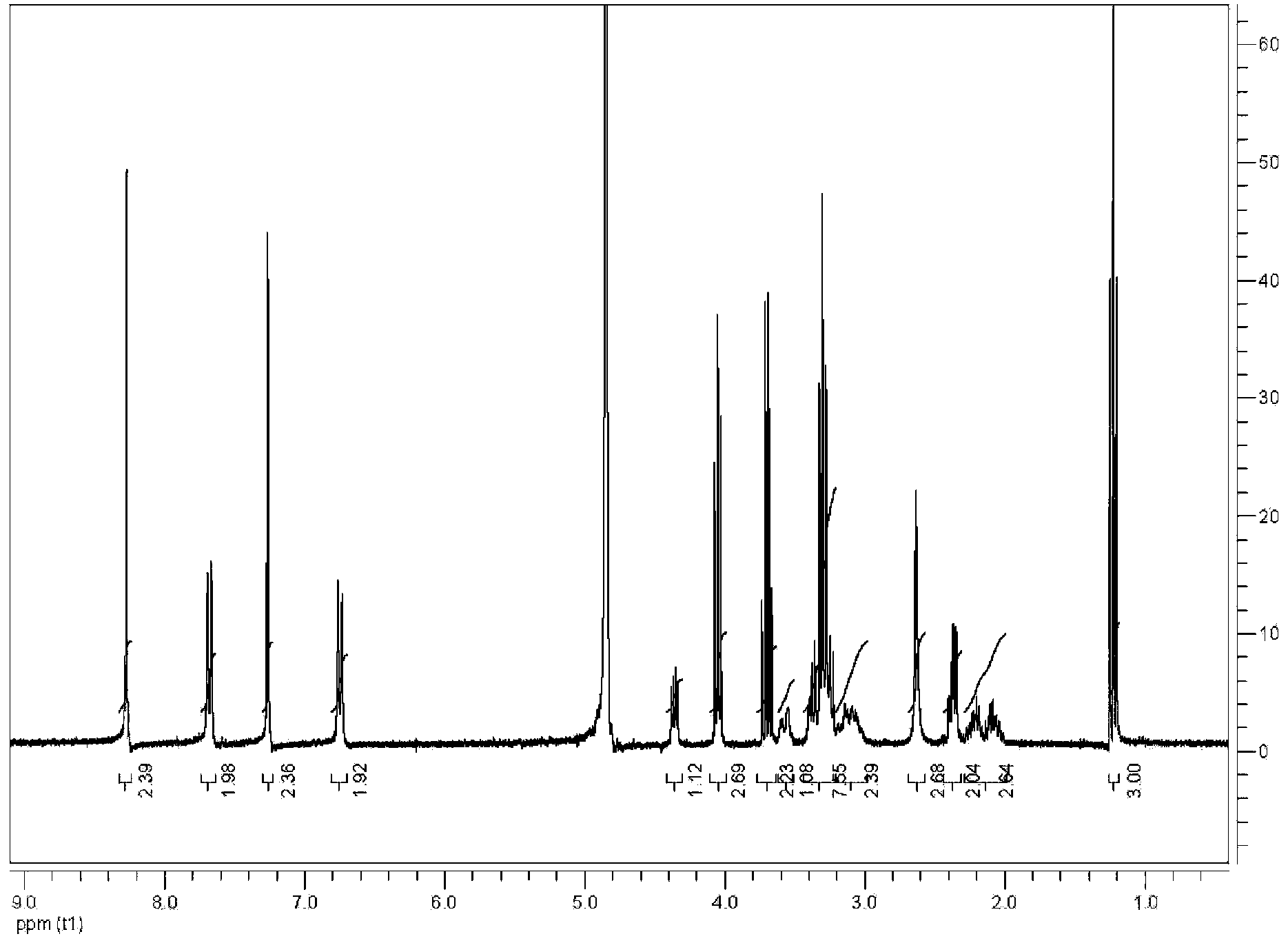
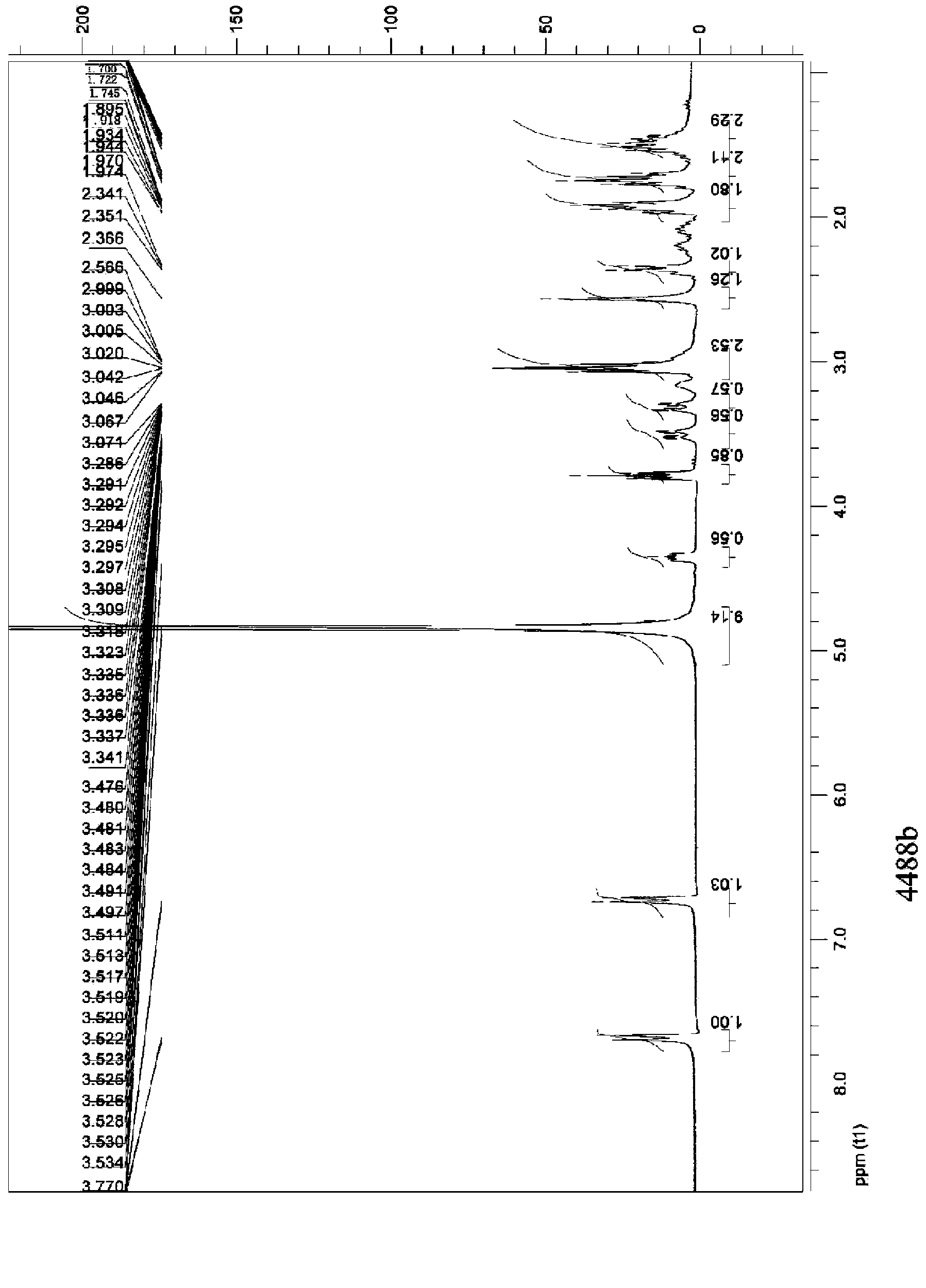
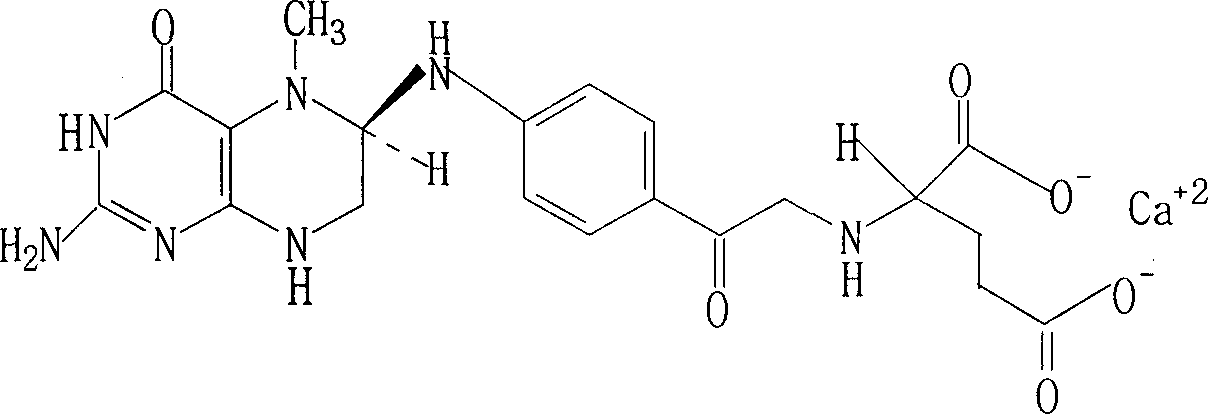
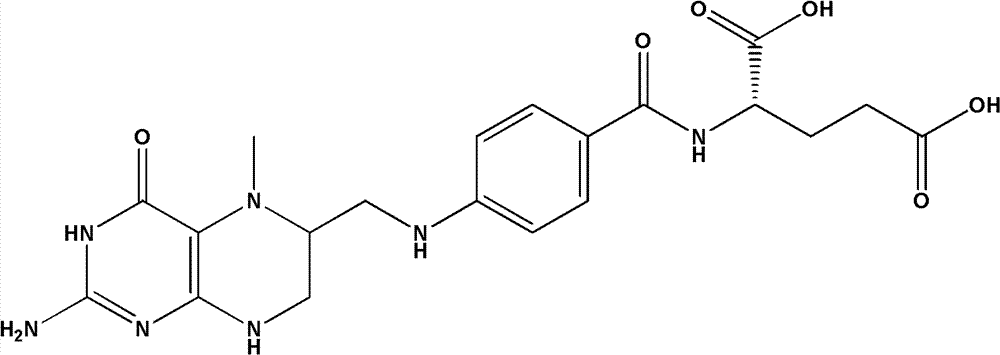
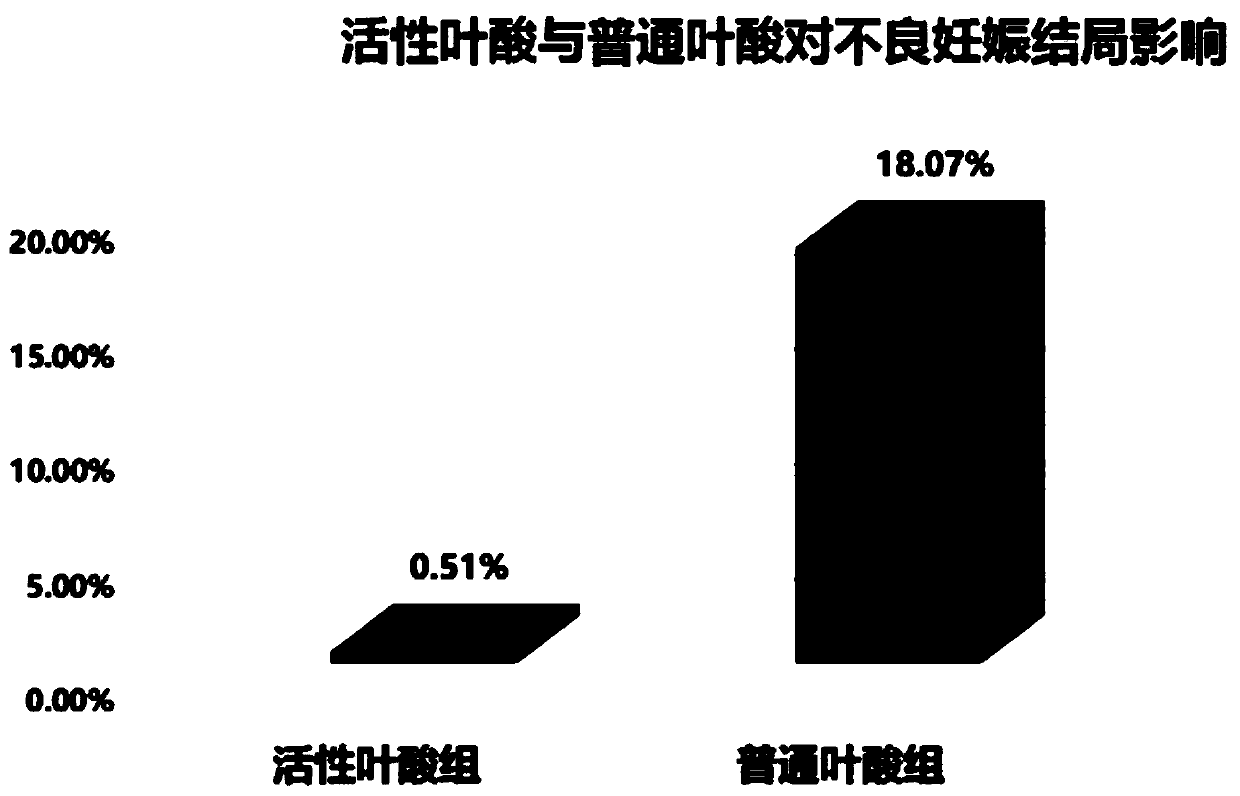
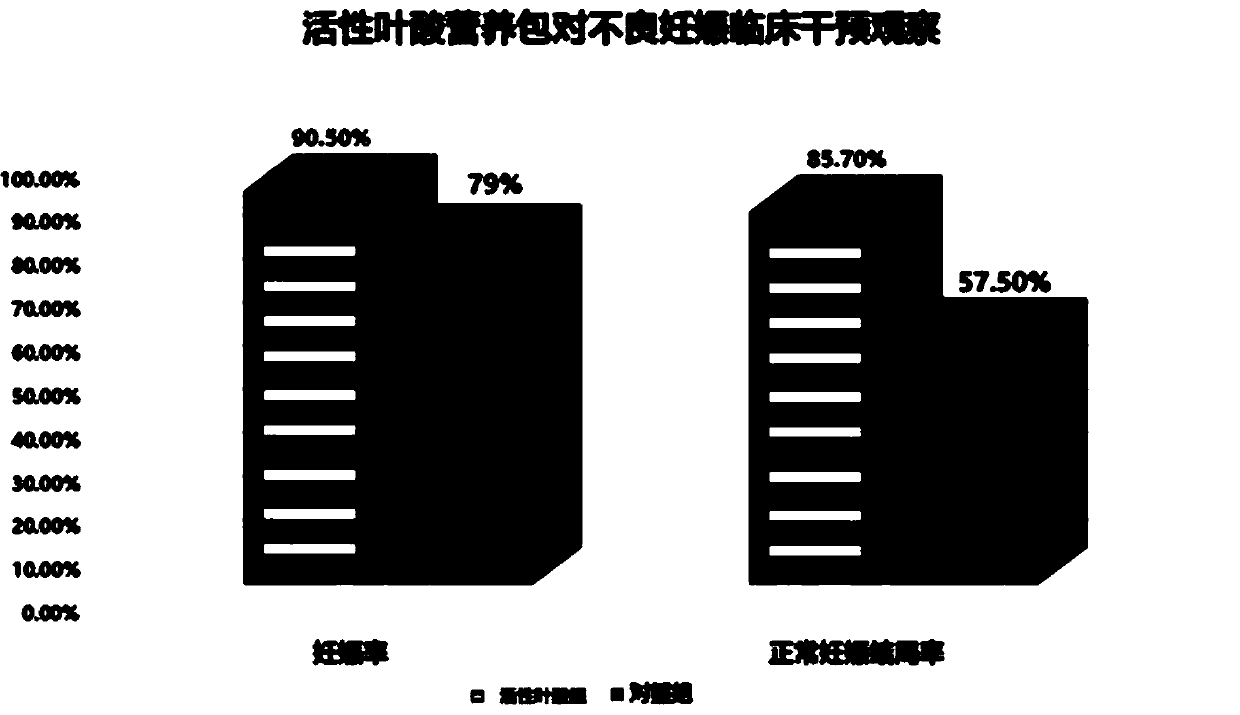
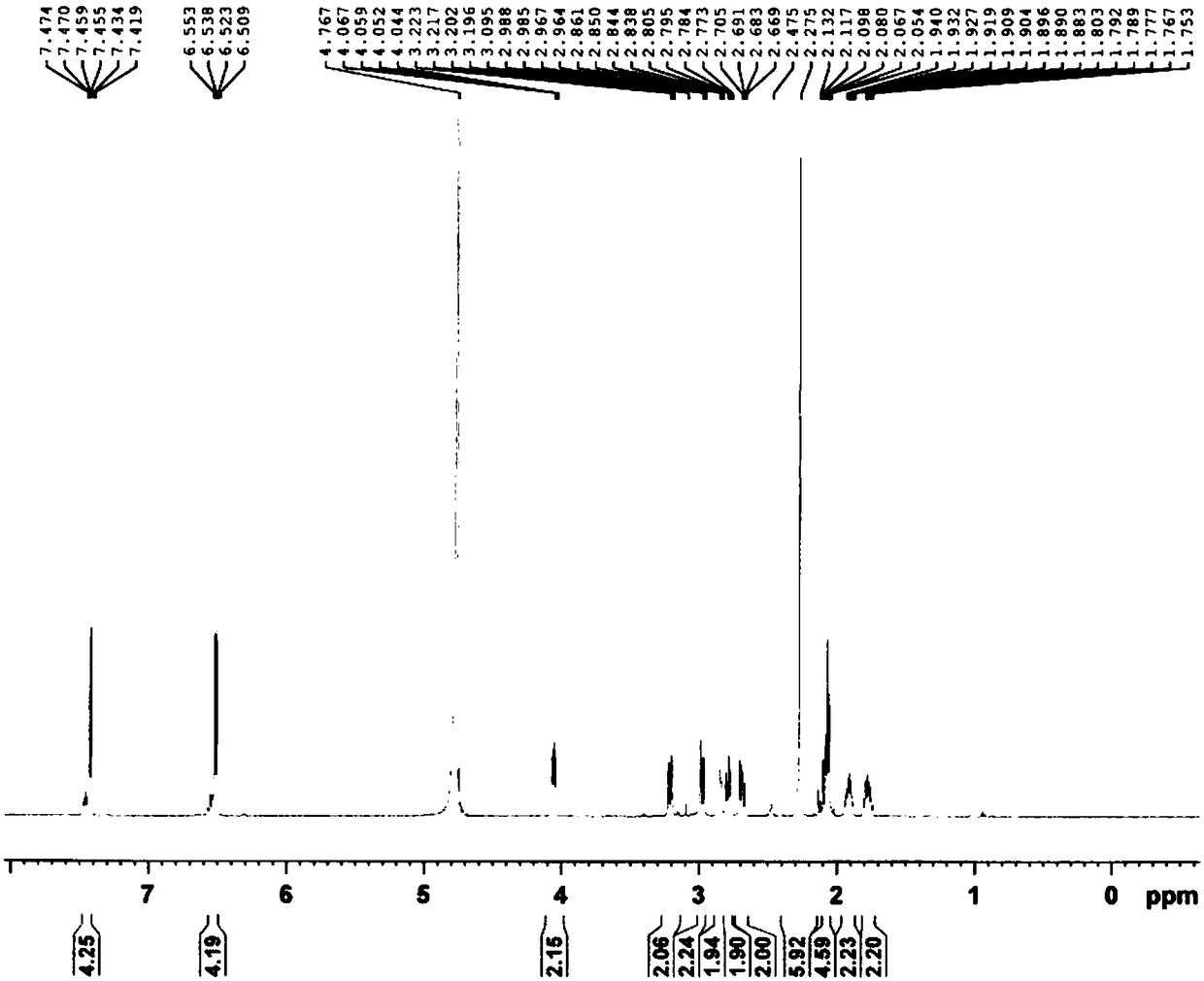
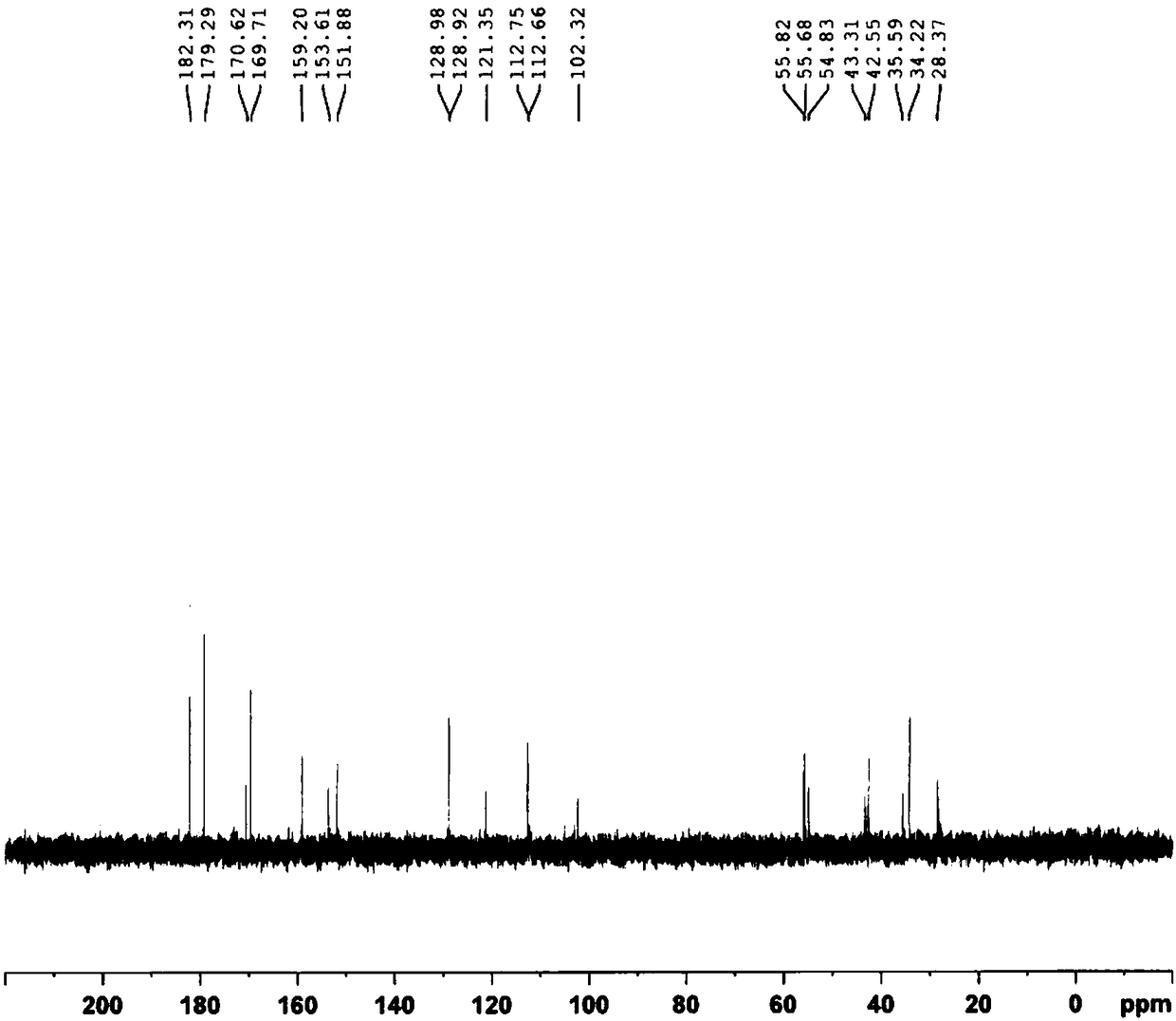
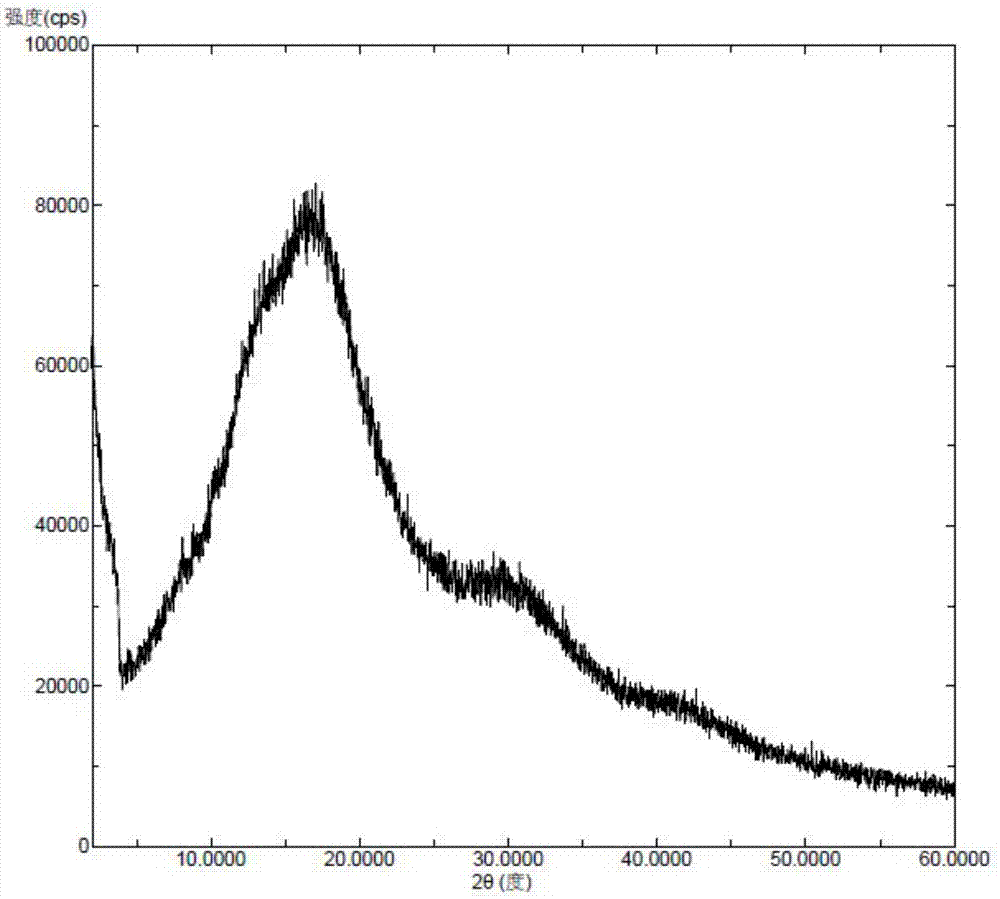
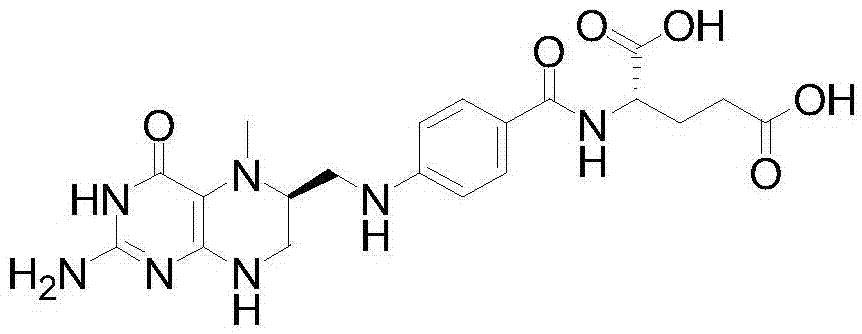
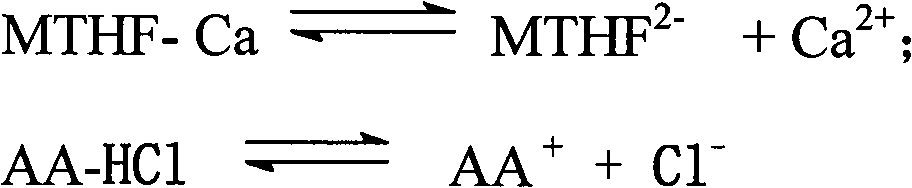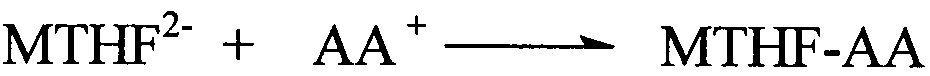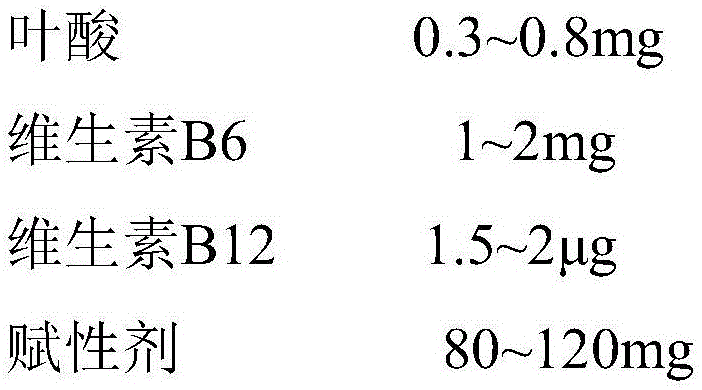Patents
Literature
49 results about "5-Methyltetrahydrofolate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
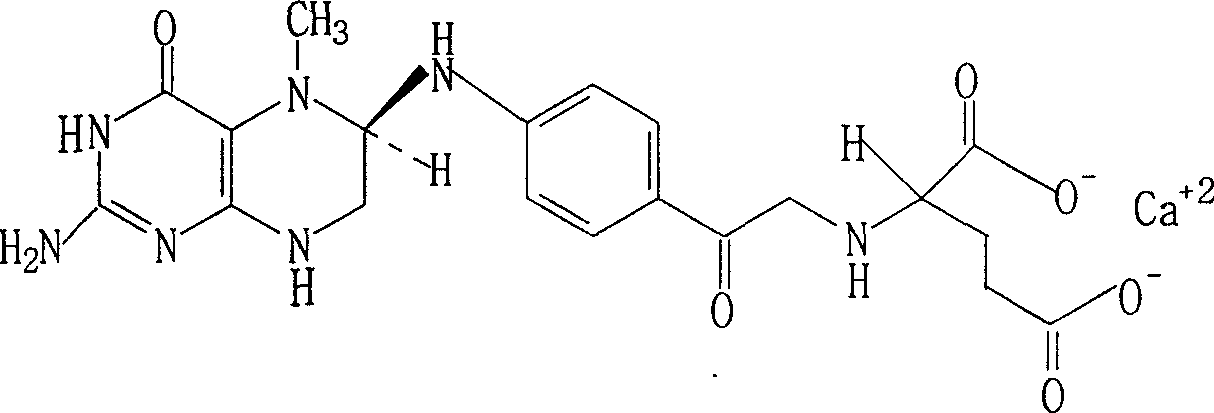
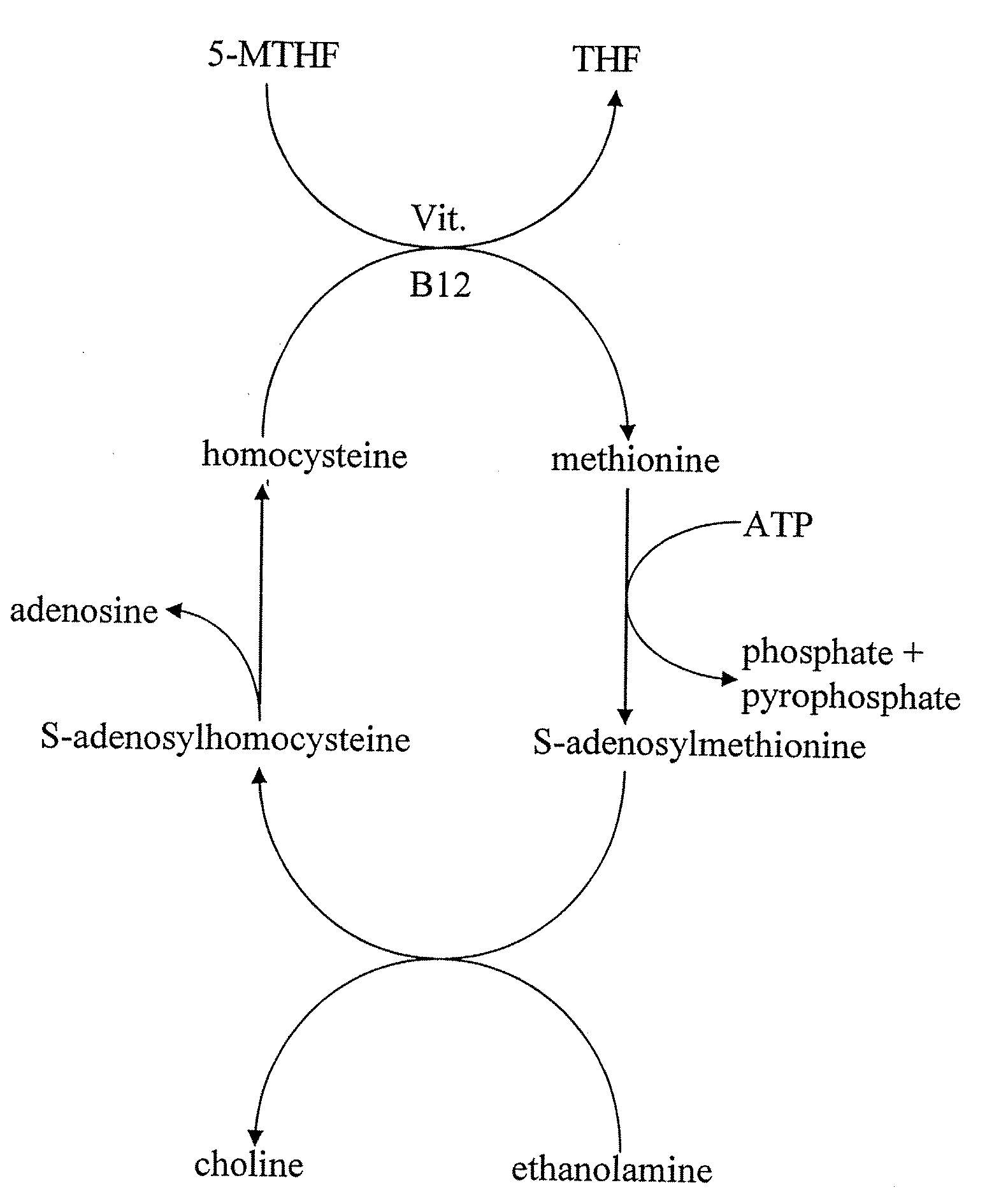
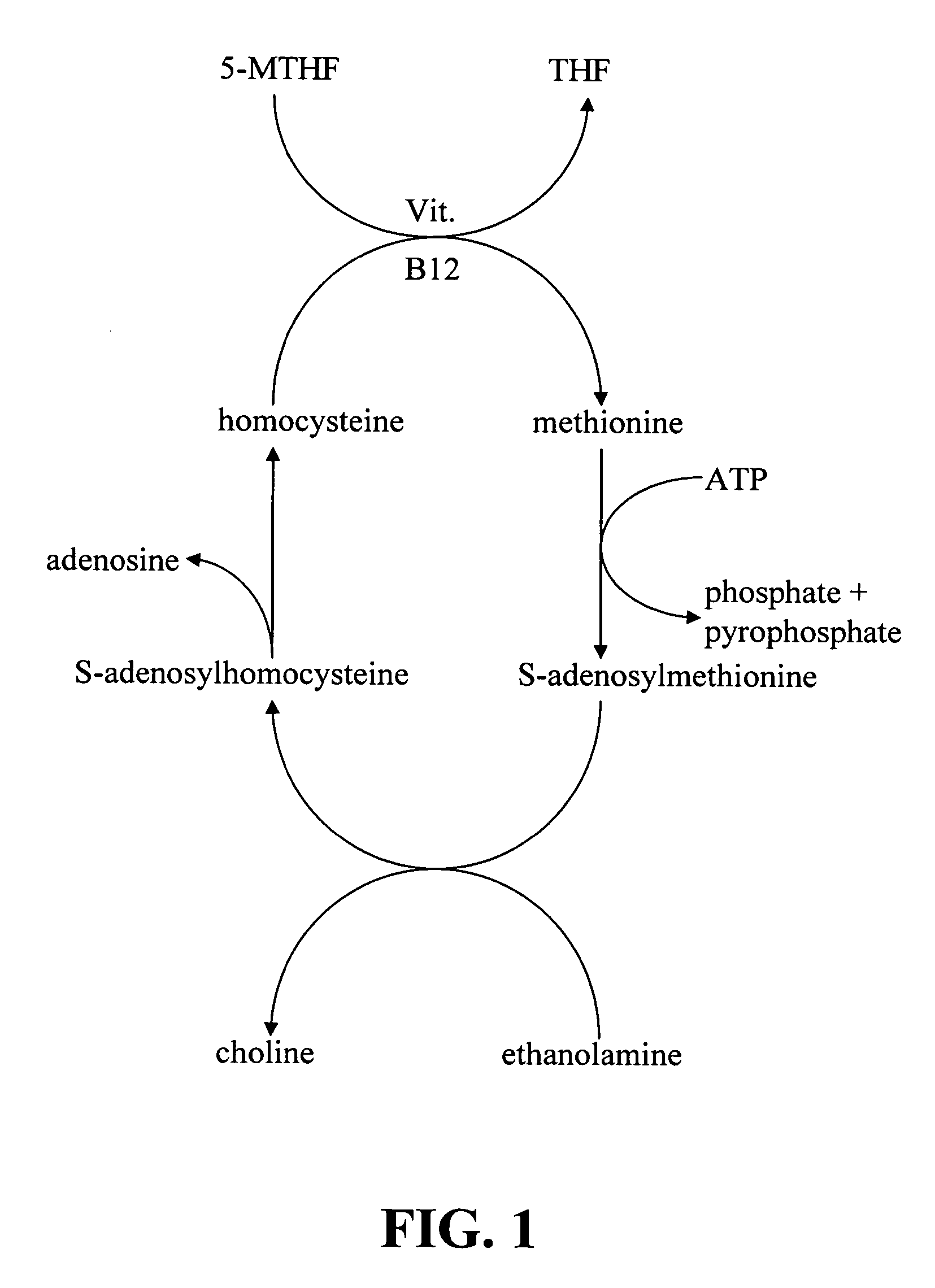
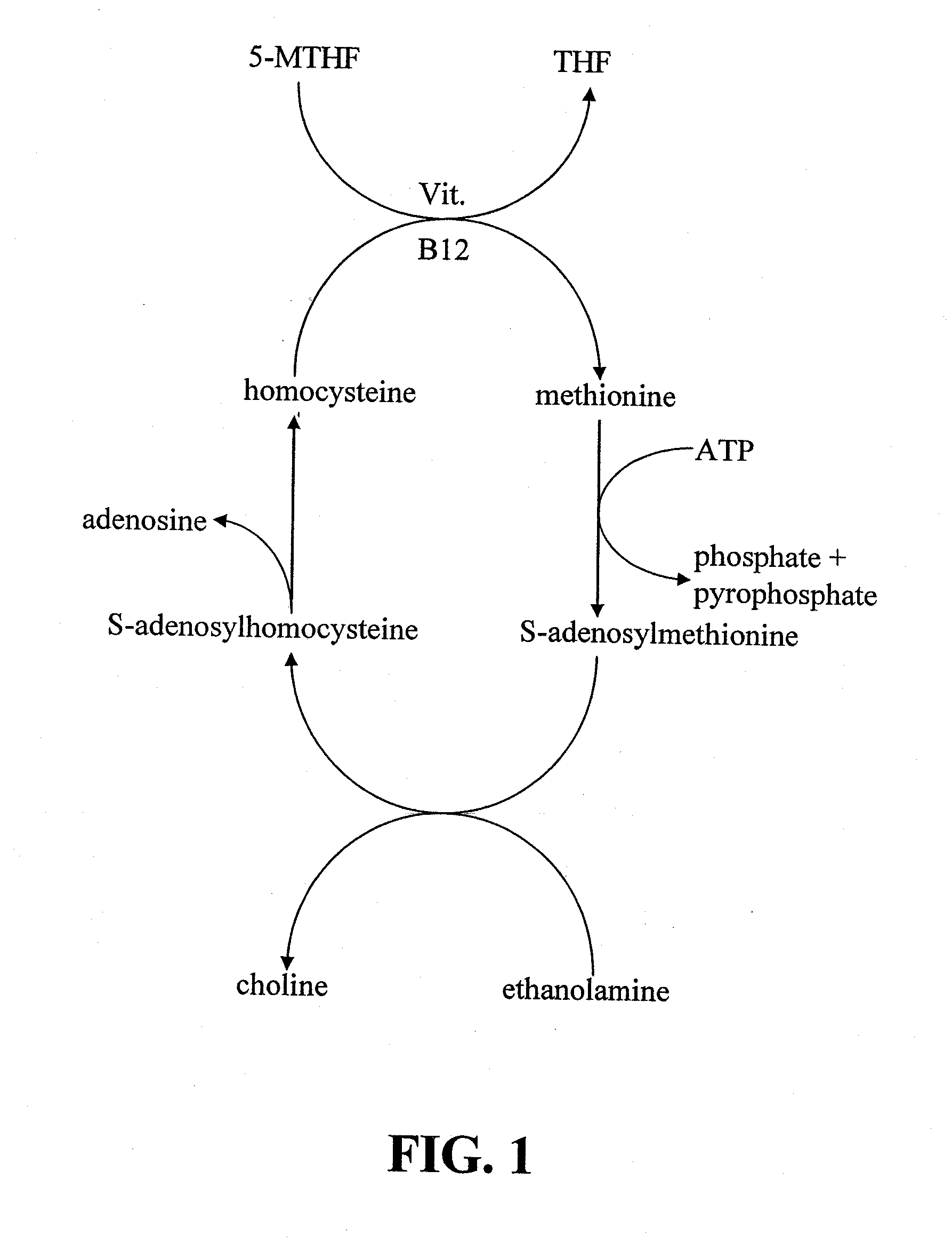
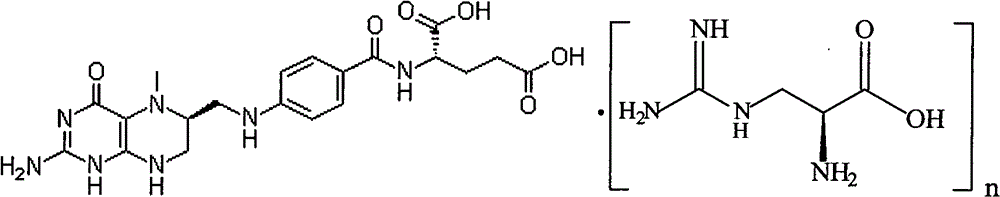
Infobox references. Levomefolic acid (INN) (also known as L-5-MTHF, L-methylfolate and L-5-methyltetrahydrofolate and (6S)-5-methyltetrahydrofolate, and (6S)-5-MTHF) is the primary biologically active form of folate used at the cellular level for DNA reproduction, the cysteine cycle and the regulation of homocysteine.
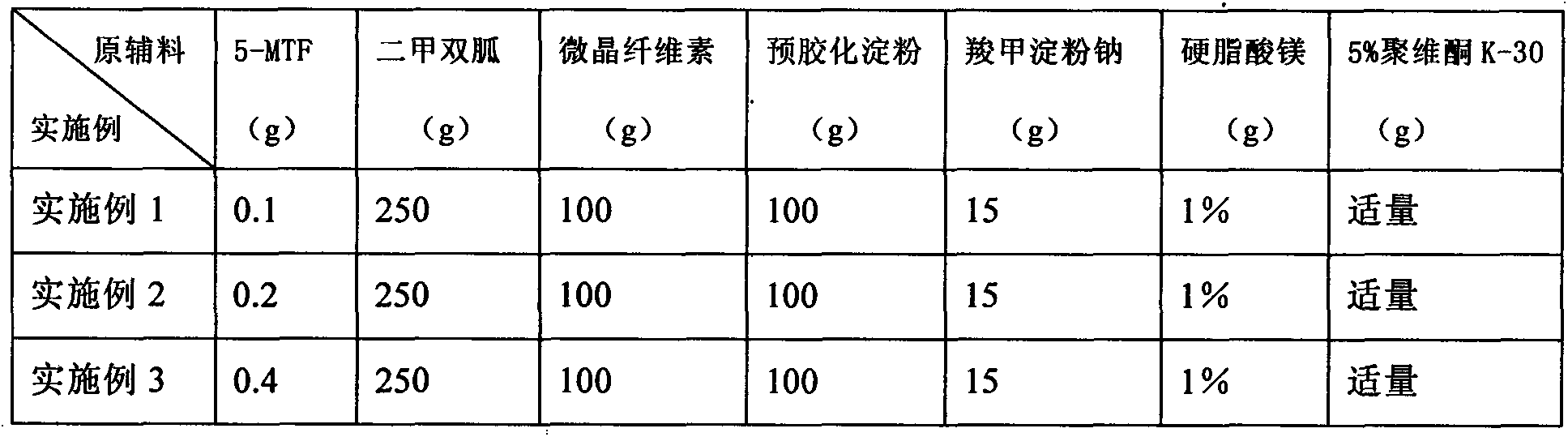
Process for producing a pharmaceutical preparation for therapeutic treatment of endometriosis containing a combination of a gestagen and (6S)-5-methyltetrahydrofolate
InactiveUS20090060997A1Reduce endometriosisEffective therapeutic treatmentBiocidePill deliveryBone densityObstetrics
A combination of an anti-androgenic gestagen at a daily dose of from an ovulation-inhibiting dose up to at most twice the ovulation-inhibiting dose and from 0.1 to 10 mg of (6S)-5-methyltetrahydrofolate are used to produce a pharmaceutical preparation for therapeutically treating endometriosis while simultaneously reducing therapy side effects including the negative effect on bone density and / or bone metabolism, reducing the risk of osteoporosis and, in the event of pregnancy, reducing the risk of congenital malformations, such as medullary tube defects, cleft lip, cleft jaw, or cleft palate, and the risk of pregnancy complications, such as detachment of the placenta and premature birth. The preparation is suitable for long-term administration, which continues daily for at least 169 days to at least two years.
Owner:BAYER SCHERING PHARMA AG
Nutritional preparations
InactiveUS20060217385A1Promote maturityReduce and alleviate fetusBiocideAnimal repellantsPregnancyAdditive ingredient
