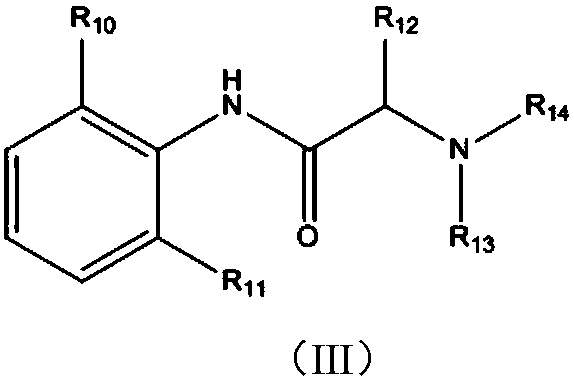
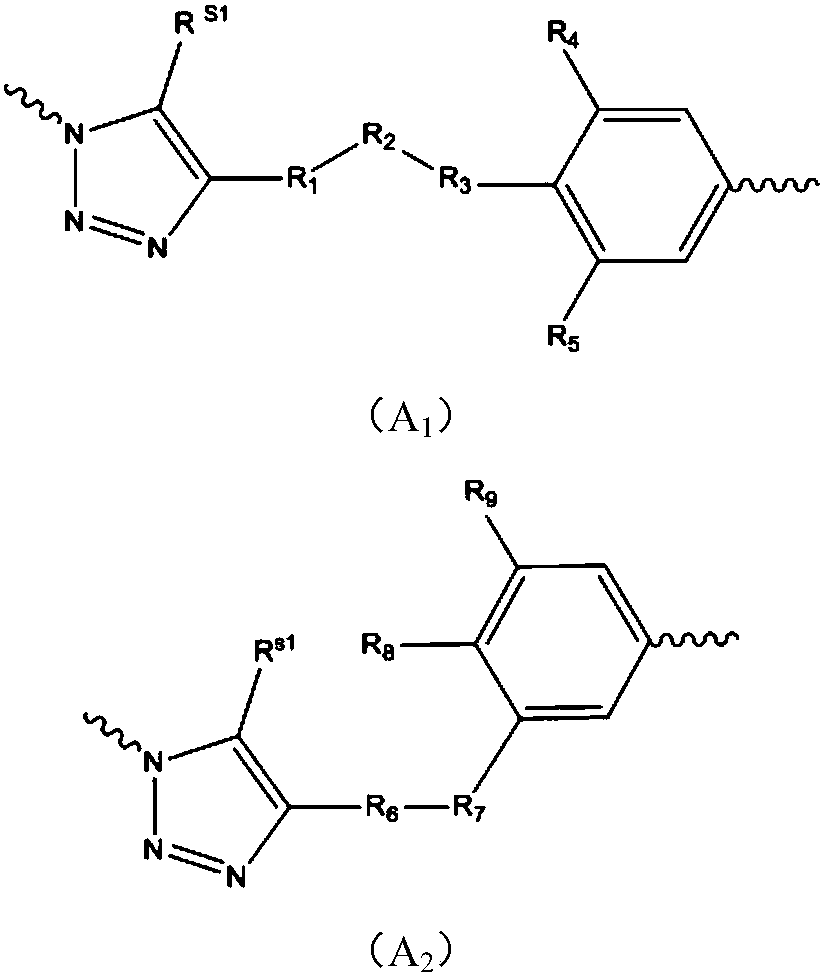
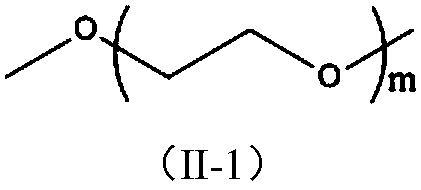
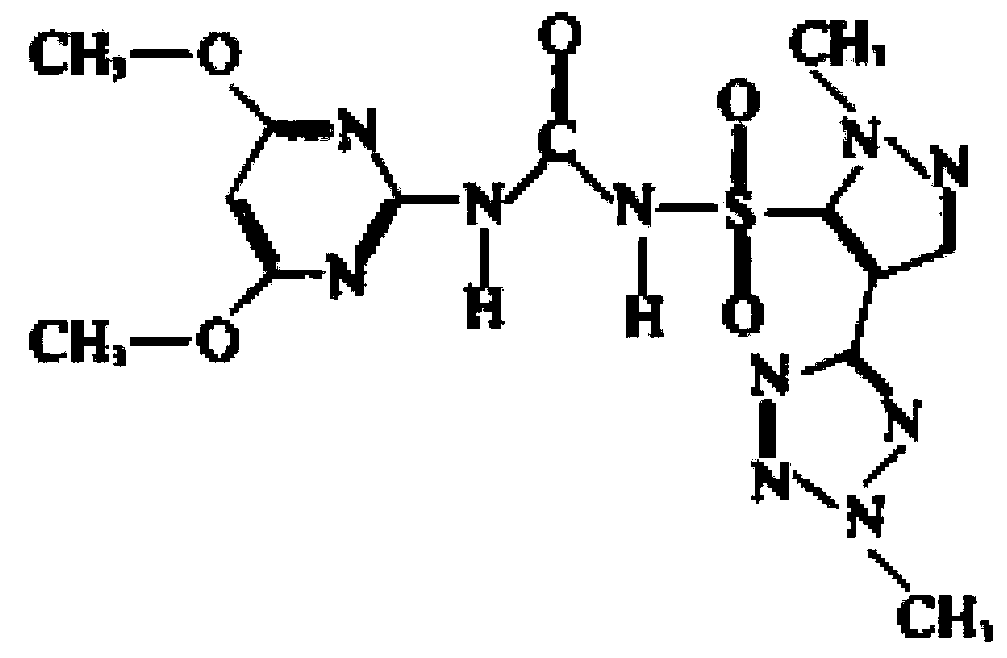
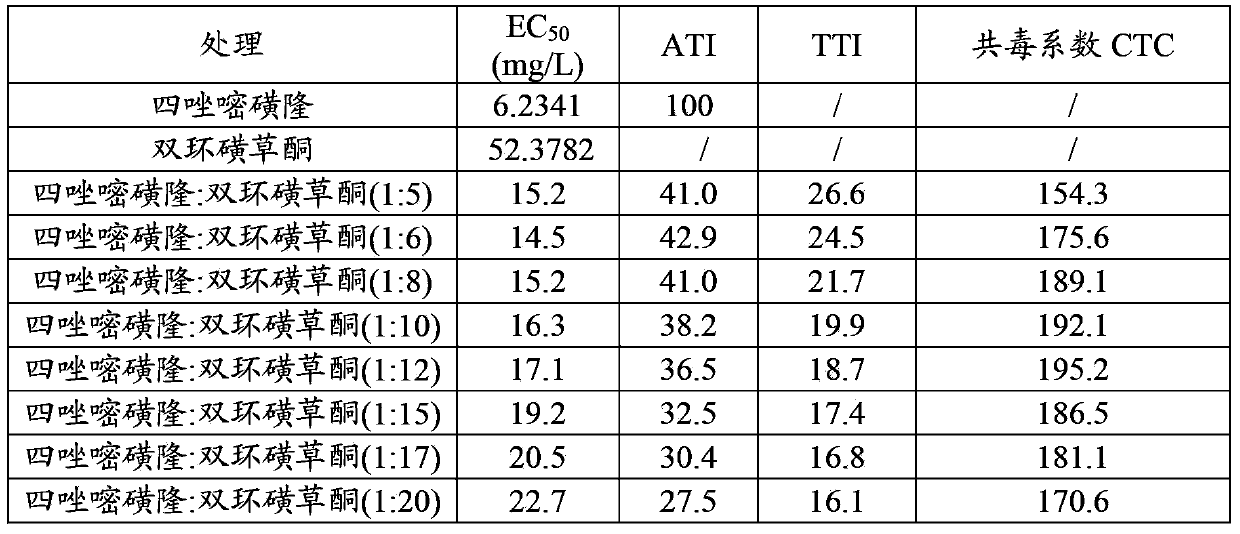
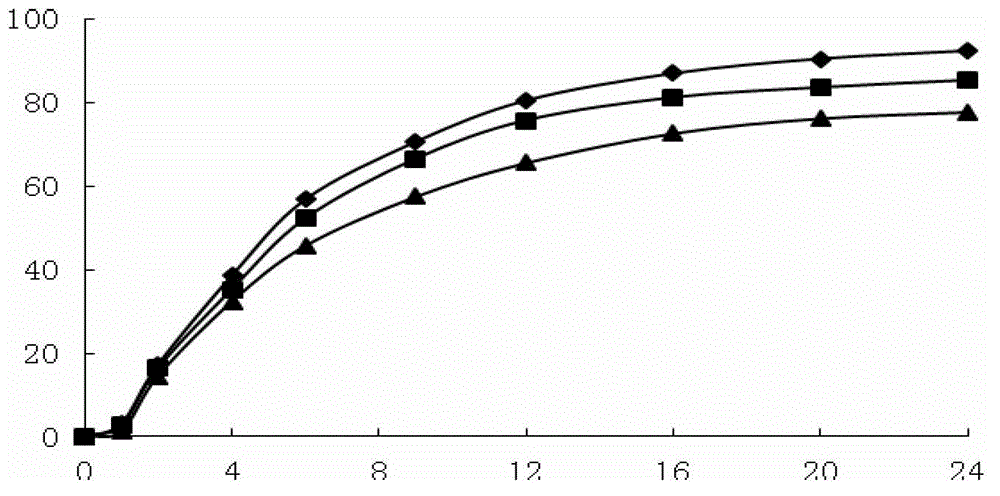
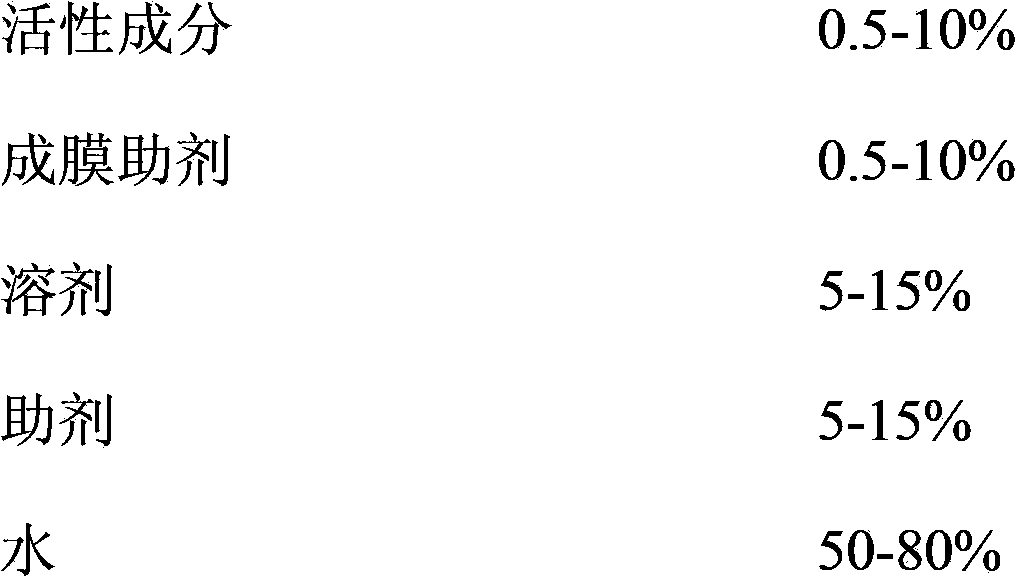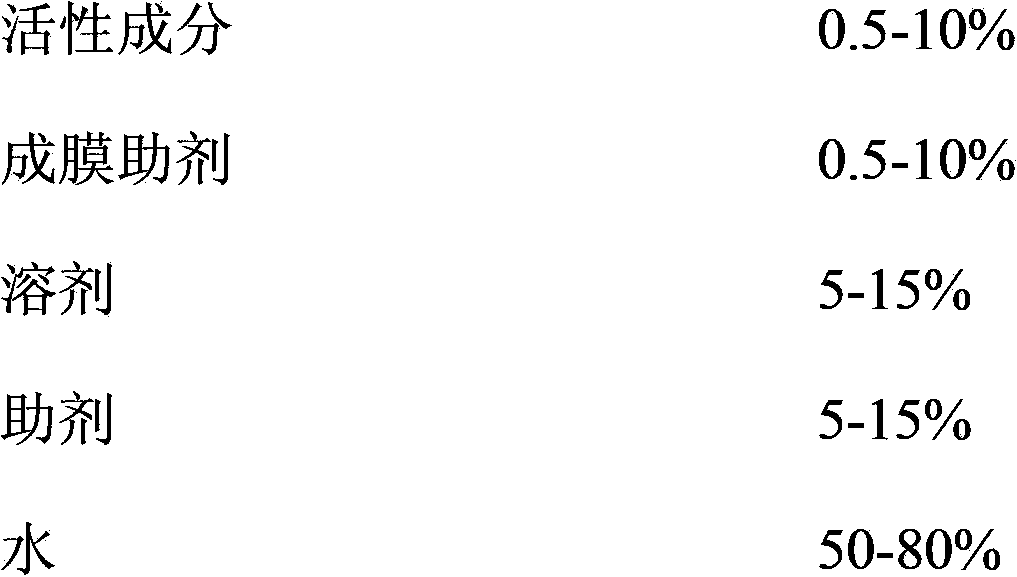Patents
