Resolution for 5-methyltetrahydrofolic acid and salifying method thereof
A technology of methyltetrahydrofolate and calcium methyltetrahydrofolate, which is applied in the directions of organic chemical methods, chemical instruments and methods, separation of optically active compounds, etc., can solve restrictions, chloride ions do not meet the limit inspection of chloride ions, and the market price is relatively low. Expensive and other issues
Inactive Publication Date: 2010-08-11
NAN JING RHINE PHARM TECH
View PDF0 Cites 3 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
And Klaus.S et al. have proposed the organic base N-ethyl-2-aminomethylpyrrolidine or its enantiomer as a resolution agent from the racemate (6R, S)-5-methyl The method for preparing (6S)-5-methyltetrahydrofolate from tetrahydrofolate (U.S 5,457,202, 1992) created a precedent for the resolution of (6R, S)-5-MTHF, but due to N-ethyl-2-amine Especially (+) or (-) enantiomeric market price of methylpyrrole is more expensive, has limited its application; Some documents used calcium chloride (Fedrico G, et al, U.S 5,124,452, 1992), and finally in the product (6S)-5-MTHF-Ca, a large amount of chloride ions are likely to remain in the product (6S)-5-MTHF-Ca, resulting in the inspection of the chloride ion limit that does not meet the requirements of drugs or food additives
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Login to View More
Abstract
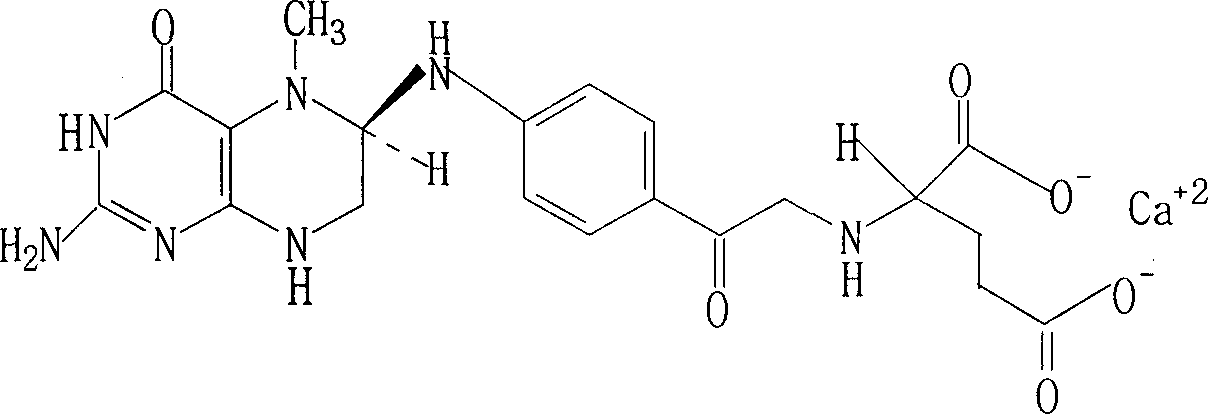
The invention brings forward a preparation method, which utilizes organic alkali Alpha-phenylethylamine to obtain (6S)-5-methyltetrahydrofolate by splitting racemate (6R, S)-5-methyltetrahydrofolate, and utilizes the hydroxide of alkaline earth, particularly calcium hydroxide, to generate (6S)-5-methyltetrahydrofolate calcium salt.
Description
Invention field The present invention relates to the field of organic chemistry, in particular to a method for the resolution and salt formation of organic medicine (6S)-5-methyltetrahydrofolate from (6R,S)-5-methyltetrahydrofolate. Background technique The chemical name of (6S)-5-methyltetrahydrofolate is N-(5-methyl)-6(S)-5,6,7,8,-tetrahydropteroyl-L-glutamic acid, abbreviated (6S)-5-MTHF, the structural formula is as follows: (6S)-5-Methyltetrahydrofolate is the main form of tissue and blood folic acid. Participate in a variety of important biochemical reactions in the body (such as purine and thymine biosynthesis, etc.). The naturally occurring 5-MTHF is only the S type, while the synthetic R type is biochemically inactive and is excreted through the kidneys. (6S)-5-MTHF does not need to go through tedious enzymatic metabolism steps in the human body and can be used directly. (Zhang Yue et al. Fine Chemicals, 13, (22), 13, 2005). Earlier, (6S)-5-MTHF as a drug has two ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Patents(China)
IPC IPC(8): C07D475/04C07B57/00
CPCC07D475/04
Inventor 陈新朱光旭陈伟
Owner NAN JING RHINE PHARM TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com