Compositions and methods for improving the nutritional and physiological status of a woman and her child during all stages of pregnancy are provided herein. This includes pre-conceptional women, pregnant women, and post-natal women (both lactating and non-lactating mothers). The compositions are particularly useful for the neurological, visual, and cognitive development of an embryo, fetus, or infant and the nutritional and physiological well-being of the mother, fetus, and infant. The compositions contain one or more folates, such as a reduced folate and / or folic acid, and one or more essential fatty acids (EFA), such as an omega-3 and / or omega-6 fatty acid. The addition of the essential fatty acid improves upon the folate containing nutritional preparations described in the prior art. The one or more folates and essential fatty acid may be administered together or in separate dosage units. The one or more folates may be selected from folic acid / folate, one or more reduced folates, or a combination of folic acid / folate and one or more reduced folates. The reduced folate is preferably 5-methyltetrahydrofolate, and most preferably 5-methyl-(6S)-tetrahydrofolic acid. The essential fatty acid is preferably an omega-3 fatty acid, and is preferably docosahexenoic acid (DHA) derived from a vegetarian or non-fish source. The compositions may optionally contain other vitamins, minerals, and ingredients, such as, emollient laxatives—all defined herein as “optional or other ingredients”.
Owner:SCIELE PHARMA CAYMAN
Nutritional preparations
InactiveUS20060217386A1Promote maturityReduce and alleviate fetusBiocideSkeletal disorderPregnancyAdditive ingredient
Compositions and methods for improving the nutritional and physiological status of a woman and her child during all stages of pregnancy are provided herein. This includes pre-conceptional women, pregnant women, and post-natal women (both lactating and non-lactating mothers). The compositions are particularly useful for the neurological, visual, and cognitive development of an embryo, fetus, or infant and the nutritional and physiological well-being of the mother, fetus, and infant. The compositions contain one or more folates, such as a reduced folate and / or folic acid, and one or more essential fatty acids (EFA), such as an omega-3 and / or omega-6 fatty acid. The addition of the essential fatty acid improves upon the folate containing nutritional preparations described in the prior art. The one or more folates and essential fatty acid may be administered together or in separate dosage units. The one or more folates may be