Literature
54 results about "Dose Frequency" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The number of times a substance is administered within a specific time period.
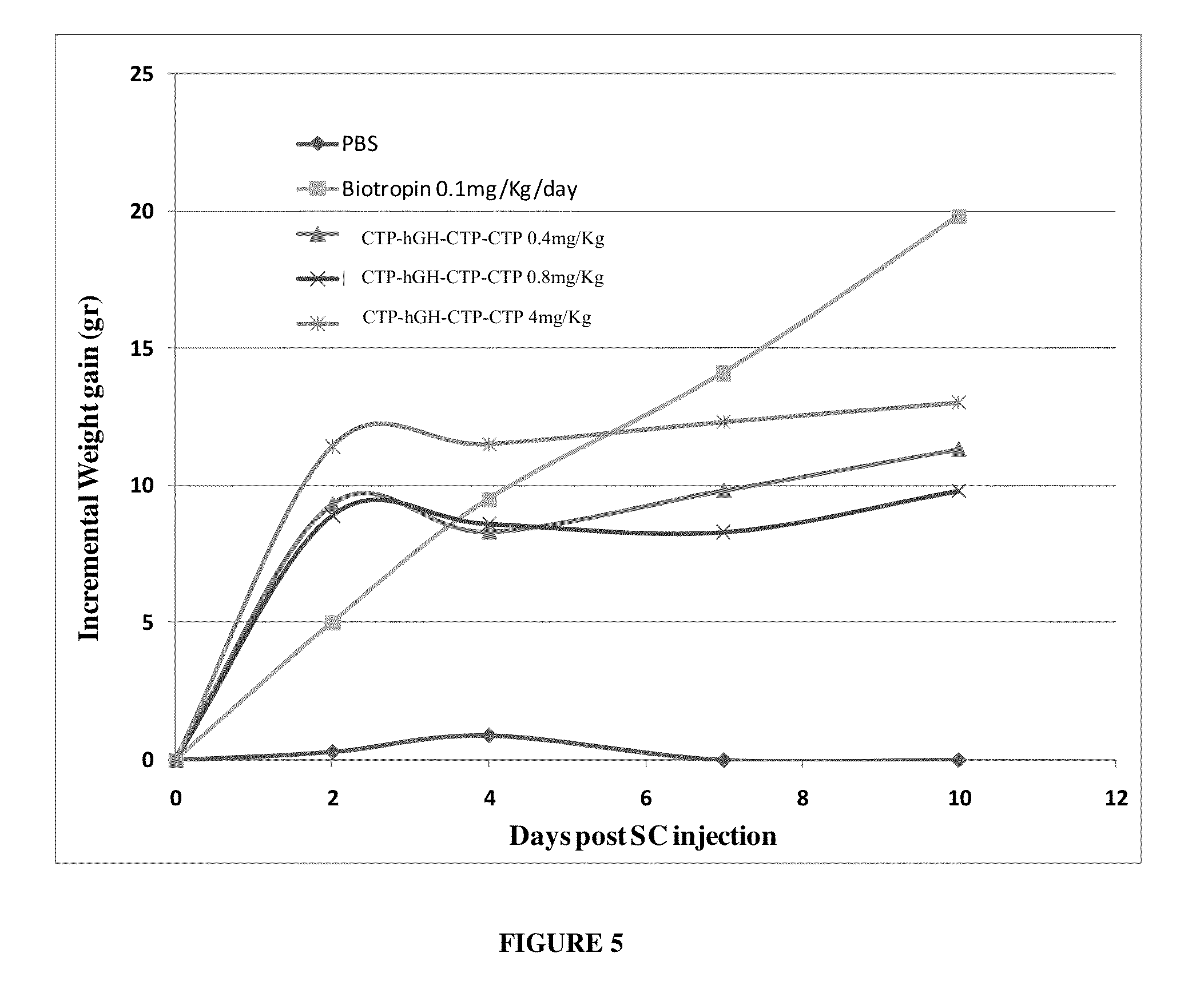
Long-acting growth hormone and methods of producing same
ActiveUS20120035101A1Decreasing body fatReduce