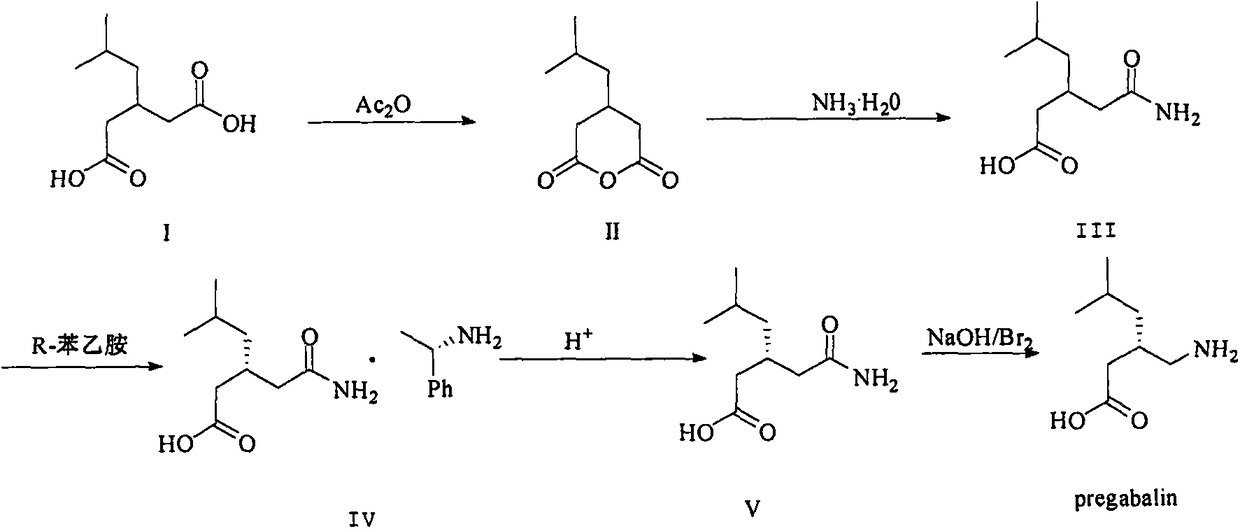
A kind of recovery method of pregabalin intermediate resolving agent (r)-(+)-alpha-phenylethylamine
An intermediate and resolving agent technology, applied in the field of medicine and chemical industry, to achieve the effects of low operating cost, easy operation and reduced emission
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0020] Take 700g of the dissociated mother liquor, raise the temperature to 40°C and stir mechanically, when the internal temperature of the system reaches 40°C, add 125g of 30% potassium carbonate solution dropwise, adjust the pH to 11.0; , 500ml, 400ml, 300ml, 200ml, stirred for 0.5h, allowed to stand for 20min to separate layers, and combined the organic layer; distilled methylene chloride under reduced pressure at 30°C, and raised the temperature to 90°C when no obvious solvent was evaporated, removed a small amount of front boiling, and evaporated Basically all of them are resolving agent (R)-(+)-α-phenethylamine; the recovery rate is 93%, the purity is 99.6%, and the optical rotation is 36.3°.
example 2
[0022] Take 700g of the dissociated mother liquor, raise the temperature to 35°C and stir mechanically, when the internal temperature of the system reaches 35°C, start to add 160g of 30% potassium carbonate solution dropwise, and adjust the pH to 10.0; , 500ml, 400ml, 300ml, 200ml, stirred for 0.5h, allowed to stand for 20min to separate layers, and combined the organic layer; distilled methylene chloride under reduced pressure at 30°C, and raised the temperature to 90°C when no obvious solvent was evaporated, removed a small amount of front boiling, and evaporated Basically all of them are resolving agent (R)-(+)-α-phenethylamine; the recovery rate is 95%, the purity is 99.3%, and the optical rotation is 36.1°.
example 3
[0024] Take 700g of the dissociated mother liquor, raise the temperature to 40°C and stir mechanically. When the internal temperature of the system reaches 40°C, add 210g of saturated sodium bicarbonate dropwise to adjust the pH to 10.0; add 600ml of dichloromethane, Stir 500ml, 400ml, 300ml, 200ml for 0-5h, let stand for 20min to separate layers, combine the organic layer; distill dichloromethane under reduced pressure at 30°C, raise the temperature to 90°C when no obvious solvent is evaporated, remove a small amount of foreboil, and distill out Basically all of them are resolving agent (R)-(+)-α-phenethylamine; the recovery rate is 90%, the purity is 99.1%, and the optical rotation is 36.5°.
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical rotation | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com