selected from folic acid / folate, one or more reduced folates, or a combination of folic acid / folate and one or more reduced folates. The reduced folate is preferably 5-methyltetrahydrofolate, and most preferably 5-methyl-(6S)-tetrahydrofolic acid. The essential fatty acid is preferably an omega-3 fatty acid, and is preferably docosahexenoic acid (DHA) derived from a vegetarian or non-fish source. The compositions may optionally contain other vitamins, minerals, and ingredients, such as, emollient laxatives-all defined herein as “optional or other ingredients”.
Owner:SCIELE PHARMA CAYMAN
Resolution for 5-methyltetrahydrofolic acid and salifying method thereof
InactiveCN101143863AOptically-active compound separationOrganic racemisationCalcium hydroxideAlkaline earth metal
The invention brings forward a preparation method, which utilizes organic alkali Alpha-phenylethylamine to obtain (6S)-5-methyltetrahydrofolate by splitting racemate (6R, S)-5-methyltetrahydrofolate, and utilizes the hydroxide of alkaline earth, particularly calcium hydroxide, to generate (6S)-5-methyltetrahydrofolate calcium salt.
Owner:NAN JING RHINE PHARM TECH
Method for detecting water-soluble vitamins in blood
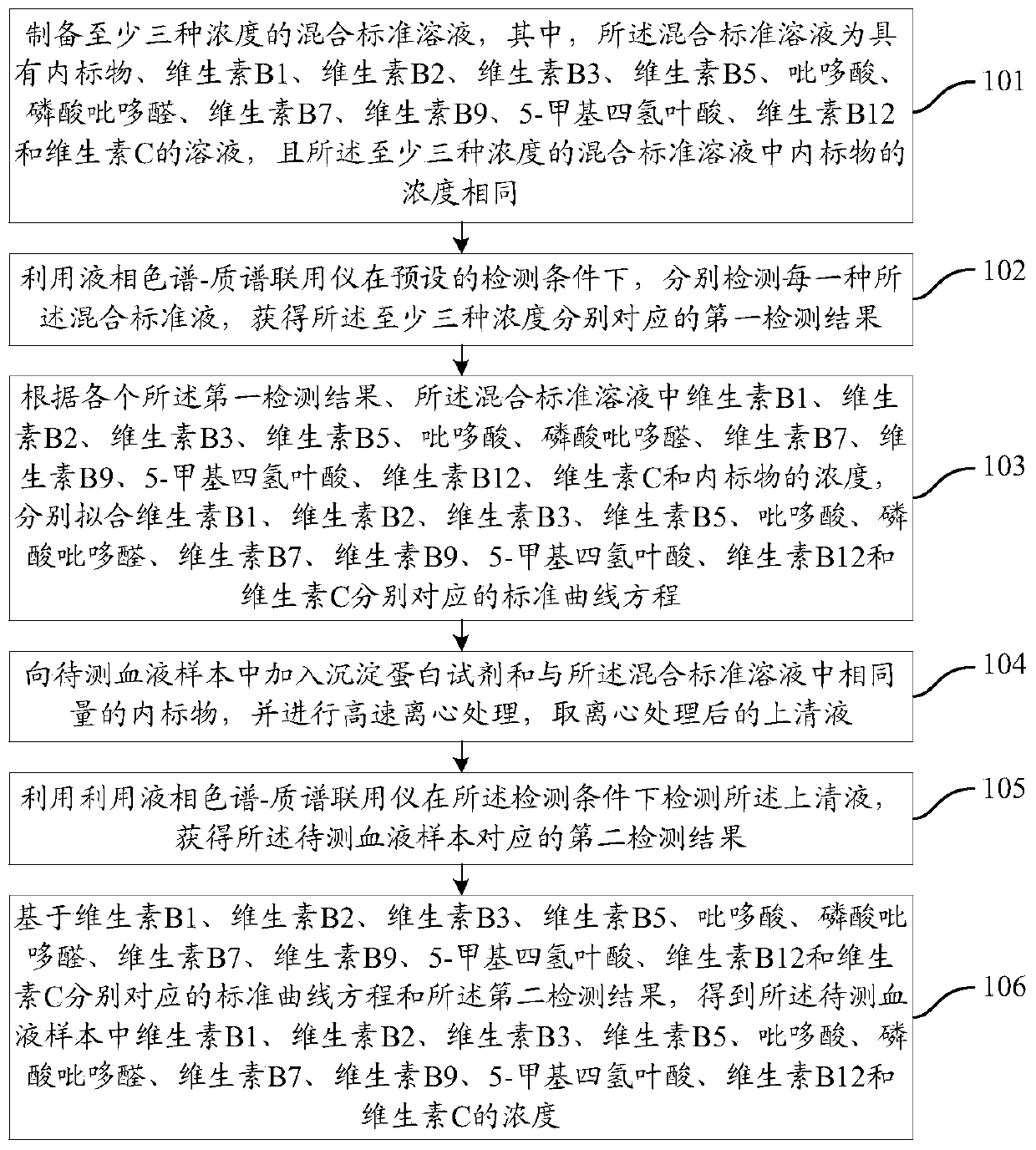
The invention provides a method for detecting water-soluble vitamins in blood. The method includes the steps of detecting a mixed standard solution with at least three concentrations through a liquidchromatograph-mass spectrometer to obtain a first detecting result, wherein the mixed standard solution comprises internal standard substances and the water-soluble vitamins, and the water-soluble vitamins include the vitamin B1, the vitamin B2, the vitamin B3, the vitamin B5, pyridoxic acid, pyridoxal phosphate, the vitamin B7, the vitamin B9, 5-methyltetrahydrofolate, the vitamin B12 and the vitamin C; conducting fitting to obtain standard curve equations corresponding to the water-soluble vitamins on the basis of the first detecting result and the concentrations of the water-soluble vitamins; centrifuging a to-be-detected blood sample with a precipitate protein agent and a certain amount of internal standard substances, and taking supernate to be detected through the liquid chromatograph-mass spectrometer to obtain a second detecting result; obtaining the concentration of the water-soluble vitamins in the to-be-detected blood sample on the basis of the standard curve equations and the second detecting result. By means of the scheme, the detecting efficiency of the blood sample can be improved.