weight lossPeptide/protein ingredientsMetabolism disorderSomatotropic hormoneNucleotide
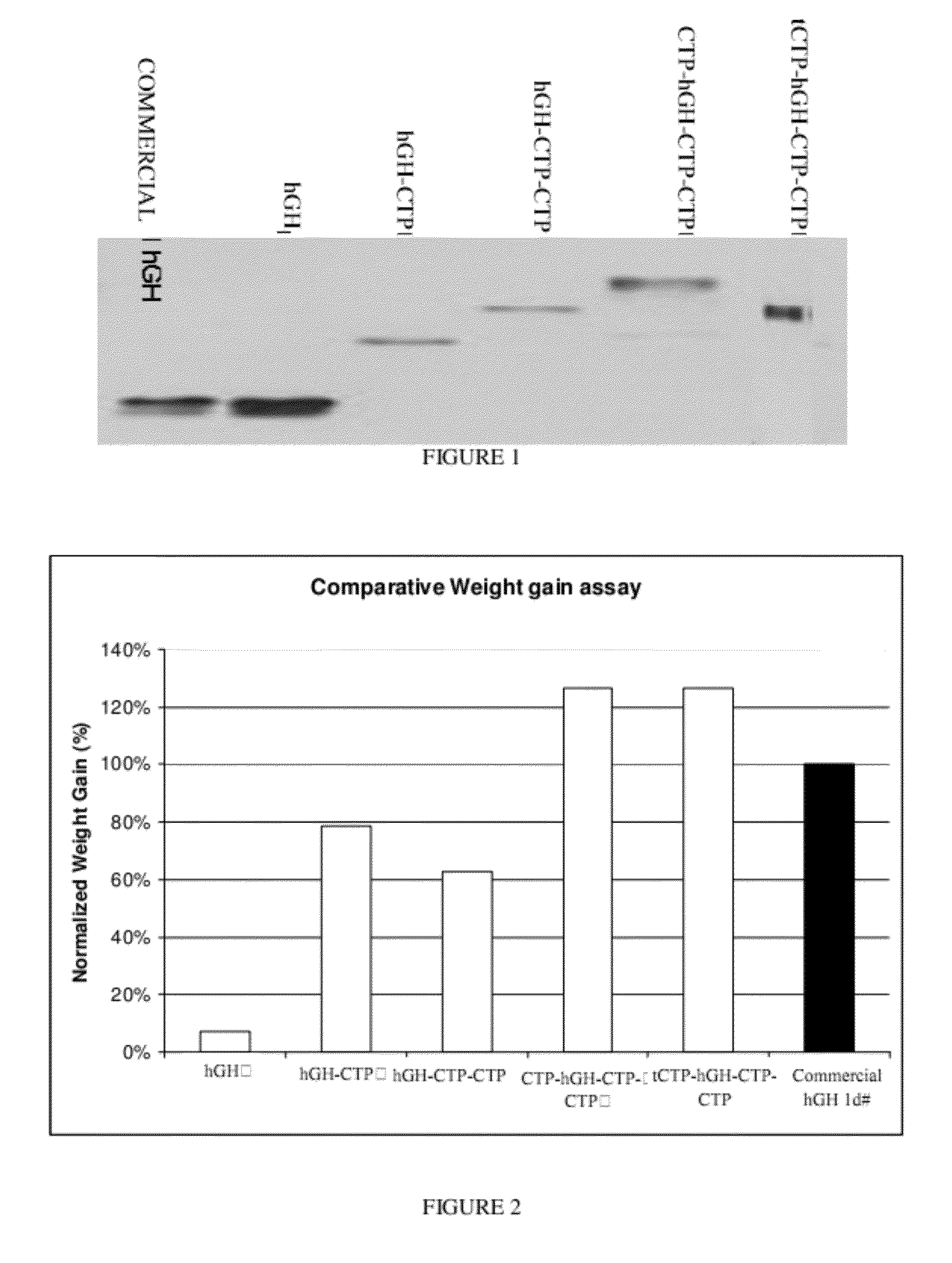
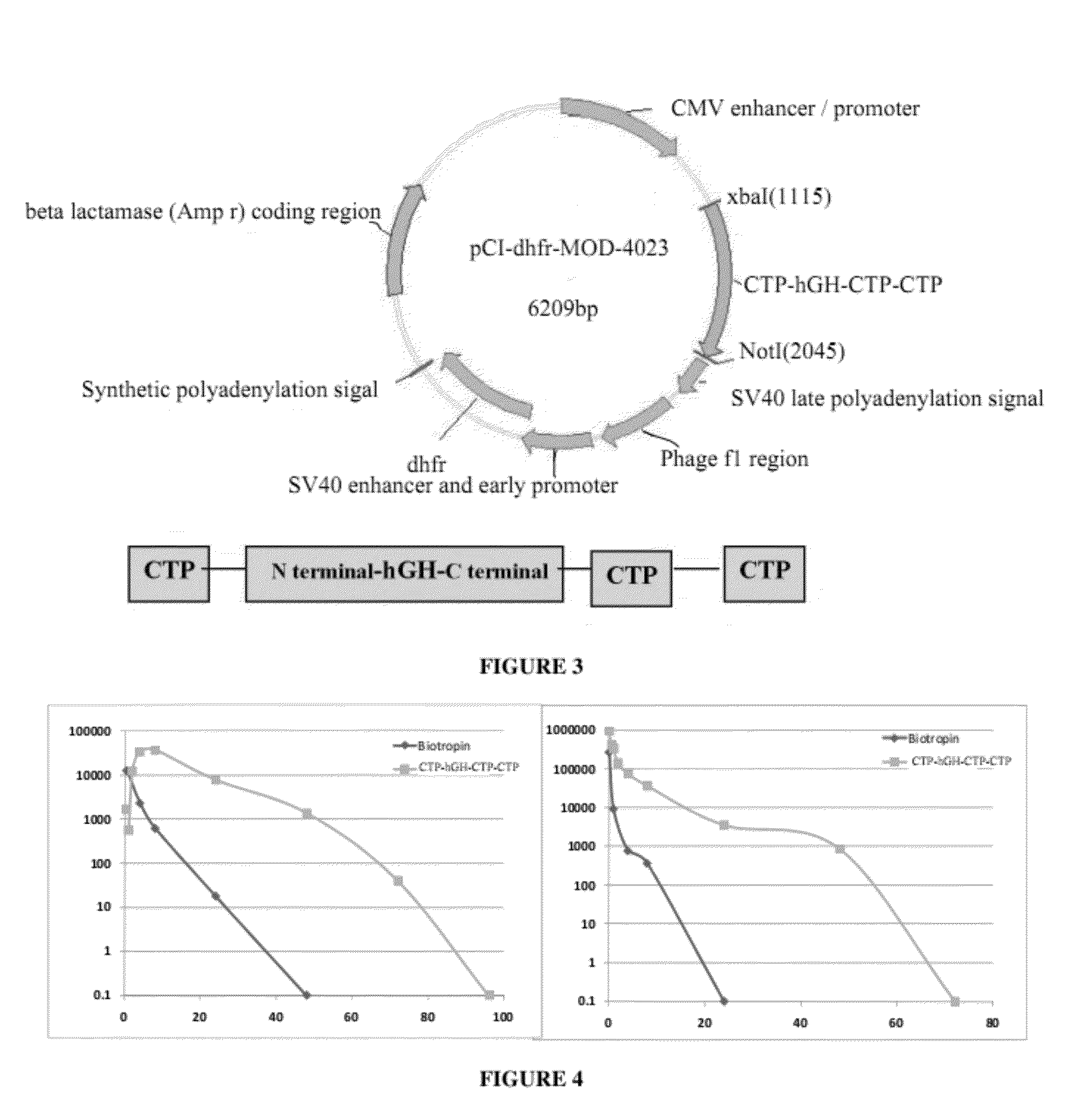
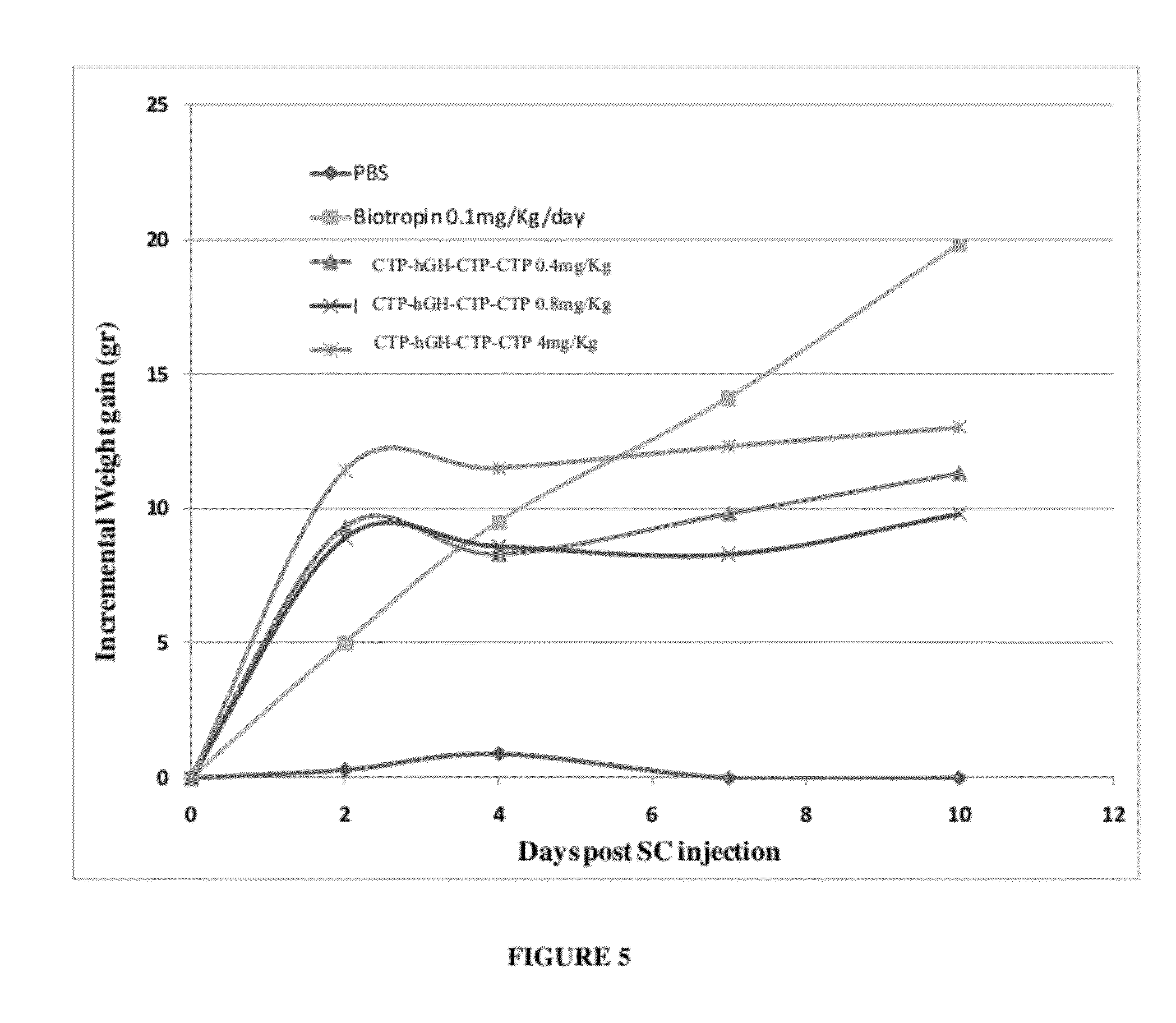
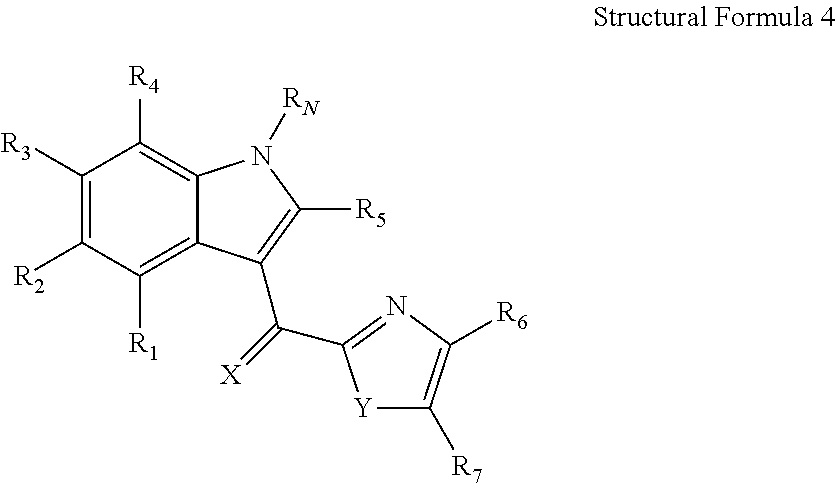
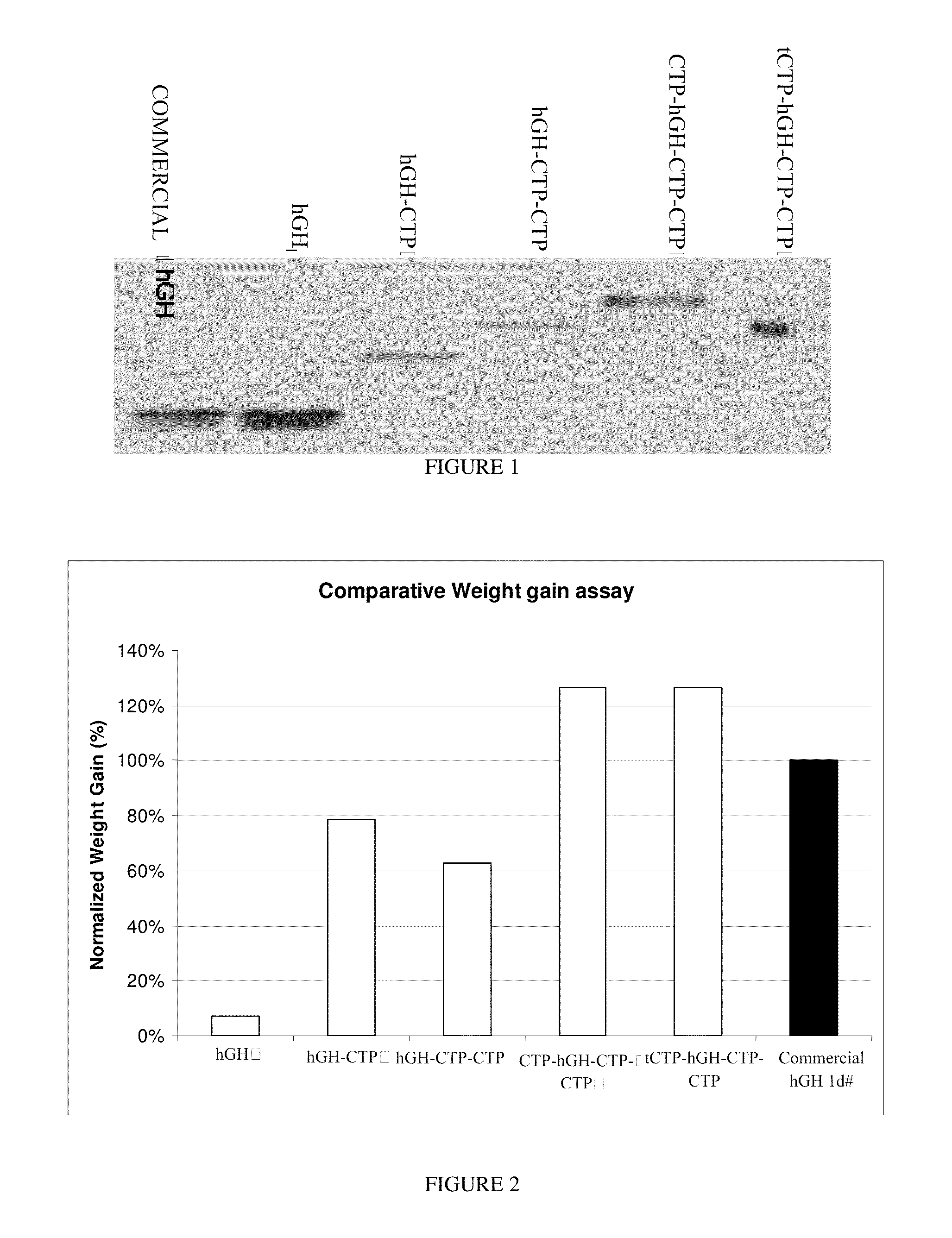
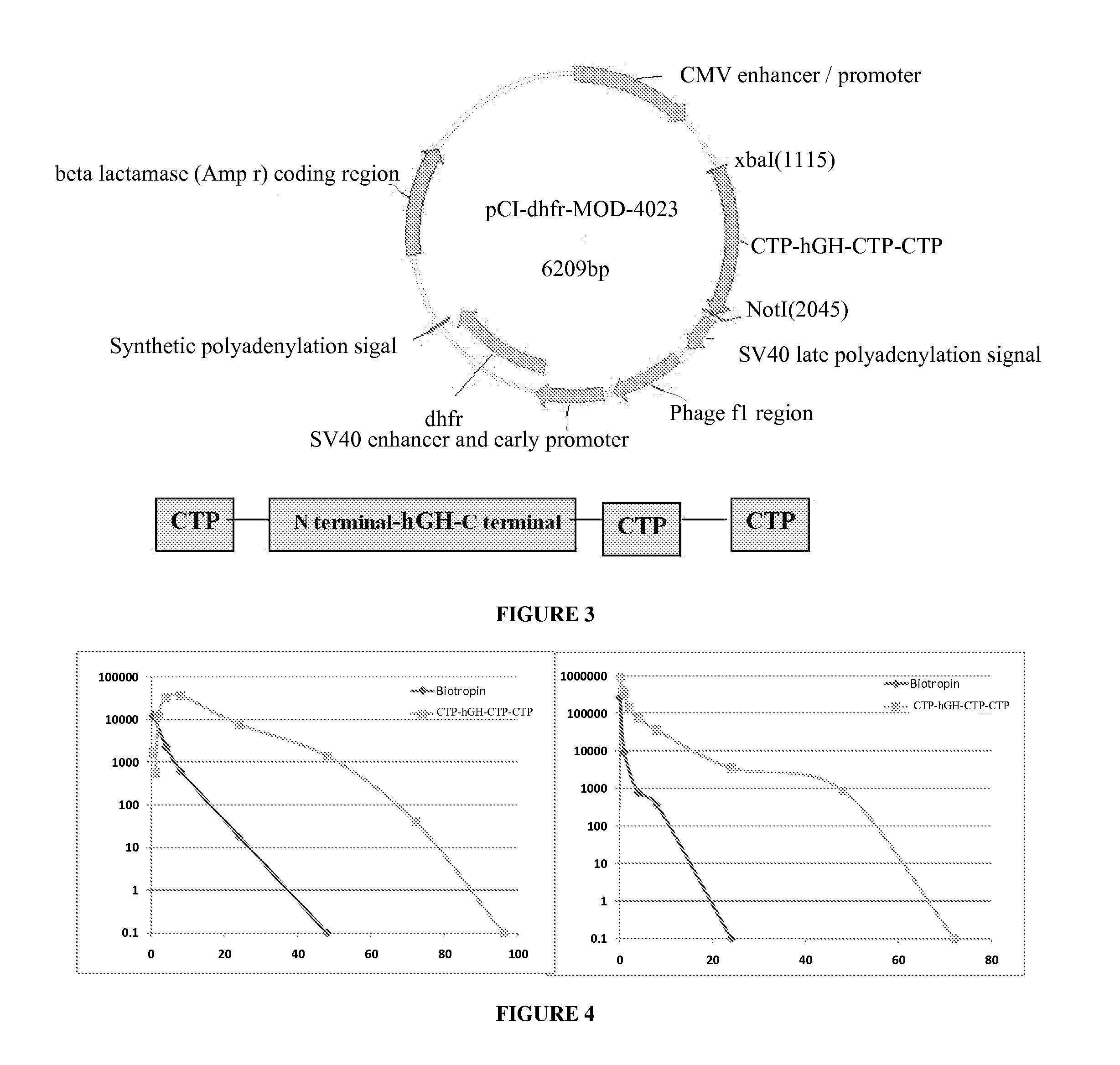
