Controlled release preparation containing 5-methyltetrahydrofolate
A technology of methyltetrahydrofolate and methyltetrahydrofolate, applied in medical preparations containing active ingredients, pill delivery, organic active ingredients, etc., can solve problems affecting male fertility and improve treatment compliance Sex, improve curative effect, reduce the effect of taking times
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
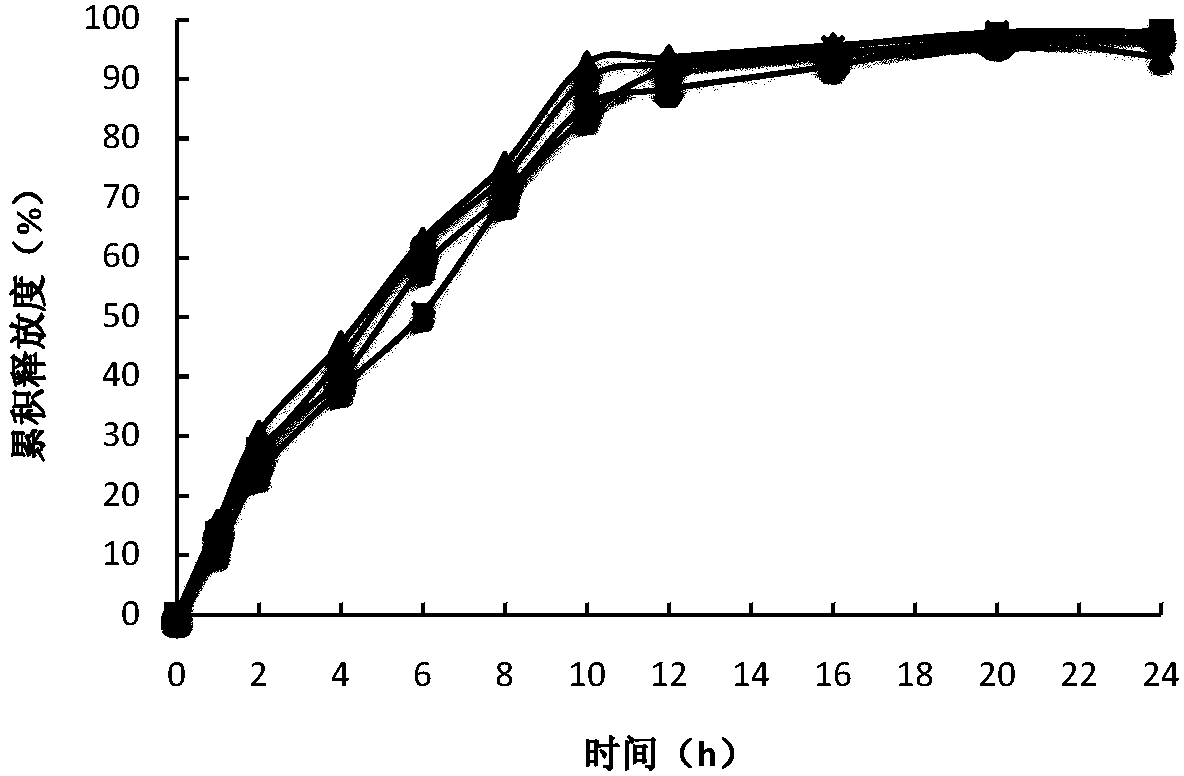
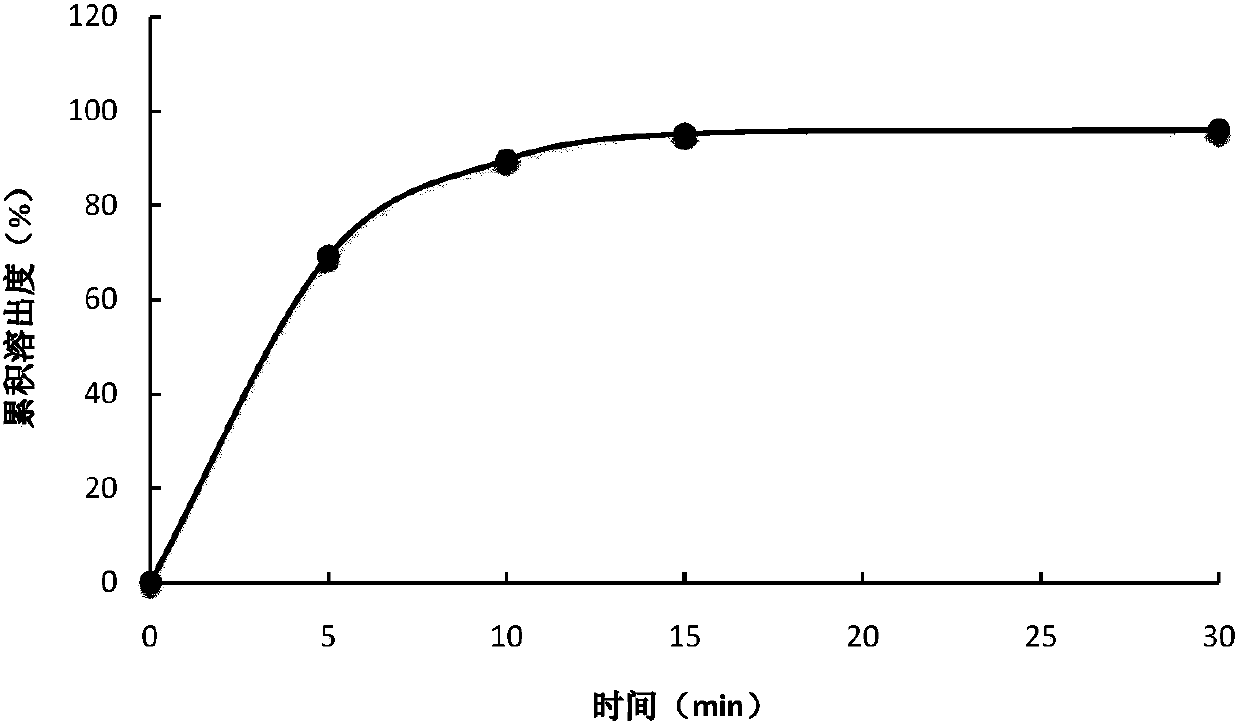
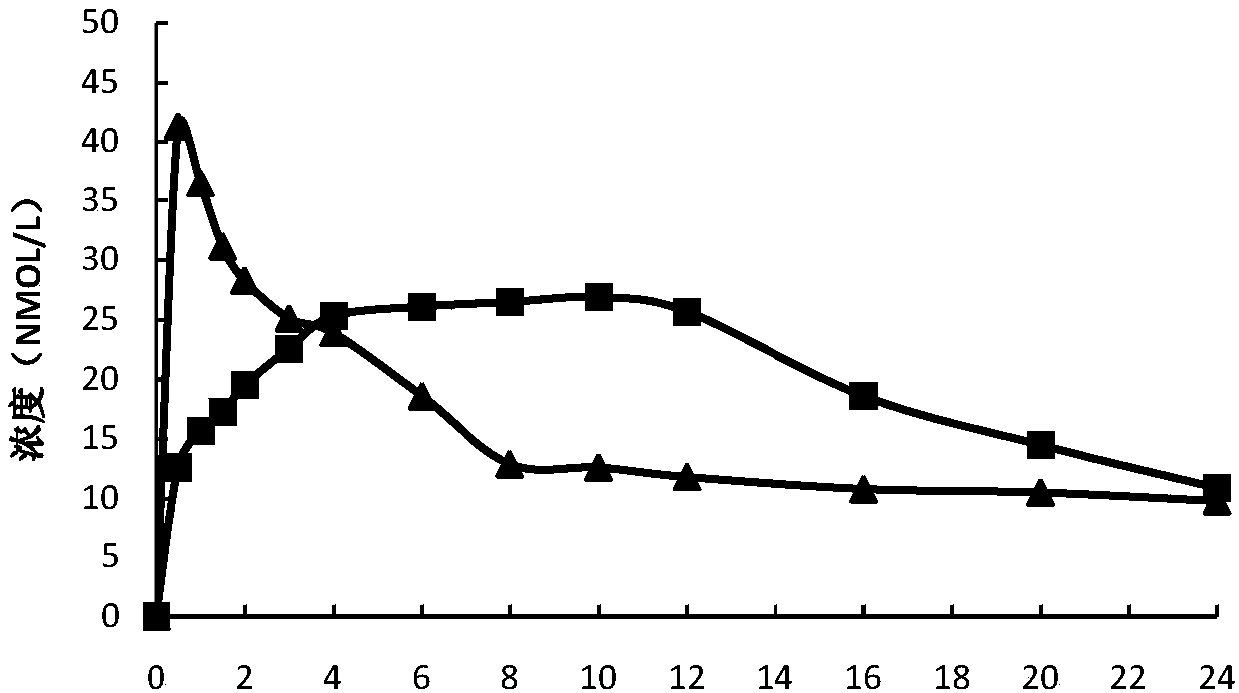
Image
Examples
Embodiment 1
[0038] Example 1: Calcium 5-Methyltetrahydrofolate Controlled Release Tablets
[0039] Single-layer tablet core prescription:
[0040]
[0041] Tablet core preparation process:
[0042] Mix microcrystalline cellulose and (6S)-5-methyltetrahydrofolate calcium, pass through a 100-mesh sieve, add lactose and sodium chloride and mix evenly, make soft material with 60% ethanol, granulate with 18-mesh sieve, 40 Dry at -45°C, granulate with 18 mesh sieve, add magnesium stearate and mix evenly, test the intermediate product, and compress into tablets.
[0043] Coating Solution Prescription:
[0044]
[0045] Coating process:
[0046] Put the tablet cores in the coating pan for coating, the flow rate of the coating solution is 5-10ml / min, the temperature of the tablet bed is 40-45°C, the rotation speed of the coating pan is 8-15rpm, until the weight gain of the outer coating of the tablet cores reaches 3.5 %, continue heating for 0.5h, then dry the coated tablet in a drying o...
Embodiment 2
[0047] Example 2: Calcium 5-Methyltetrahydrofolate Controlled Release Tablets
[0048] Single-layer tablet core prescription:
[0049]
[0050] Preparation Process:
[0051] Mix microcrystalline cellulose and (6R,S)-5-methyltetrahydrofolate calcium, pass through a 100-mesh sieve, then add pregelatinized starch and magnesium chloride and mix evenly, add 10% povidone K30 soft material, 18 Granulate with a mesh sieve, dry at 40-45°C, granulate with a 18-mesh sieve, add magnesium stearate and mix evenly, test the intermediate product, and compress into tablets.
[0052] Coating Solution Prescription:
[0053]
[0054] Coating process: put the tablet core in the coating pot for coating, the flow rate of the coating solution is 5-10ml / min, the temperature of the tablet bed is 40-45°C, the speed of the coating pot is 8-15rpm, and the coating is carried out until the core is coated Continue heating for 0.5h until the weight gain reaches 4.0%, and then dry the coated tablet in...
Embodiment 3
[0055] Example 3: Calcium 5-methyltetrahydrofolate controlled-release tablets
[0056] Single-layer tablet core prescription:
[0057]
[0058] Preparation Process:
[0059] Mix microcrystalline cellulose and (6S) 5-methyltetrahydrofolate calcium, pass through a 100-mesh sieve, then add pregelatinized starch and magnesium chloride, mix evenly, add 10% povidone K30 soft material, and 18-mesh sieve Granules, dried at 40-45°C, granulated with 18 mesh sieve, added with magnesium stearate, mixed evenly, intermediate product inspection, compressed into tablets.
[0060] Coating Solution Prescription:
[0061]
[0062] Coating process:
[0063] Put the tablet cores in the coating pan for coating, the flow rate of the coating solution is 5-10ml / min, the temperature of the tablet bed is 40-45°C, the rotation speed of the coating pan is 8-15rpm, until the weight gain of the outer coating of the tablet cores reaches 5.0 %, continue heating for 0.5h, then dry the coated tablet i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com