Owner:BEIJING HARMONY HEALTH MEDICAL DIAGNOSTICS CO LTD
Compositions and methods for the treatment of osteoporosis and inflammatory joint disease
InactiveUS20060216361A1Avoid problemsUseful in treatmentBiocideCarbohydrate active ingredientsAdditive ingredientVitamin B6 synthesis
Compositions and methods for the treatment of osteoporosis and / or inflammatory joint disease are provided herein. The compositions contain a folate, such as a reduced folate, and folic acid. The folate is preferably 5-methyltetrahydrofolate, and most preferably 5-methyl-(6S)-tetrahydrofolic acid. The folate and folic acid can be given in the same dosage unit or separate dosage units, and more than one dosage unit can be given per dose. The compositions may also contain one or more vitamins and minerals selected from vitamin B12, vitamin B6, vitamin D3, calcium, magnesium, and polyunsaturated fatty acids (PUFAs). These ingredients are optional, but preferable (especially the vitamins and minerals). The compositions may further contain one or more additional ingredients such as vitamins, minerals, and laxatives. The compositions are useful in the treatment of all forms of osteoporosis, including primary osteoporosis and secondary osteoporosis, and / or inflammatory joint diseases, especially in patients having a folic acid metabolism deficiency. The compositions are particularly useful in the treatment of inflammatory joint diseases, with complications that include bone loss, fracture, and osteoporosis. In addition, the compositions are beneficial for the prevention of osteoporosis in subjects who do not yet have the disease, but who are at risk for getting osteoporosis, such as post-menopausal women, subjects with osteopenia (mid thinning of the bone mass), subjects with an inflammatory joint disease, or people who are over the age of 70.
Owner:SCIELE PHARMA CAYMAN
Homocysteine measuring method and reagent
InactiveCN102095696AHigh detection sensitivitySimple and fast operationMicrobiological testing/measurementColor/spectral properties measurementsLyaseL-homoserine
The invention discloses a method for measuring homocysteine concentration. The method comprises the following steps of: placing a sample to be measured into an enzymatic circular reaction system, performing reaction shown as formulas 1 to 3, measuring and calculating the production rate of ammonia produced in the enzymatic circular reaction system so as to obtain the content of homocysteine in the sample to be measured; and the enzymatic circular reaction system comprises methionine synthase, methionine gama-lyase, O-acetyl homoserine aminocarboxypropyl transferase, 5-methyltetrahydrofolate or salt of the 5-methyltetrahydrofolate, and O-acetyl-L-homoserine. The invention also discloses a corresponding measuring reagent. The invention provides a novel homocysteine enzymological measuring method and a measuring reagent. The method does not need any special instrument, is easy and convenient to operate, has high sensitivity, is low in cost, can realize the clinical, quick and high-flow sample detection, ensures that the homocysteine possibly becomes the clinical conventional detection item, and has high scientific and economic value. The formulas 1 to 3 are shown in the specifications.
Owner:王学忠
Preparation method of L-5-calcium methyltetrahydrofolate
ActiveCN108164531AReduce usageRaw materials are cheap and easy to getOrganic chemistry methodsHydrogenWastewater
The invention discloses a preparation method of L-5-calcium methyltetrahydrofolate. The preparation method comprises the following steps of (1) adopting folic acid as a raw material, and reducing to preparing an intermediate 6-(R,S)-tetrahydrofolic acid; (2) carrying out chiral resolution on the 6-(R,S)-tetrahydrofolic acid, and obtaining an intermediate 6-S-tetrahydrofolate; (3) carrying out methylation on the 6-S-tetrahydrofolate to obtain an intermediate L-5-methyltetrahydrofolate; (4) reacting the L-5-methyltetrahydrofolate and salt containing calcium to obtain the L-5-calcium methyltetrahydrofolate. According to the method provided by the invention, the problem that hydrogen, wastewater and waste residues are easily generated by using a large number of sodium borohydride is avoided.
Owner:无锡紫杉药业股份有限公司
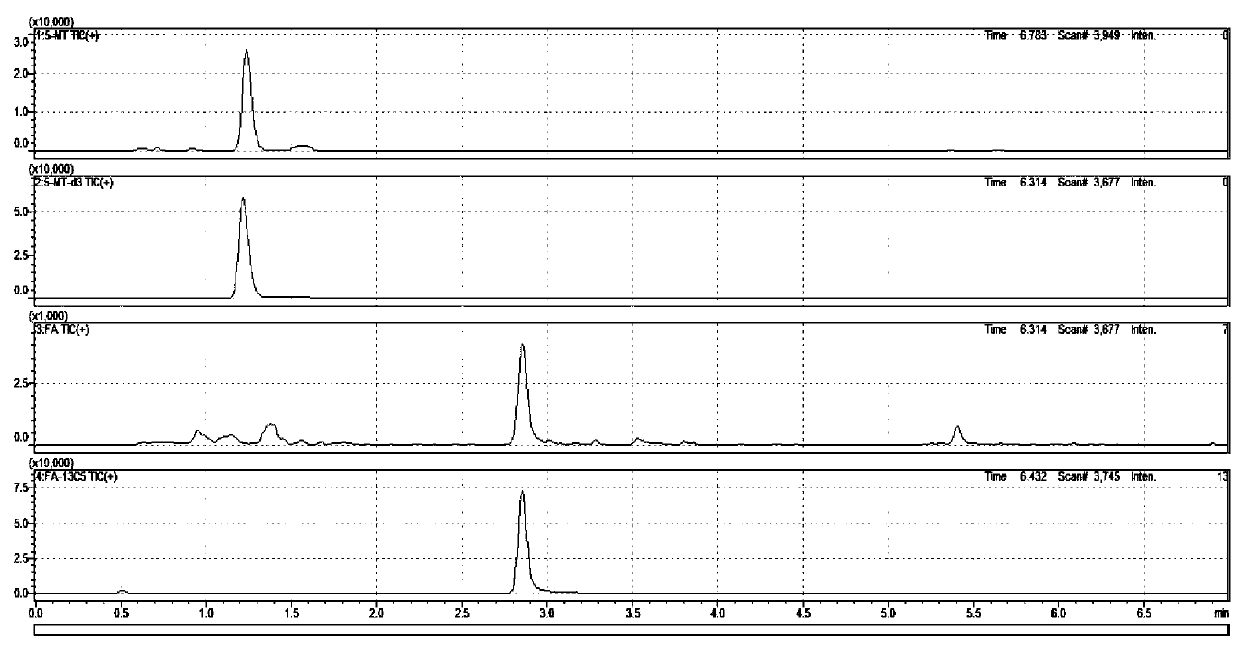
Kit for detecting folic acids in blood spots through high performance liquid chromatography-tandem mass spectrometry