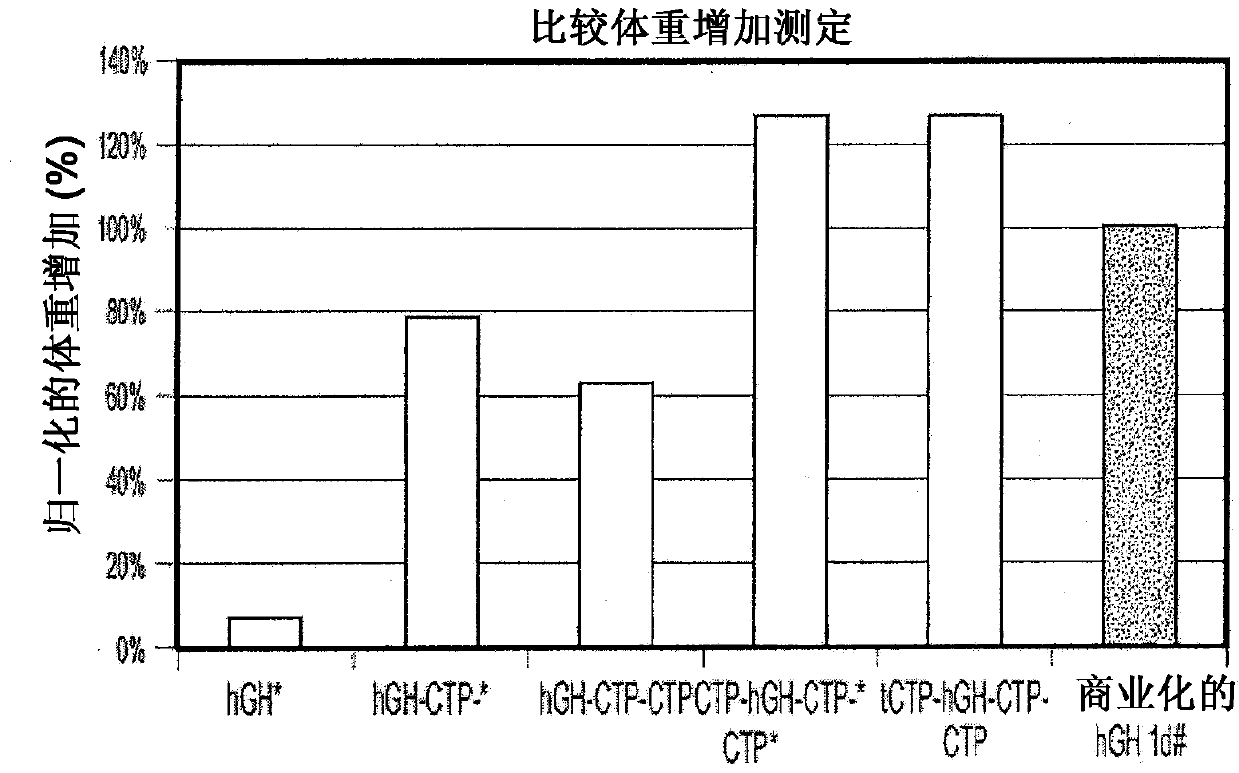
Use of a growth hormone protein and polynucleotides encoding same comprising an amino-terminal carboxy-terminal peptide (CTP) of chorionic gonadotrophin and two carboxy-terminal chorionic gonadotrophin CTPs attached to the growth hormone in methods of inducing growth or weight gain, method of increasing insulin-like growth factor (IGF-1) levels, and methods of reducing the dosing frequency of a growth hormone in a human subject are disclosed. Pharmaceutical compositions comprising the growth hormone and polynucleotides encoding the growth hormone of the invention and methods of using same are also disclosed.
