy-[1,1′-bis(cyclohexyl)]-2-carboxylic acid
ActiveCN108069846BAvoid high pressure hydrogenation of benzene ring operationReduce processing costsPreparation from carboxylic acid saltsOrganic compound preparationCombinatorial chemistryCarboxylic acid
Owner:SHANGHAI INST OF PHARMA IND CO LTD +1
Zinc nitride and copper nitride compound of chiral alpha-phenylethylamine and use thereof
InactiveCN101830919BAmino preparation from aminesOrganic-compounds/hydrides/coordination-complexes catalystsZinc nitrideCopper chloride
The invention discloses a zinc nitride and copper nitride compound of chiral alpha-phenylethylamine, which comprises a (S) alpha-phenylethylamine zinc nitride and copper nitride compound and a (R) alpha-phenylethylamine zinc nitride and copper nitride compound, which are prepared from the alpha-phenylethylamine, zinc acetate dihydrate, copper acetate monohydrate and copper chloride dihydrate and have the following formulas. In the formulas, ML is Zn(OOCCH3)2, Cu(OOCCH3)2 or CuCl2. The compound serves as a chiral catalyst in a Henry reaction.
Owner:HEFEI UNIV OF TECH
Fosfomycin disodium preparation method
InactiveCN109694389APrecisely control the amount addedReduce dosageGroup 5/15 element organic compoundsSolubilityTurbidity
The invention discloses a fosfomycin disodium preparation method. According to the method, based on the high solubility of fosfomycin monosodium in industrial ethanol and the almost insolubility of fosfomycin disodium in industrial ethanol, industrial ethanol is used as the single solvent, and sodium hydroxide reacts with L-cis-1,2-epoxypropylphosphonic acid-D-alpha-phenylethylamine in industrialethanol, wherein fosfomycin disodium can be formed to cause turbidity when the added sodium hydroxide is excessive so as to accurately control the adding amount of sodium hydroxide based on the condition, such that the adding of sodium hydroxide is stopped before the formation of fosfomycin disodium; then active carbon is added to decolorize, and impurities are removed to obtain the mixed solutionof impurity-free fosfomycin monosodium and industrial ethanol; and a sodium hydroxide ethanol solution is added to the mixed solution of impurity-free fosfomycin monosodium and industrial ethanol, and a reaction is performed to obtain the final product fosfomycin disodium, wherein no impurity exist, and the filtrates in the steps c and d can be continuously and repeatedly used after centrifugal filtration, such that the consumption of the solvent ethanol is substantially reduced, and the production cost is reduced.
Owner:HUBEI XUNDA PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com


![Resolution method and intermediate of cis-1-hydroxy-[1,1′-bis(cyclohexyl)]-2-carboxylic acid Resolution method and intermediate of cis-1-hydroxy-[1,1′-bis(cyclohexyl)]-2-carboxylic acid](https://images-eureka.patsnap.com/patent_img/926517e1-4d1b-4cb6-8409-e3c005bdd56f/HDA0001155312230000011.png)
![Resolution method and intermediate of cis-1-hydroxy-[1,1′-bis(cyclohexyl)]-2-carboxylic acid Resolution method and intermediate of cis-1-hydroxy-[1,1′-bis(cyclohexyl)]-2-carboxylic acid](https://images-eureka.patsnap.com/patent_img/926517e1-4d1b-4cb6-8409-e3c005bdd56f/HDA0001155312230000012.png)
![Resolution method and intermediate of cis-1-hydroxy-[1,1′-bis(cyclohexyl)]-2-carboxylic acid Resolution method and intermediate of cis-1-hydroxy-[1,1′-bis(cyclohexyl)]-2-carboxylic acid](https://images-eureka.patsnap.com/patent_img/926517e1-4d1b-4cb6-8409-e3c005bdd56f/BDA0001155312220000011.png)