The invention discloses a kit for detecting folic acids in blood spots through a high performance liquid chromatography-tandem mass spectrometry. The kit comprises a standard product, a quality control product, a mixed internal standard solution, an eluent A, an eluent B, an extract, an extract additive, a combined antioxidant and a complex solution. The folic acids comprise a folic acid and 5-methyltetrahydrofolate. According to the invention, the kit is used for detection, which has the advantages of high sensitivity, high specificity, accuracy, simple pretreatment method, short time and greatly saved time cost; and the precision and recovery rate basically meet requirements.
Owner:上海可力梅塔生物医药科技有限公司 +3
Method for determining folic acids in blood spots through high performance liquid chromatography-tandem mass spectrometry
InactiveCN110146626AEasy to manufactureEasy to storeComponent separationInternal standardMass Spectrometry-Mass Spectrometry
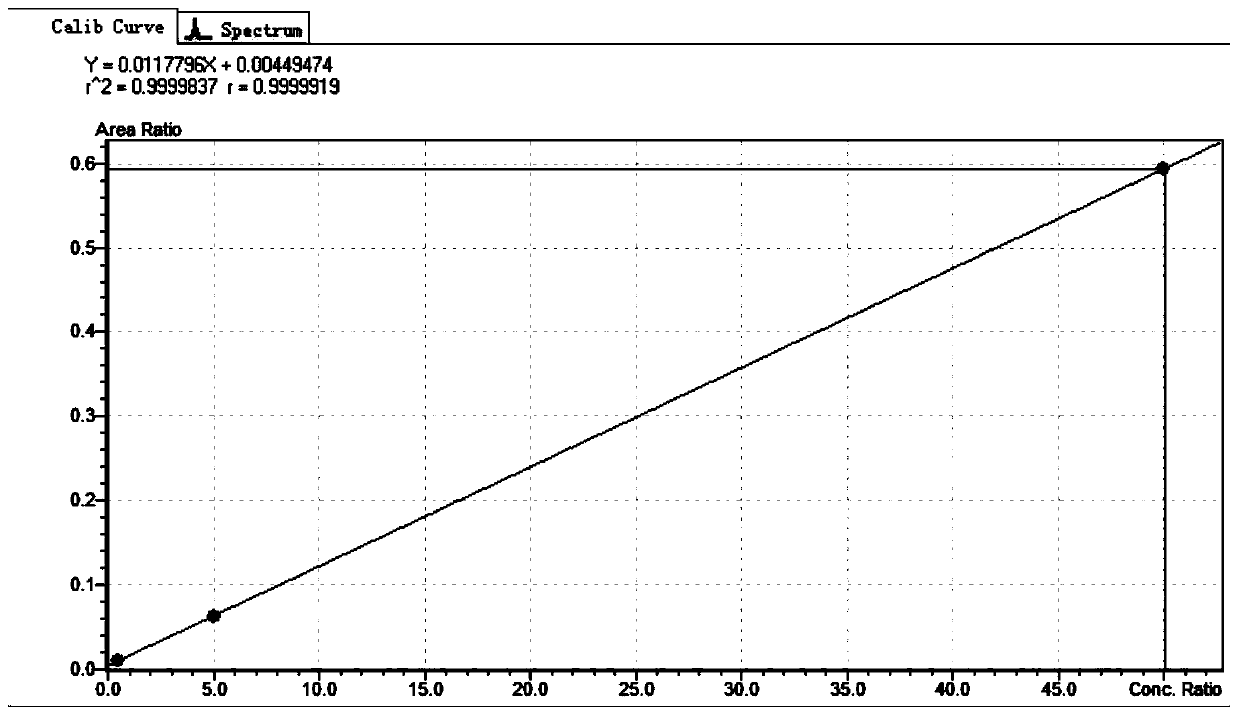
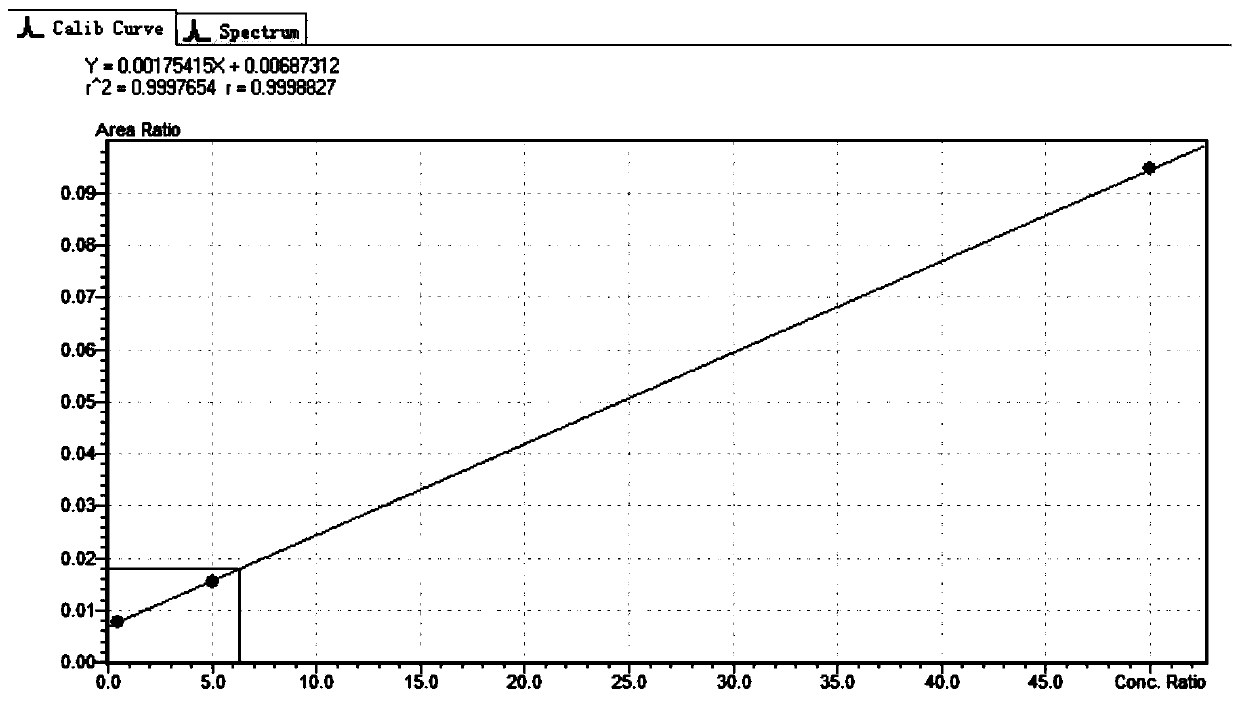
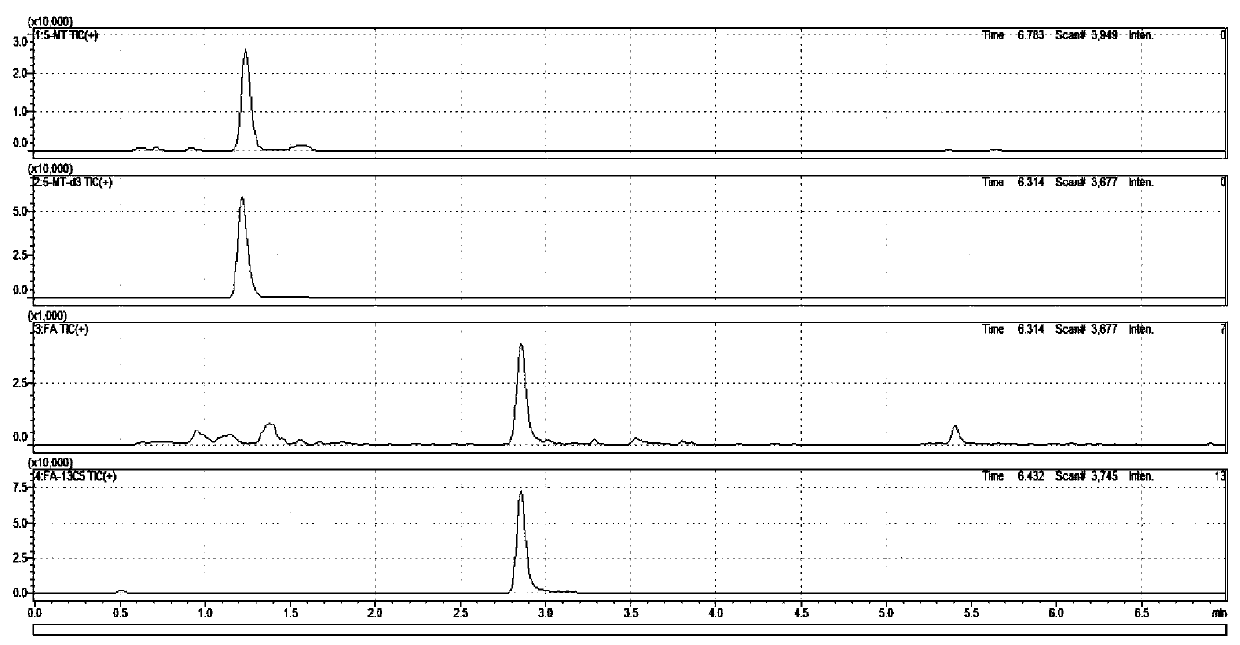
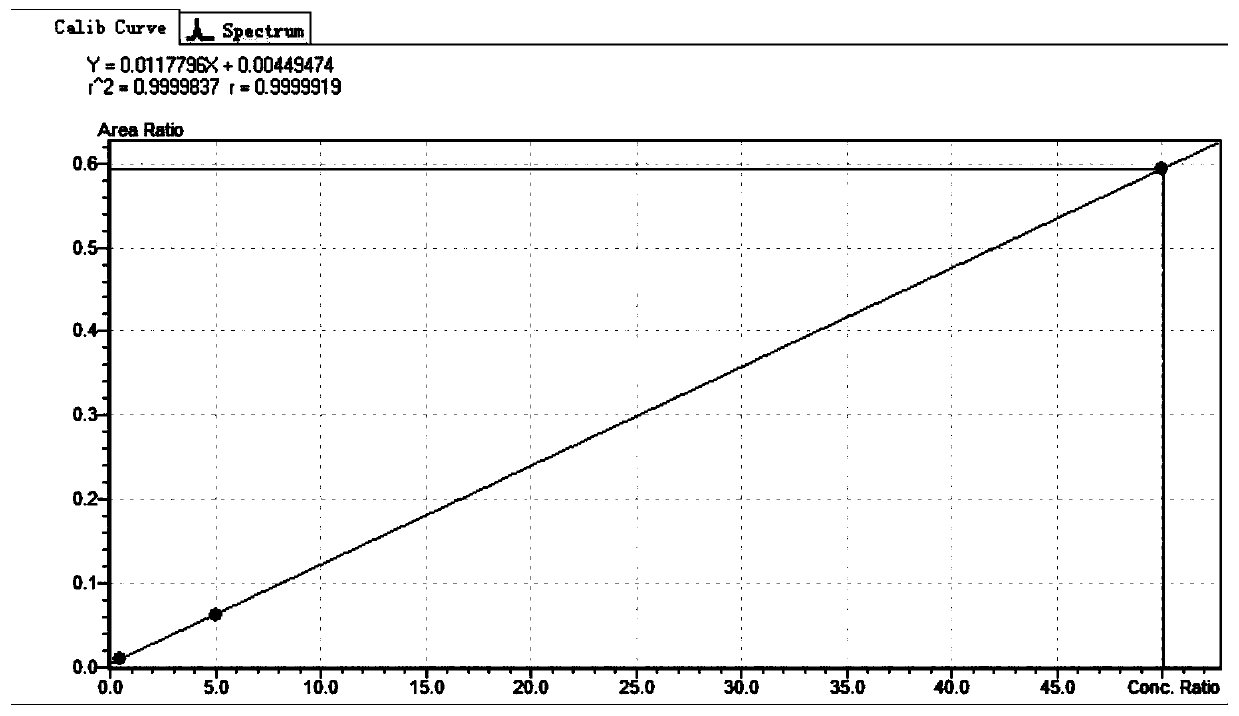
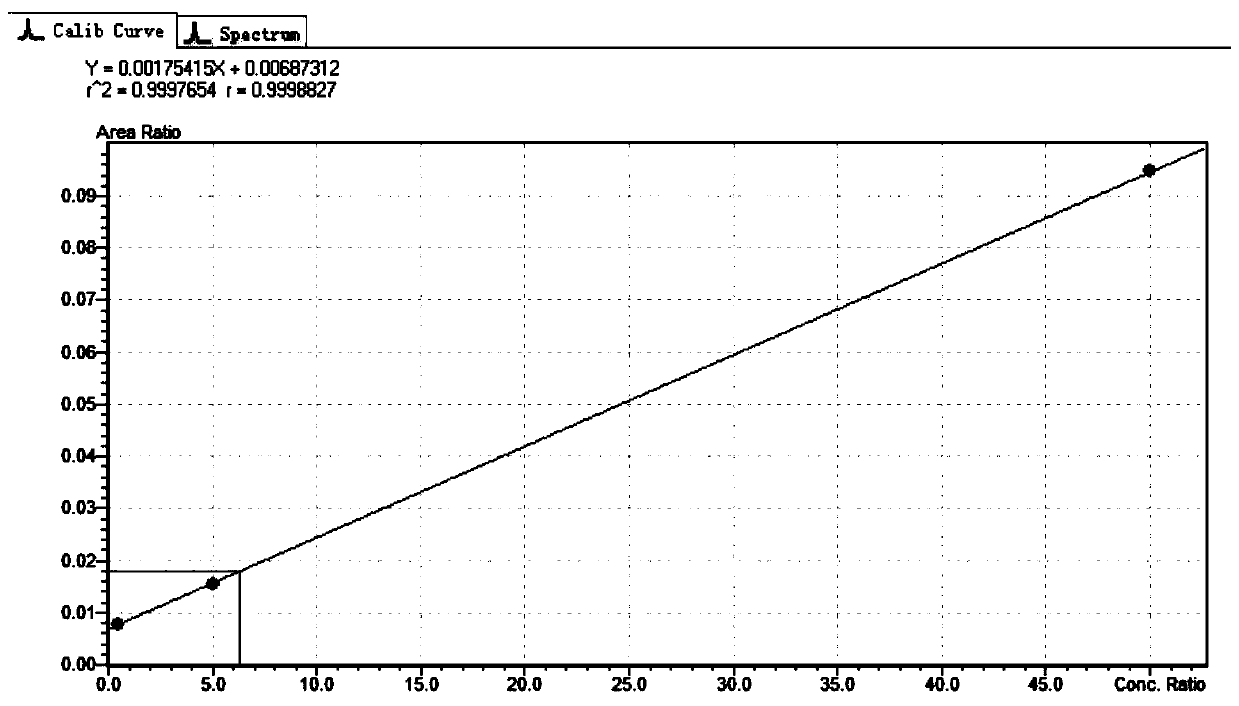
The invention provides a method for determining folic acids in blood spots through high performance liquid chromatography-tandem mass spectrometry. The folic acid and 5-methyltetrahydrofolate are separated by high performance liquid chromatography, and then mass spectrometry isotope internal standard quantitation is used. The concentration of the standard is taken as the X-axis, and the peak arearatio of the standard to the internal standard is the Y-axis to establish a calibration curve. The content of the folic acid and 5-methyltetrahydrofolate is calculated. The method provided by the invention has the advantages of high sensitivity, high specificity, accuracy, simple pretreatment process, short time and greatly saved time cost.
Owner:上海可力梅塔生物医药科技有限公司 +3
Biological method for improving yield of L-5-methyltetrahydrofolate by virtue of two-plasmid engineering bacteria
InactiveCN105861534AIncrease cumulativeIncrease profitTransferasesNucleic acid vectorBiotechnologySynthesis methods
The invention provides a biological synthesis method for increasing the accumulation of L-5-methyltetrahydrofolate, an L-5-methyltetrahydrofolate synthetase system co-expressed recombinant plasmid and a construction method and application thereof. The L-5-methyltetrahydrofolate synthetase system co-expressed recombinant plasmid comprises serine hydroxymethyltransferase (SHMT) gene GlyA and methylenetetrahydrofolate reductase (MTHFR) gene MetF sequences. The biological synthesis method for increasing the accumulation of the L-5-methyltetrahydrofolate comprises the following steps: converting for accumulating an original strain of the L-5-methyltetrahydrofolate to obtain a recombinant strain by virtue of the L-5-methyltetrahydrofolate synthetase system co-expressed recombinant plasmid, and fermenting the recombinant strain. The accumulation of the L-5-methyltetrahydrofolate in the recombinant strain in a final fermentation product is remarkably higher than that of the original strain, the utilization rate of the raw material is increased, and production cost and energy consumption are reduced.
Owner:CHINA PHARM UNIV
Methods of producing edible fungi containing activated folates and nutritional supplements containing activated folates
ActiveUS20080292596A1Easy to ingestPromote absorptionOrganic active ingredientsBiocideSoftgelNutrition supplementation
Owner:JARROW FORMULAS INC
Methods of producing edible fungi containing activated folates and nutritional supplements containing activated folates
ActiveUS7354590B2Easy to ingestPromote absorptionOrganic active ingredientsBiocideSoftgelNutrition supplementation
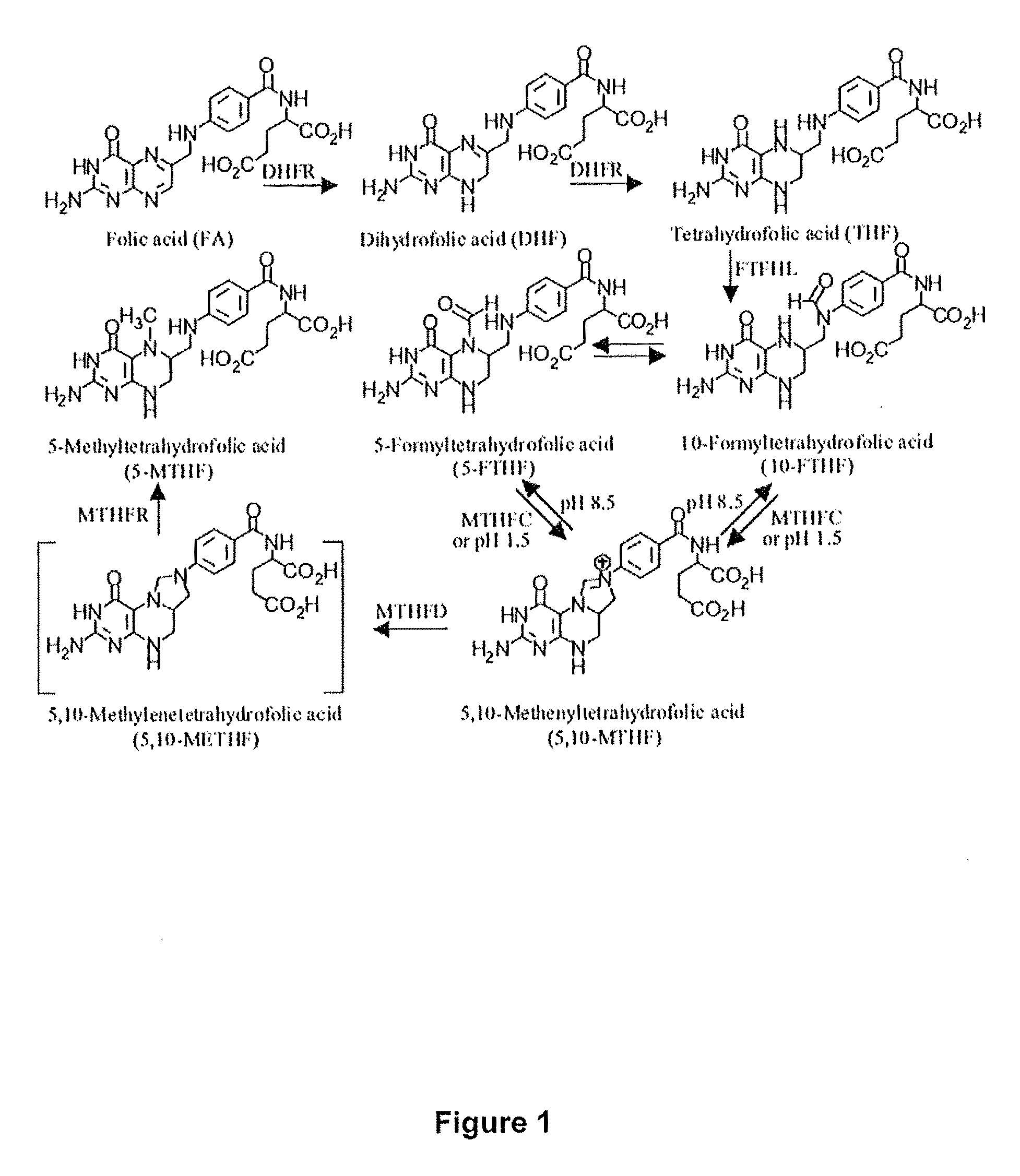
Methods are described for producing reduced, methylated, biologically active forms of folate, including 5-methyltetrahydrofolate (i.e. 5-methyltetrahydropteroylglutamic acid), 5-formyltetrahydrofolate, 10-formyltetrahydrofolate and tetrahydrofolate. Edible mushroom-producing fungi are cultivated to enhance the uptake of pteroylmonoglutamate (synthetic folate) into edible portions of the plants. The mushroom-producing fungi reduce and methylate the pteroylmonoglutamates into activated folates. The cultivated mycelia or mushrooms of the mushroom-producing fungi are harvested, and may be consumed directly or processed into oral dosage formats (capsules, tablets, softgels, powders, gel packets, liquids, nutritional bars, beverages) for use as functional foods or nutritional supplements.