Owner:OPKO BIOLOGICS
Method of Cancer Treatment with 2-(1H-Indole-3-Carbonyl)-Thiazole-4-Carboxylic Acid Methyl Ester
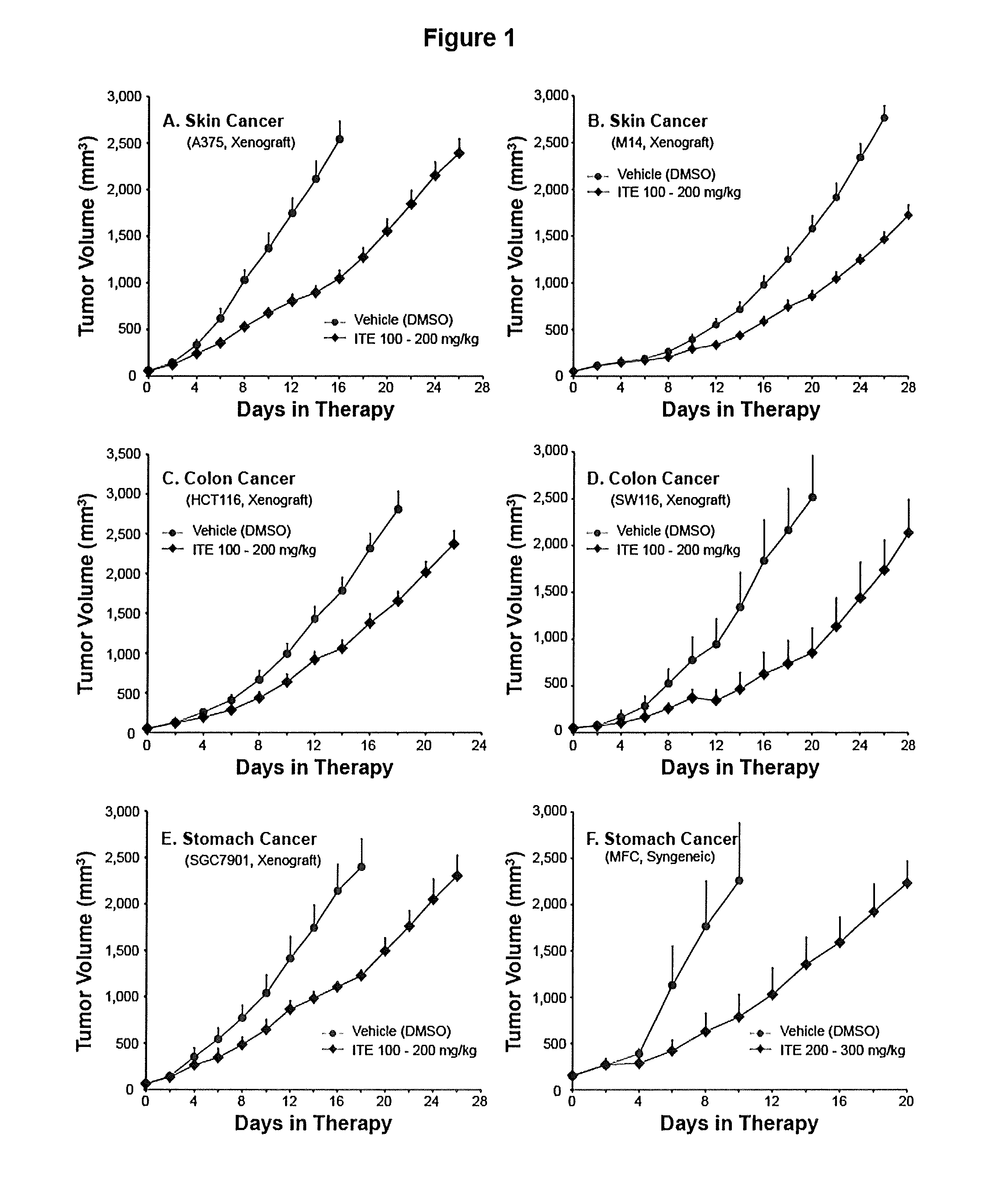
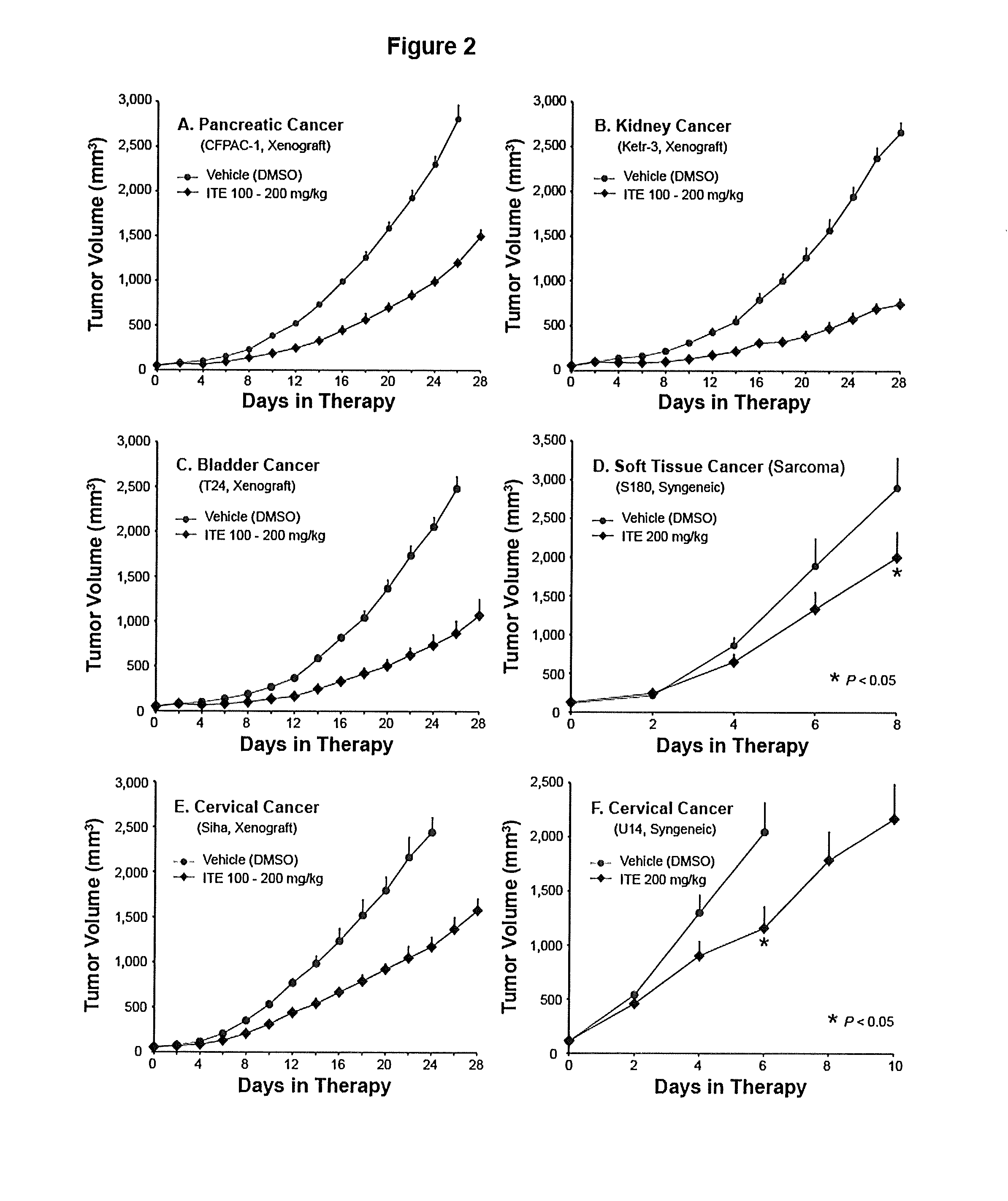
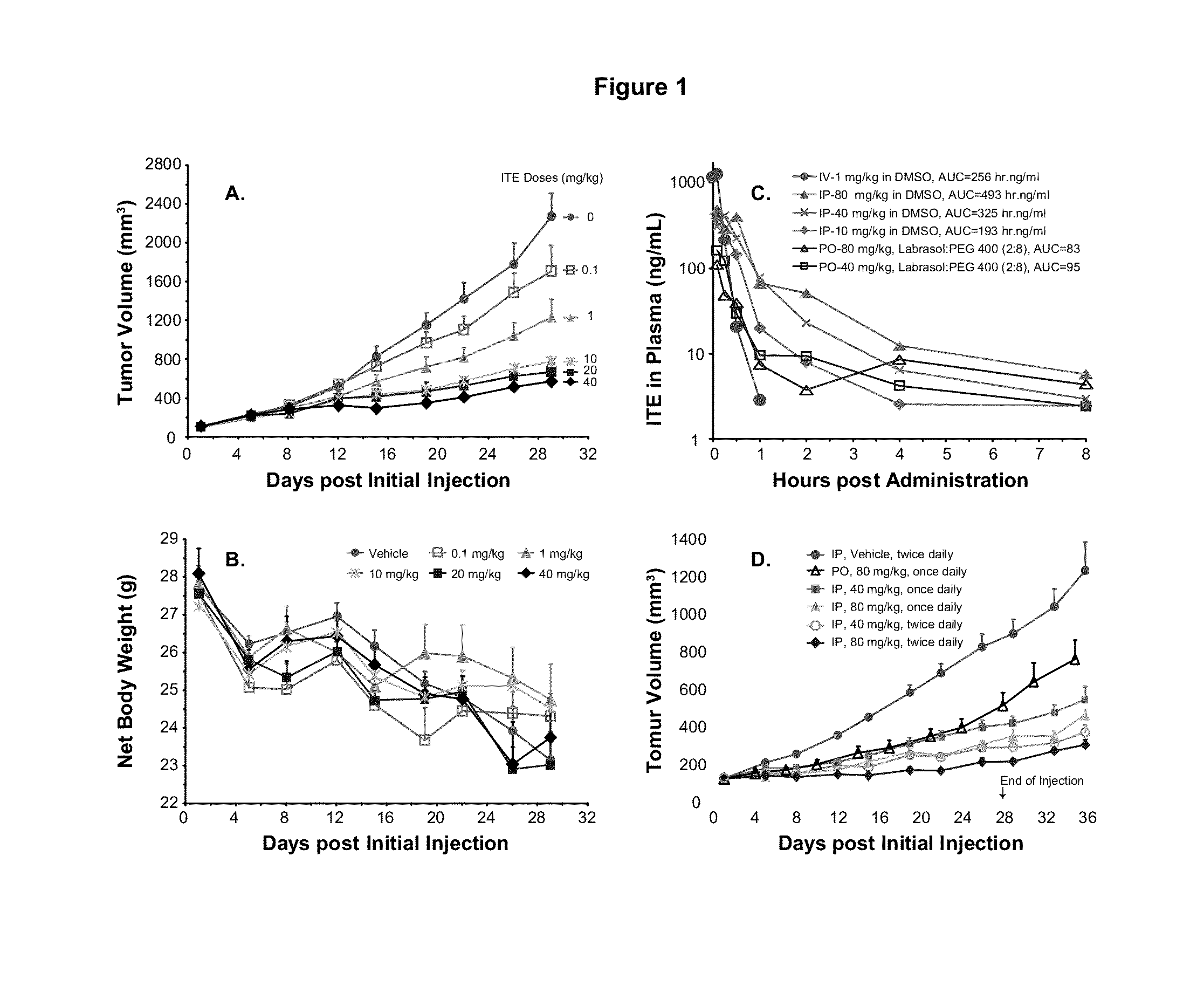
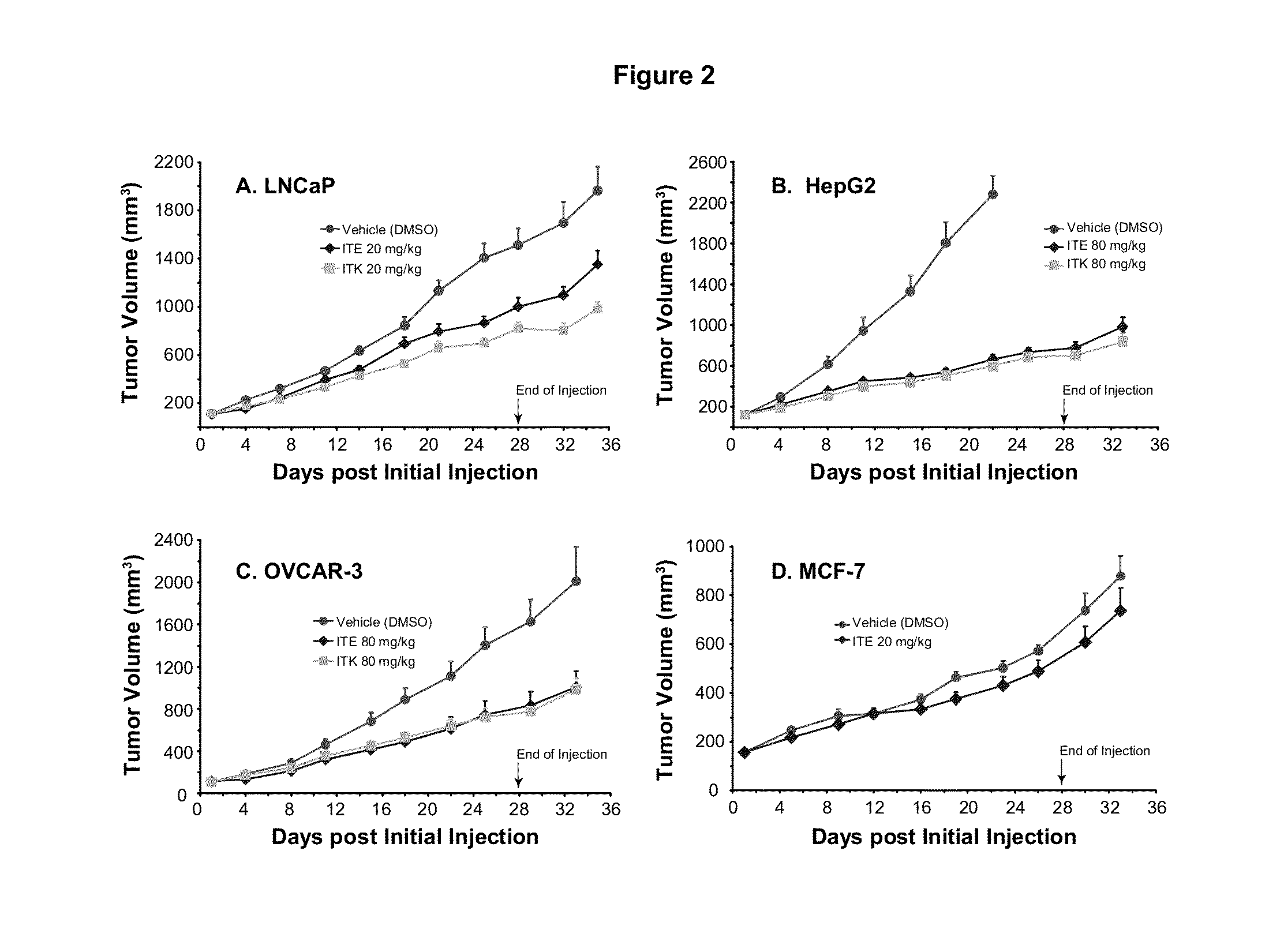
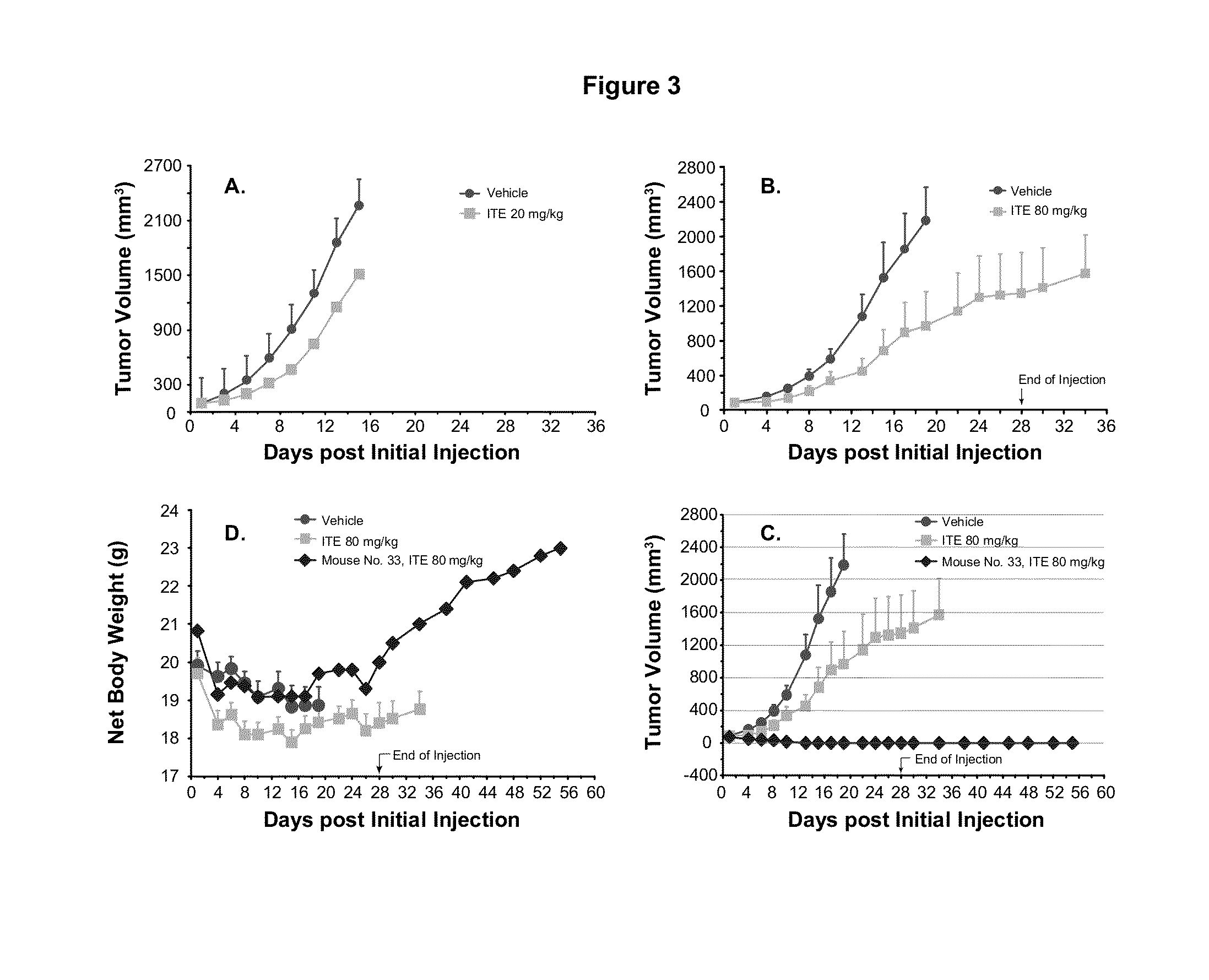
A method of cancer treatment is provided that includes administering an effective amount of an endogenous ligand for the aryl hydrocarbon (Ah) receptor (AhR) named ITE or one of its structural analogs (the active ingredient) to a subject with cancer is disclosed. An effective dose and dosing frequency of the active ingredient are determined by measuring