Owner:JARROW FORMULAS INC
Method for Assessment of Folate Phenotypes, Disease Risk and Response to Therapy
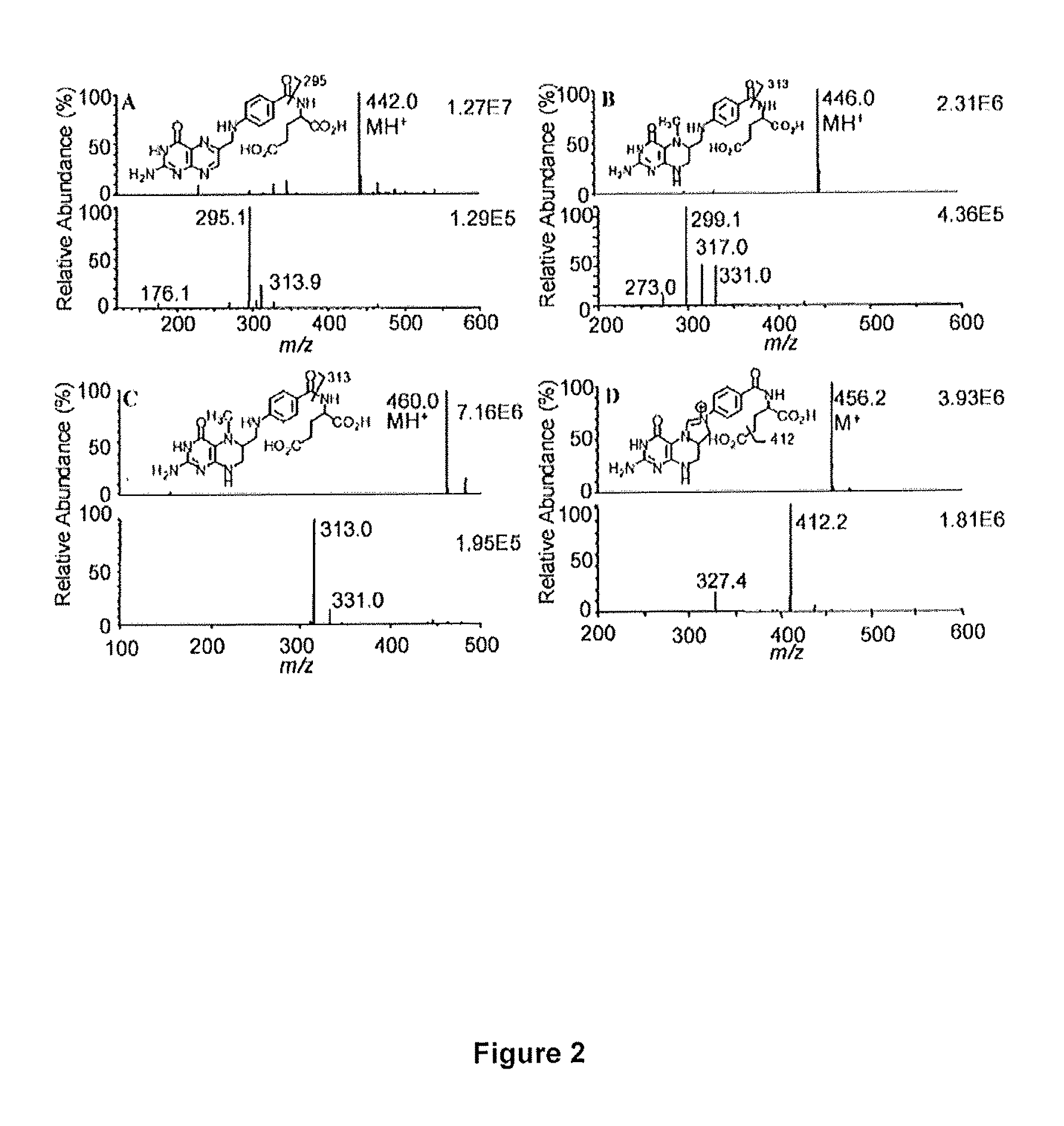
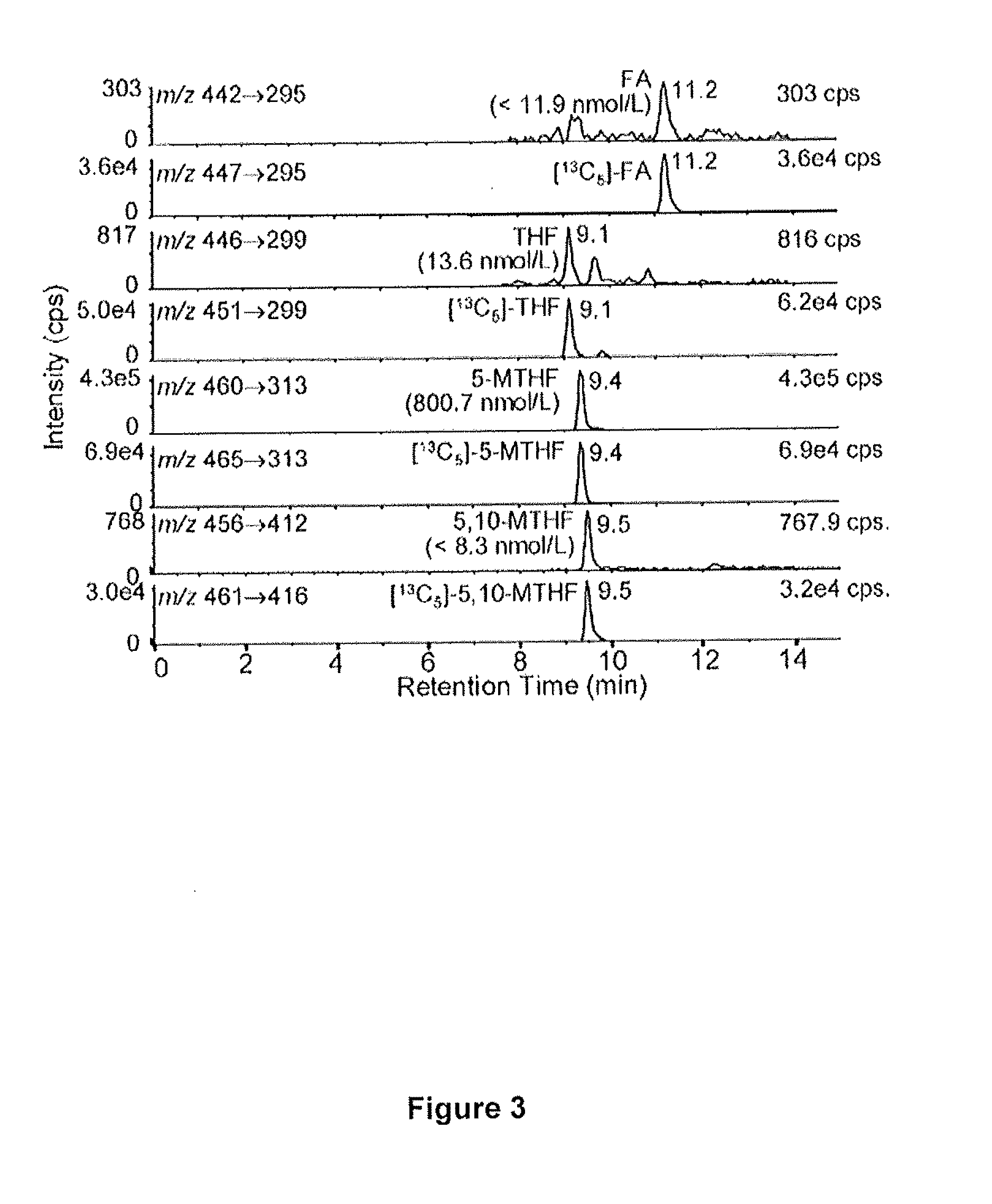
The invention provides a method for measuring the levels of 5-methyltetrahydrofolate (5-MTHF), tetrahydrofolate (THF), and 5,10-MTHF in a biological sample. The method includes employing an isotope dilution liquid chromatography-multiple reaction monitoring / mass spectrometry (LC-MRM / MS) methodology.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
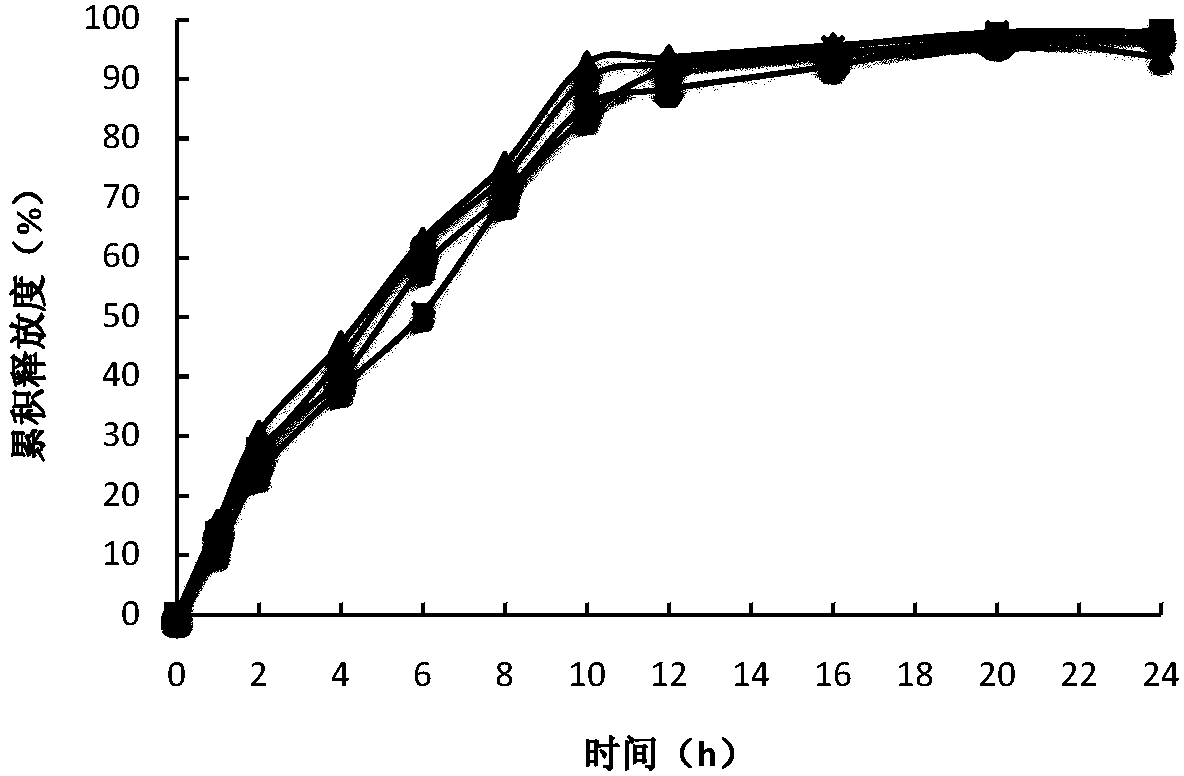
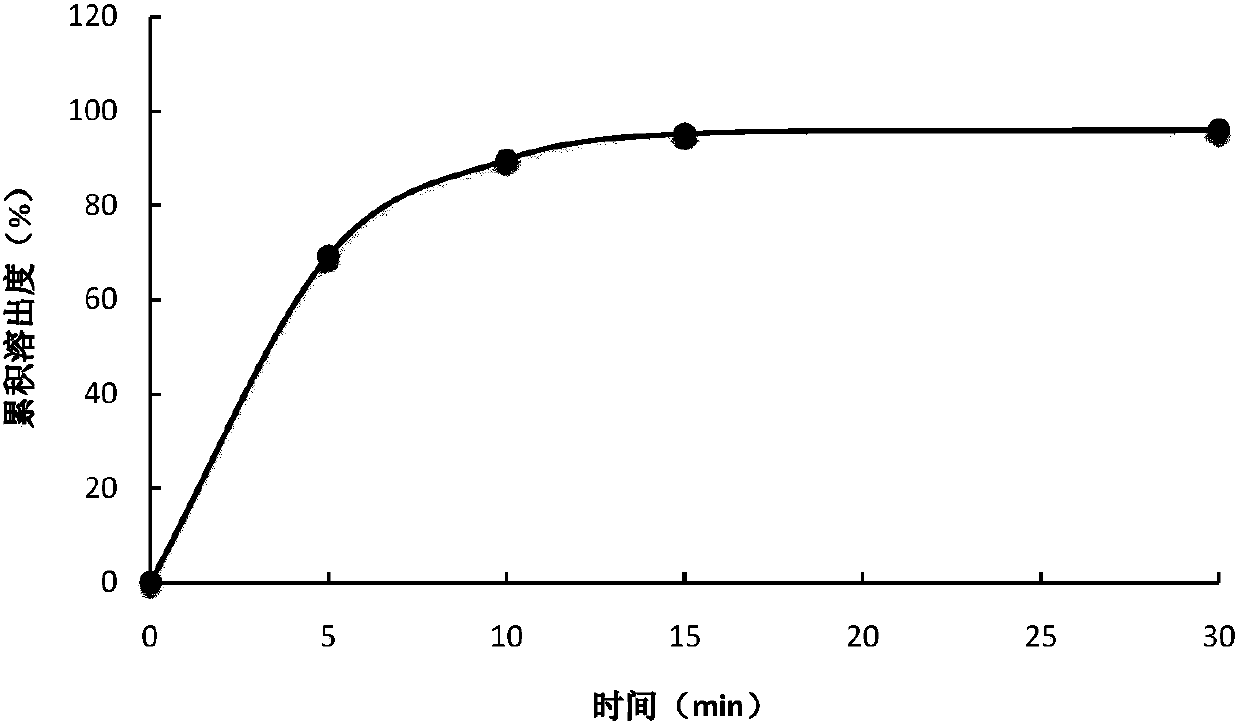
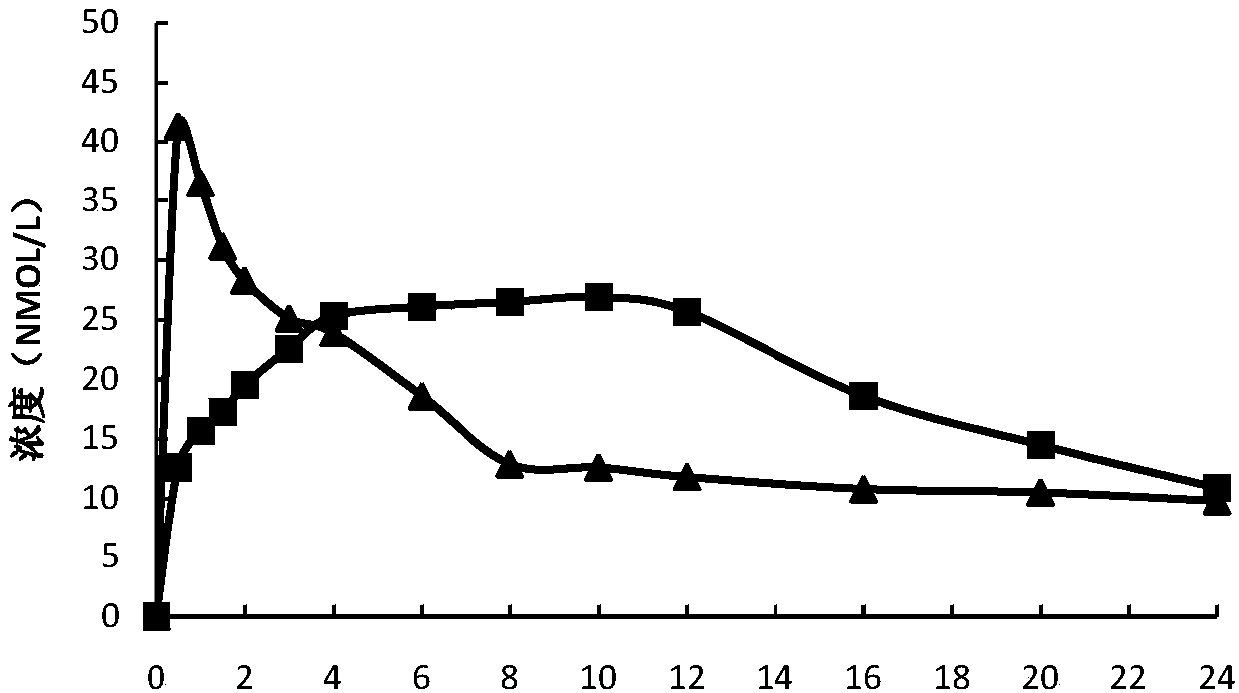
Controlled release preparation containing 5-methyltetrahydrofolate
ActiveCN109939077AGood curative effectAvoid the "peak" and "trough" phenomenon of blood drug concentrationOrganic active ingredientsPill deliveryDosing FrequencyOsmotic pump
The invention relates to a controlled release preparation containing 5-methyltetrahydrofolate. The controlled release preparation comprises a 5-methyltetrahydrofolate osmotic pump tablet core and a polymeric semi-permeable membrane coating layer. The semi-permeable membrane coating layer accounts for 2-15% of the weight of the tablet core, and the 5-methyltetrahydrofolate osmotic pump tablet corecan be a single-layer tablet or a double-layer tablet. By means of the preparation, 5-methyltetrahydrofolate can be slowly and stably released for a long time, the dosing frequency is reduced, and themedication compliance of a patient is improved.
Owner:SHENZHEN AUSA PHARM CO LTD +1
Preparation method for 5-methyltetrahydrofolate basic amino acid salt compound, 5-methyltetrahydrofolate basic amino acid salt compound and application thereof
InactiveCN104356134AImprove solubilityImprove stabilityOrganic active ingredientsNervous disorderFood additiveSolubility
The invention discloses a preparation method for a 5-methyltetrahydrofolate basic amino acid salt compound. The method comprises the following step of stirring basic amino acid and 5-methyltetrahydrofolate to prepare the 5-methyltetrahydrofolate basic amino acid salt compound under the action of a solvent. The invention also discloses the 5-methyltetrahydrofolate basic amino acid salt compound prepared by the preparation method and the application of the compound as a food additive, a nutritional supplement and a medicament. The 5-methyltetrahydrofolate basic amino acid salt compound has stable chemical properties at room temperature, and the solubility of the 5-methyltetrahydrofolate basic amino acid salt compound in water is over 50 percent.
Owner:GUANGDONG WEILUO BIOTECH
Resolution for 5-methyltetrahydrofolic acid and salifying method thereof
InactiveCN101143863BOptically-active compound separationOrganic racemisationCalcium hydroxideAlkaline earth metal
The invention brings forward a preparation method, which utilizes organic alkali Alpha-phenylethylamine to obtain (6S)-5-methyltetrahydrofolate by splitting racemate (6R, S)-5-methyltetrahydrofolate, and utilizes the hydroxide of alkaline earth, particularly calcium hydroxide, to generate (6S)-5-methyltetrahydrofolate calcium salt.
Owner:NAN JING RHINE PHARM TECH
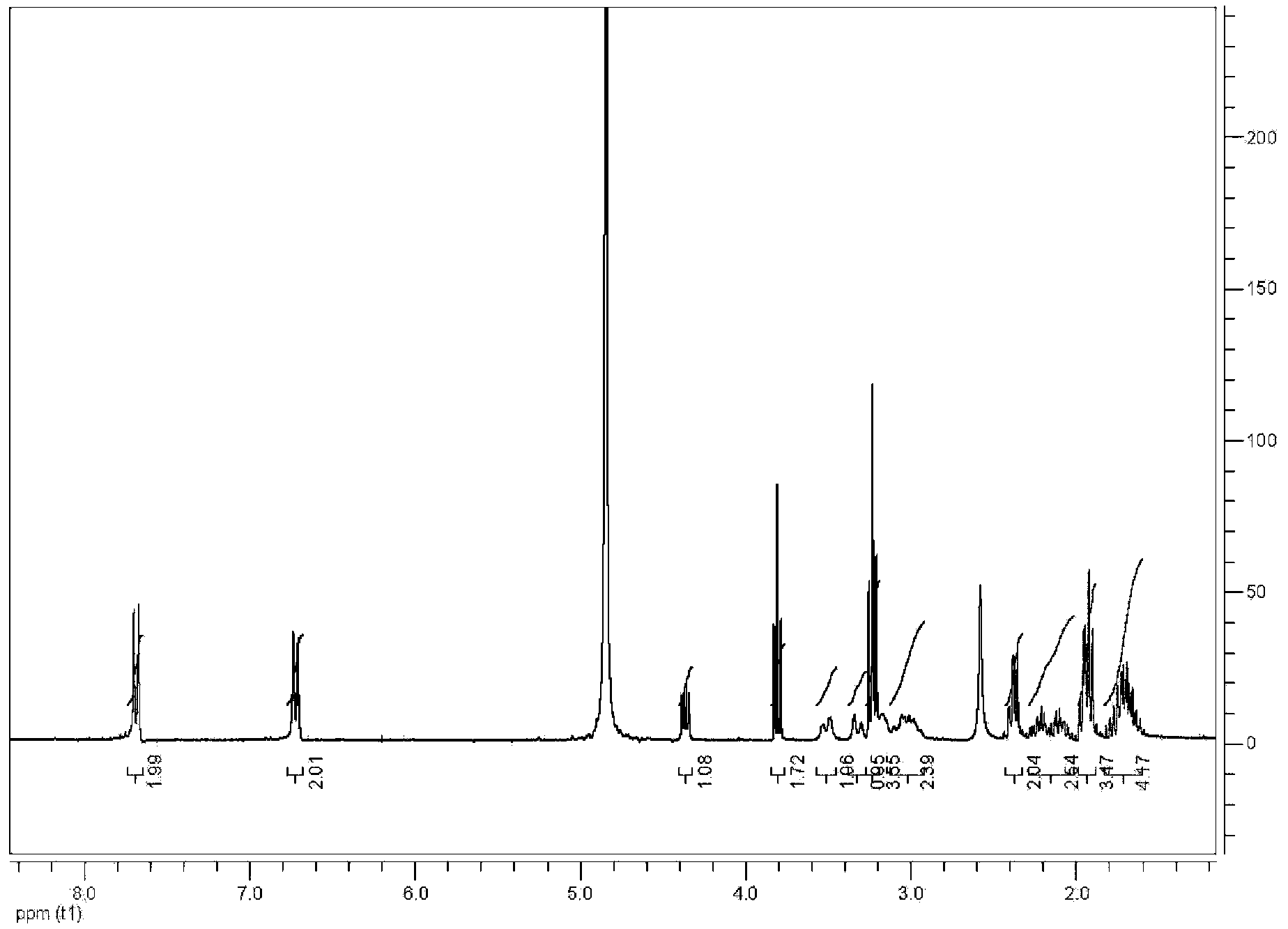
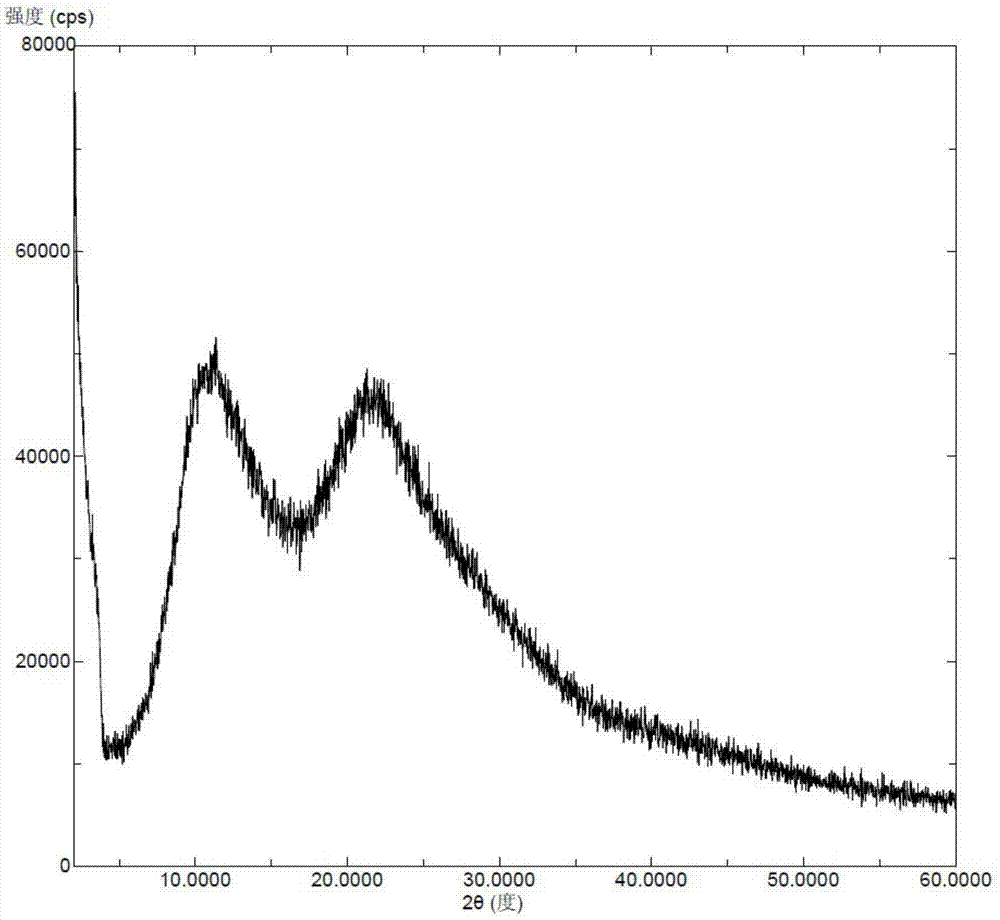
Stable 5-methyltetrahydrofolate crystal form and preparation method thereof
ActiveCN102775407ANo pollution in the processThe reaction steps are simpleOrganic active ingredientsNervous disorderAlkaline earth metalDissolution
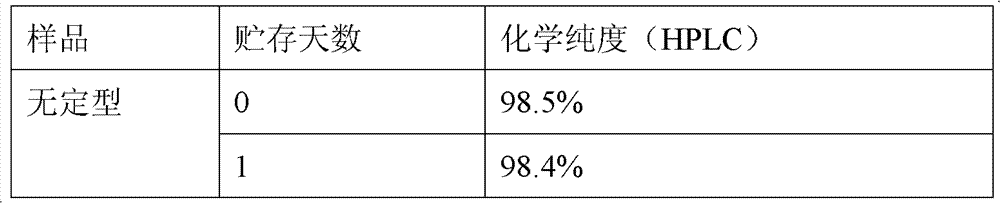
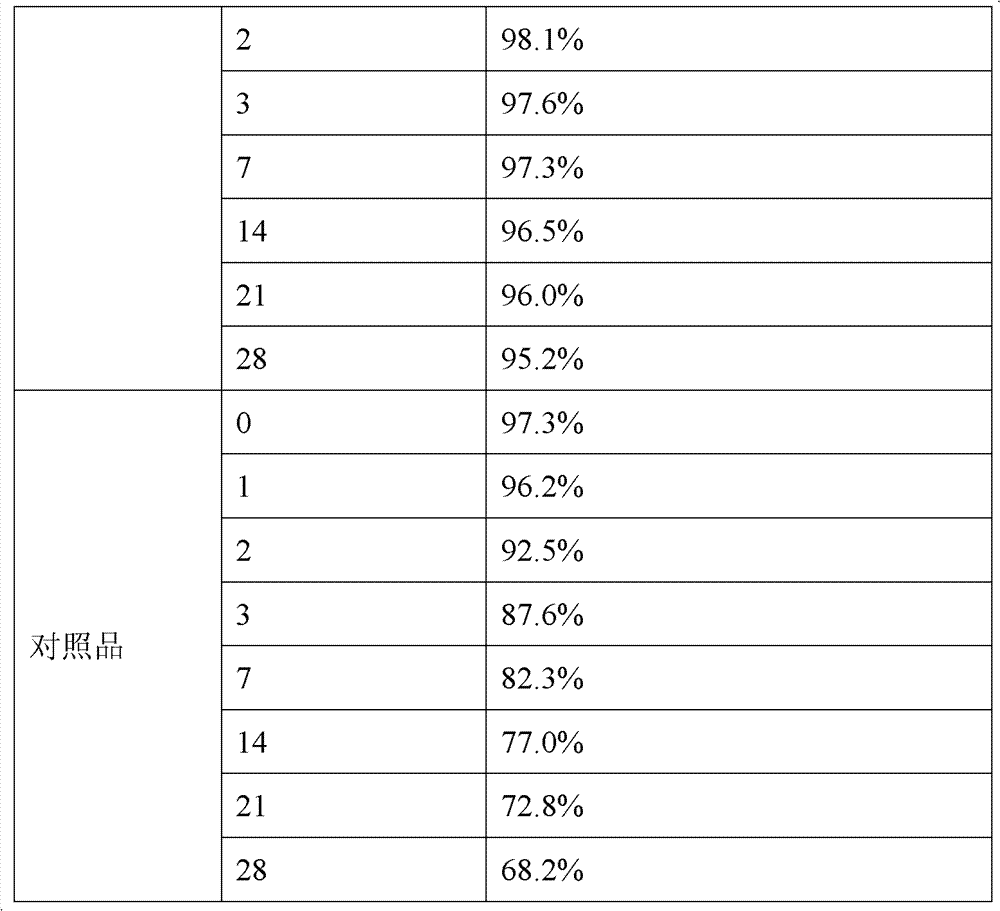
The invention discloses a stable amorphous 5-methyltetrahydrofolate salt and a preparation method of the 5-methyltetrahydrofolate salt; the method comprises the steps of: firstly, neutralizing 5-methyltetrahydrofolate with alkali in aqueous medium until complete dissolution, adding alkaline-earth metal salt or alkaline-earth metal salt solution into the neutralized solution, controlling the temperature of the mixed solution to be within the range of 0-20 DEG C, carrying out ultrasonic treatment, and separating out the amorphous 5-methyltetrahydrofolate salt. The amorphous 5-methyltetrahydrofolate salt has higher stability; and the preparation method is simple in reaction steps, short in time, free from pollution, high in purity, good in stability and more suitable for industrial scale application.
Owner:北京金康和信药业科技有限公司
Angiotensin II receptor blocker/thiazide diuretics/5-methyltetrahydrofolate pharmaceutical composition
InactiveCN103721259AGood synergyAvoid damageOrganic active ingredientsUrinary disorderCurative effectThiazide diuretic
The invention relates to a pharmaceutical composition containing an angiotensin II receptor blocker (ARB) / thiazide diuretics / 5-methyltetrahydrofolate and an application of the pharmaceutical composition. The pharmaceutical composition comprises the ARB with pharmaceutical dosage, the thiazide diuretics with pharmaceutical dosage, the 5-methyltetrahydrofolate with pharmaceutical dosage, and a pharmaceutically acceptable carrier. The invention provides the application of the pharmaceutical composition in the preparation of medicines used for treating hypertension as well as preventing, treating or delaying target-organ damage caused by the hypertension. The invention also provides the application of the pharmaceutical composition in the preparation of medicines used for reducing the risk of cardiovascular and cerebrovascular events caused by the hypertension. Through the implementation of the invention, the pharmaceutical composition with the specific purposes is provided for patients, the medication compliance of the patients also can be increased, and the curative effect is improved.
Owner:SHENZHEN AUSA PHARMA
Method for preparing L-5-calcium methyl tetrahydrofolate through enzymic method
InactiveCN109627244AMild responseHigh yieldOrganic chemistryFermentationEnzymatic synthesisDihydrofolic acid
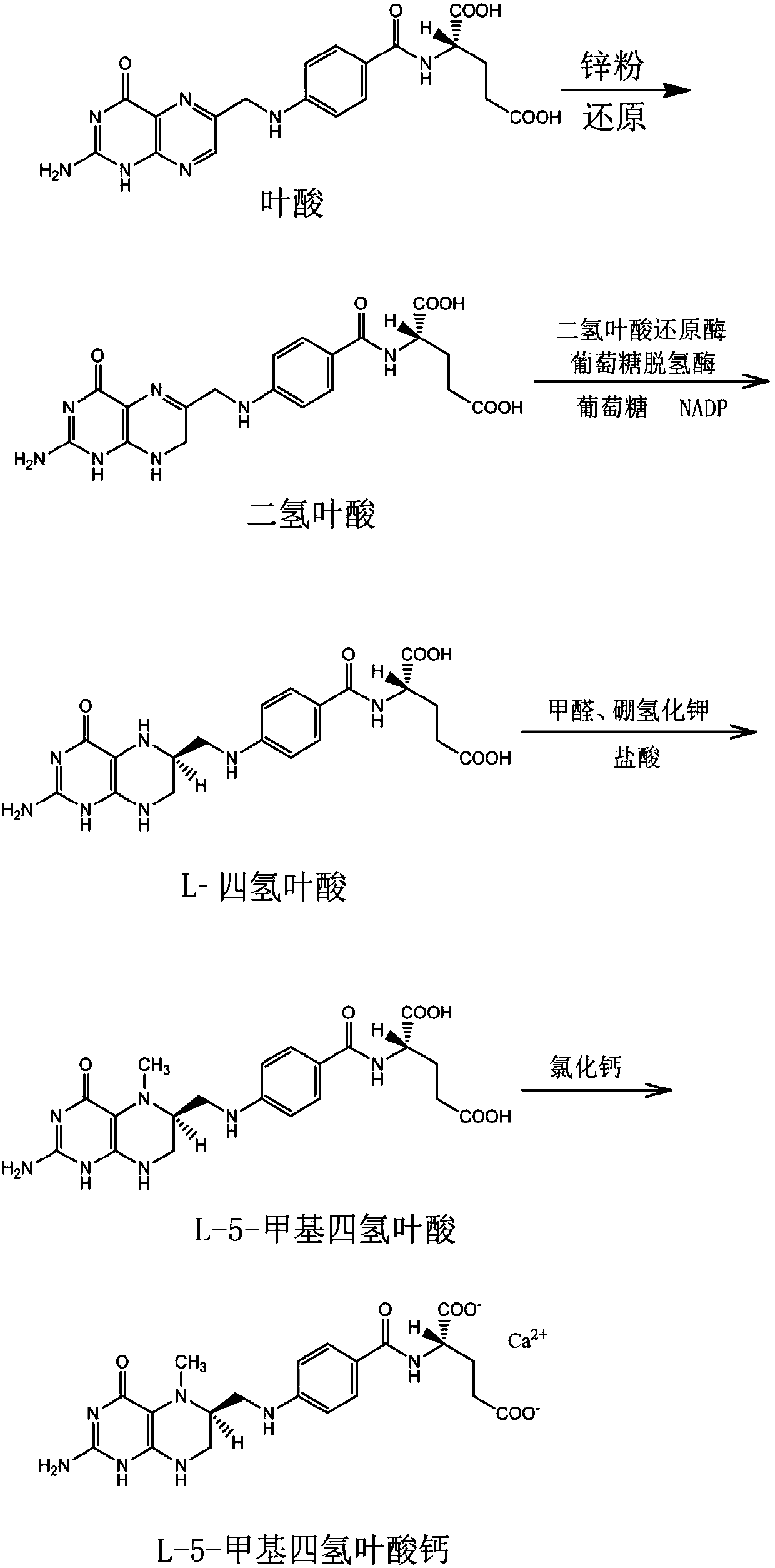
The invention belongs to the technical field of enzymatic synthesis, and relates to a method for preparing L-5-calcium methyl tetrahydrofolate through an enzymic method. The method comprises the following steps that 1, folic acid serves as the raw material, and zinc powder is used for making zinc powder reduced to be dihydrofolic acid; 2, dihydrofolic acid reductase and glucose dehydrogenase are added in dihydrofolic acid, and a reaction is conducted to obtain L-tetrahydrofolic acid; 3, formaldehyde and hydroboron are added in L-tetrahydrofolic acid, and a reaction is conducted for obtaining L-5-methyltetrahydrofolate; 4, L-5-methyltetrahydrofolate is salified, and L-5-calcium methyl tetrahydrofolate is obtained. Accordingly, the effective method for preparing L-5-calcium methyl tetrahydrofolate through the enzymic method is provided, the preparation method is free of splitting, mild in reaction, high in yield, high in product purity and friendly to environment, and has a very good industrial prospect.
Owner:ZHEJIANG SHENGDA BIO PHARM
Fruit and vegetable active folic acid capable of reducing homocysteine
The invention discloses a fruit and vegetable active folic acid capable of reducing homocysteine. The fruit and vegetable active folic acid consists of main raw materials and auxiliary raw materials, wherein the main raw materials and the auxiliary raw materials are blended according to a raw material quality; the main raw materials comprise the following raw material powders in corresponding parts by weight: 300-800ug of 5-methyltetrahydrofolate, 100-300mg of glycine betaine, 0.1-1mg of vitamin B6, 0.1-2ug of vitamin B12, 3-10mg of vitamin E, 1-10mg of active Fe and 2-8mg of active zinc; and the auxiliary raw materials comprise the following raw materials in corresponding parts by weight: seaweed powder, composite natural fruit and vegetable powder, anhydrous dextrose powder, black soya bean powder and instant yeast powder are evenly mixed to obtain powder of which the specification is 2g / bag. The active folic acid disclosed by the invention obtains appointed nutritional ingredients from natural foods or vegetables, and meets a situation that the active folic acid is easy in metabolic absorption and the mutation possibility of a user is lowered while the homocysteine is reduced.
Owner:医维他(山东)健康产业集团有限公司
Method for preparing (6S)-5-methyltetrahydrofolate calcium
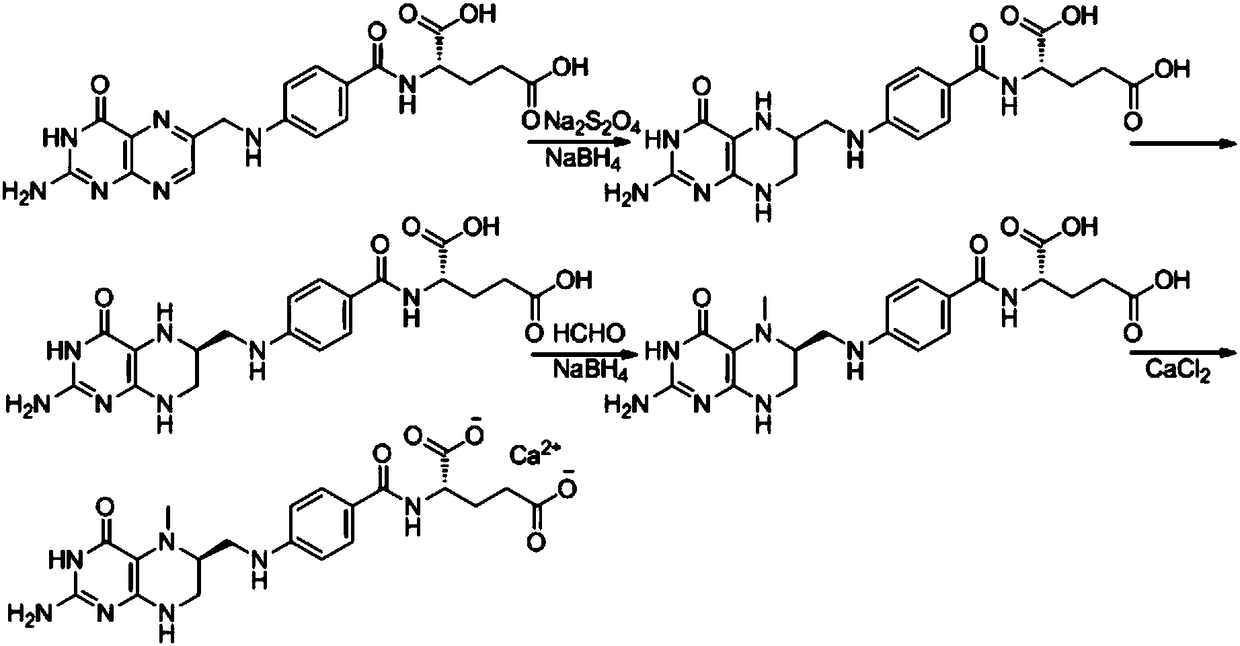
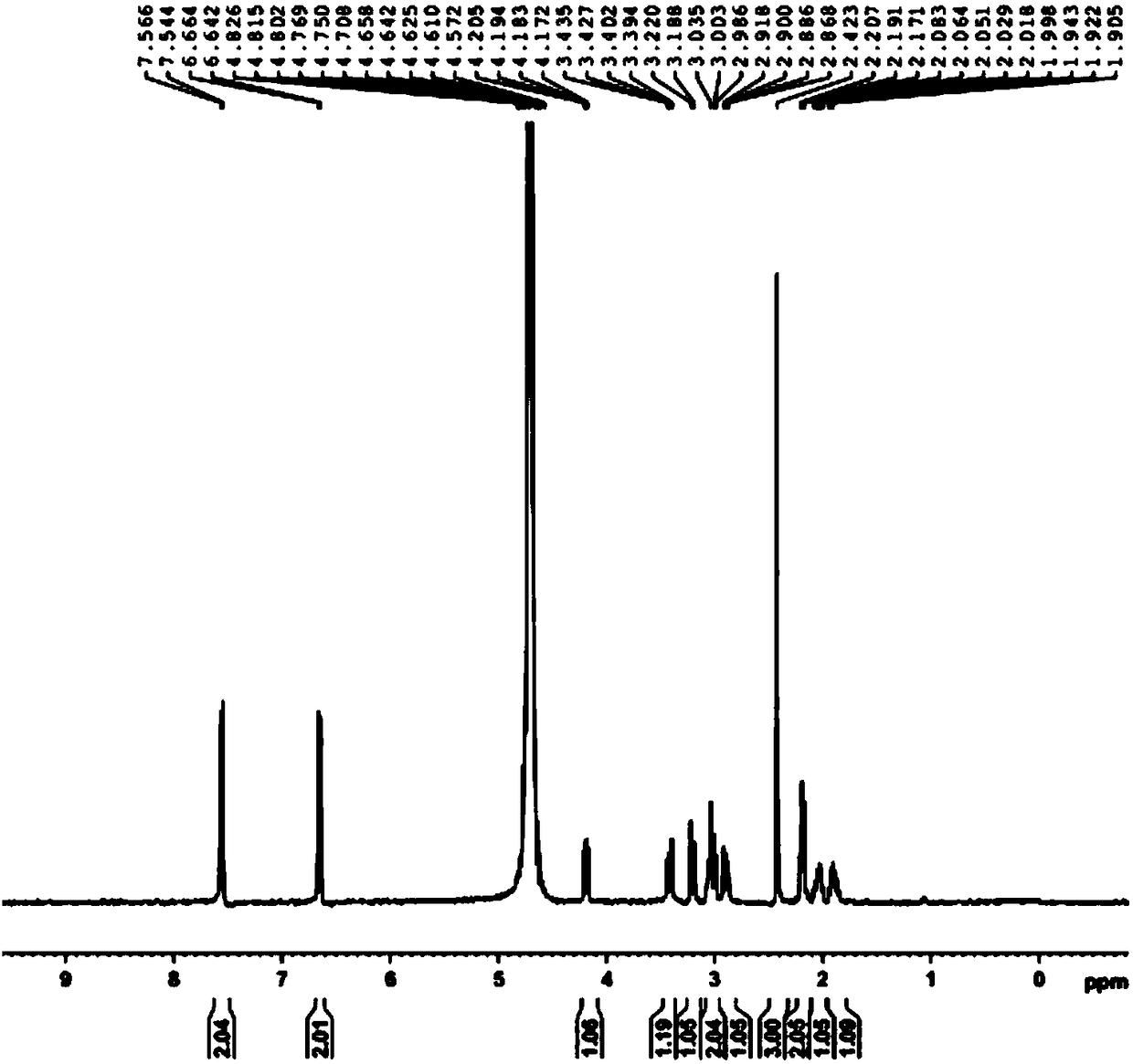
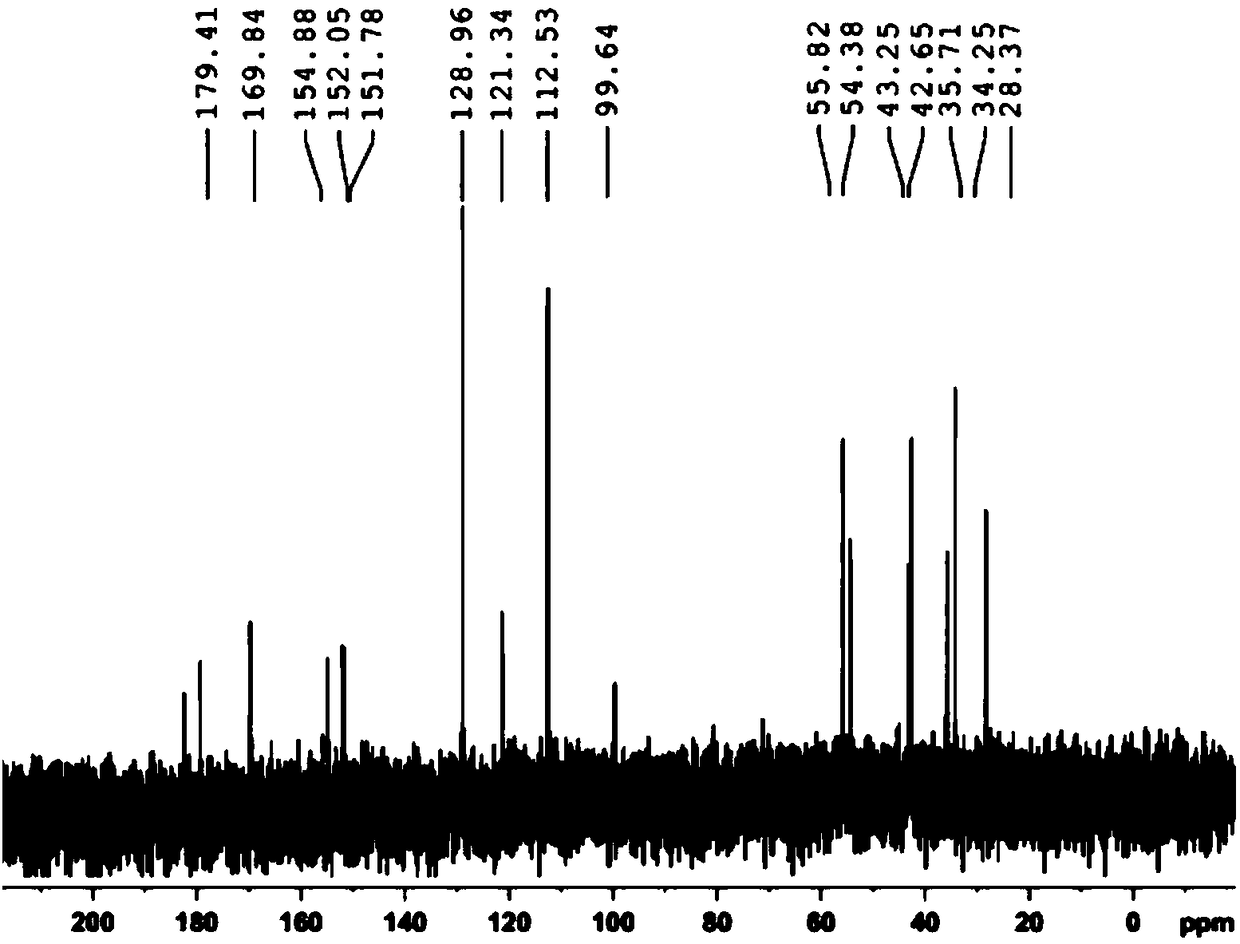
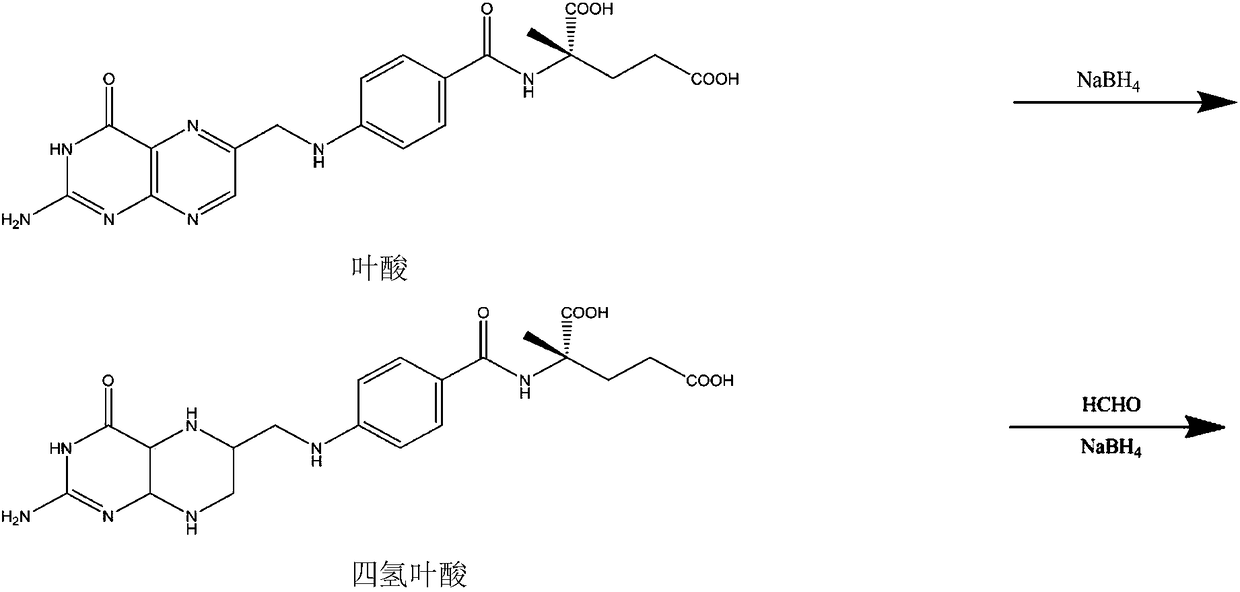
The invention belongs to the technical field of organic synthesis and relates to a method for preparing (6S)-5-methyltetrahydrofolate calcium. The method comprises the following steps: in an alkali environment, adding sodium borohydride, dropping a mixture of folic acid and water at a normal temperature, and performing a heating reaction to generate tetrahydrofolic acid; firstly, dropping a formaldehyde and sodium borohydride solution to implement a reaction, after the reaction is completed, cooling, leaving to stand for a certain time, and performing suction filtration to remove boron; dropping a calcium chloride solution into filtrate, performing stirring crystallization, and performing suction filtration so as to obtain 5-methyltetrahydrofolate calcium; mixing the obtained crystal withwater, adding EDTA (Ethylene Diamine Tetraacetic Acid) and S-(-)-N-ethyl-2-aminomethyl pyrrolidine, performing a resolution reaction, after the reaction is completed, cooling to separate an R-type antipode and an S-type antipode in sequence, continuously dropping the calcium chloride solution into the obtained S-type antipode, performing stirring crystallization, and performing suction filtration,thereby obtaining (6S)-5-methyltetrahydrofolate calcium. The (6S)-5-methyltetrahydrofolate calcium produced by using the method provided by the invention is high in product purity, stable in reactionand good in environment protection.
Owner:ZHEJIANG SHENGDA BIO PHARM
Homocysteine diagnosis/determination reagent (kit) and homocysteine concentration determination method
InactiveCN101762522AFast measurementImprove accuracyMicrobiological testing/measurementColor/spectral properties measurementsEnzymatic ColorimetryWavelength
The invention relates to a homocysteine diagnosis / determination reagent (kit) using enzyme-colorimetry and enzyme-linked method technology. Meanwhile, the invention also relates to a homocysteine concentration determination method, reagent composition and component, which belongs to the technical filed of medical test and determination. The reagent (box) of the invention mainly includes: buffer solution, coenzyme, 5-methyltetrahydrofolate, methionine synzyme, dihydrofolate reductase and stabilizing agent; samples and reagent are mixed according to a certain volume ratio to generate a series of enzymatic reactions; then the reactants are arranged under an ultraviolet / visible light analyzer to detect the increasing degree of absorbance at the 340nm position of a dominant wave so as to measure and calculate the concentration of the homocysteine.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
METHODS OF PRODUCING NUTRITIONAL SUPPLEMENTS USING MUSHROOMS FROM THE SPECIES Poria cocos OR Flammulina velutipes
InactiveUS20140271704A1Readily apparentEasy to ingestBiocideLichen medical ingredientsSoftgel5-Methyltetrahydropteroylglutamic acid
Methods are described for producing reduced, methylated, biologically active forms of folate, including 5-methyltetrahydrofolate (i.e. 5-methyltetrahydropteroylglutamic acid), 5-formyltetrahydrofolate, 10-formyltetrahydrofolate and tetrahydrofolate. Edible mushroom-producing fungi are cultivated to enhance the uptake of pteroylmonoglutamate (synthetic folate) into edible portions of the plants. The mushroom-producing fungi reduce and methylate the pteroylmonoglutamates into activated folates. The cultivated mycelia or mushrooms of the mushroom-producing fungi are harvested, and may be consumed directly or processed into oral dosage formats (capsules, tablets, softgels, powders, gel packets, liquids, nutritional bars, beverages) for use as functional foods or nutritional supplements.
Owner:JARROW FORMULAS INC
New application of pharmaceutical composition of metformin and 5-methyltetrahydrofolate
InactiveCN103263419AReduce dosageEliminate side effectsOrganic active ingredientsMetabolism disorderDiabetic complicationBlood sugar
The invention relates to new application of a pharmaceutical composition containing officinal dose of metformin and 5-methyltetrahydrofolate for preventing and treating complications of diabetic microangiopathy. The pharmaceutical composition is characterized in that the dose of the metformin is 250-1000 mg, and the dose of the 5-methyltetrahydrofolate is 0.4-1.6 mg. The application has the advantages that the pharmaceutical composition has obvious beneficial effects of preventing and treating the diabetic microangiopathy on the basis that the blood sugar is effectively lowered, so that the pharmaceutical composition is beneficial for preventing or delaying diabetic complications and lowering the disability rate and the fatality rate.
Owner:SHENZHEN AUSA PHARMA
Composition of L-5-methyltetrahydrofolate or pharmaceutically acceptable salt thereof and pharmaceutical adjuvants and preparing method thereof
InactiveCN107028950ALow priceImprove solubilityOrganic active ingredientsNervous disorderDispersityBioavailability
Owner:CHANGZHOU AINUOXINRUI PHARMA LTD
Lactococcus lactis accumulating L-5-methyltetrahydrofolate and construction method thereof
The invention discloses lactococcus lactis accumulating L-5-methyltetrahydrofolate and a construction method thereof, and belongs to the field of genetic engineering. The recombinant lactococcus lactis provided by the invention can realize the intracellular accumulation of L-5-methyltetrahydrofolate, and the concentration of the L-5-methyltetrahydrofolate in microbial cells can reach 65 [mu]g / g DCW after 10 hours of fermentation so as to lay the foundation for further metabolic engineering of lactococcus lactis to produce L-5-methyltetrahydrofolate. The method for constructing the recombinantlactococcus lactis provided by the invention is simple, convenient to use, and has a good application prospect.
Owner:JIANGNAN UNIV
The preparation method of 1-5-methyltetrahydrofolate amino acid salt
Owner:NAN JING RHINE PHARM TECH
Homotype semicystinol diagnostic kit and homotype semicystinol concentration investigating method
InactiveCN101089603AFast measurementHigh sensitivityMaterial analysis by observing effect on chemical indicatorMicrobiological testing/measurementBuffer solutionMethyltransferase
A diafnosis kit of homocysteine consists of compositions as buffer solution; 5-methyltetrahydrofolate; methyltetrahydrodioperin-homocysteine- methyltransferase; amino acid oxidase; 10 ( carboxymethyl-N2 -acetyl) -3, 7-dual ( dimethylamino) phenothiazine (DA-67) and stabilizer. Its method for determining concentration of homosteine is also disclosed.
Owner:SUZHOU ANJ BIOTECHNOLOGY CO LTD
Multivitamin pellet containing 5-methyltetrahydrofolate
ActiveCN105748503AImprove stabilityLong validity periodOrganic active ingredientsNervous disorderAdditive ingredientVitamin B12
The invention belongs to the field of pharmaceutical preparation and discloses a multivitamin pellet containing 5-methyltetrahydrofolate.The multivitamin pellet consists of a pellet core and a coating, and the diameter of the multivitamin pellet is 200-400 micrometers.The multivitamin pellet comprises, by unit dose, 0.3-0.8mg of folic acid, 1-2mg of vitamin B, 1.5-2microgram of vitamin B12, 80-120mg of an excipient and 6-10mg of a coating agent.The multivitamin pellet containing the 5-methyltetrahydrofolate has the advantages that stability of main ingredients under conditions of high temperature, high humidity and high light can be evidently improved, and accordingly medicine validity period is prolonged.
Owner:FERGUSON WUHAN BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com