its blood levels of the subject after dosing. The active ingredient formulated with a carrier system is applied topically, enterally, or parenterally to the subject. An oral dose of water, in addition to normal water drinking, is administered to help alleviate feces hardening, a complication of ITE dosing. Subjects with cancers of skin, colon (or rectum), stomach, pancreas, kidney, bladder, soft tissue, and cervix, are preferably accepted for treatment or intervention.
Owner:ARIAGEN INC
Long-acting growth hormone and methods of producing same
ActiveUS8450269B2Decreasing body fatReduce weight lossPeptide/protein ingredientsMetabolism disorderGrowth hormoneDosing Frequency
Use of a growth hormone protein and polynucleotides encoding same comprising an amino-terminal carboxy-terminal peptide (CTP) of chorionic gonadotrophin and two carboxy-terminal chorionic gonadotrophin CTPs attached to the growth hormone in methods of inducing growth or weight gain, method of increasing insulin-like growth factor (IGF-1) levels, and methods of reducing the dosing frequency of a growth hormone in a human subject are disclosed. Pharmaceutical compositions comprising the growth hormone and polynucleotides encoding the growth hormone of the invention and methods of using same are also disclosed.
Owner:OPKO BIOLOGICS
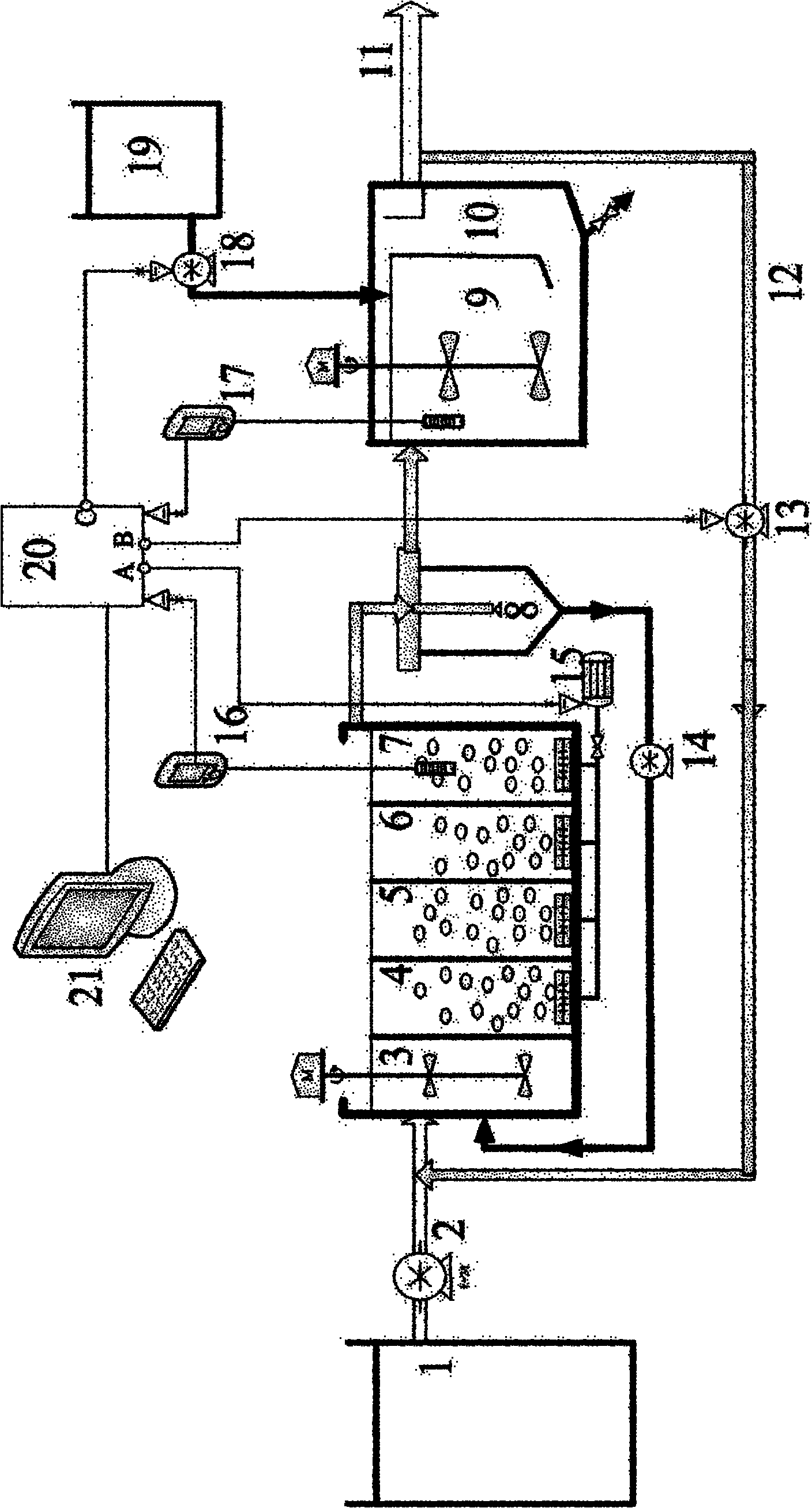
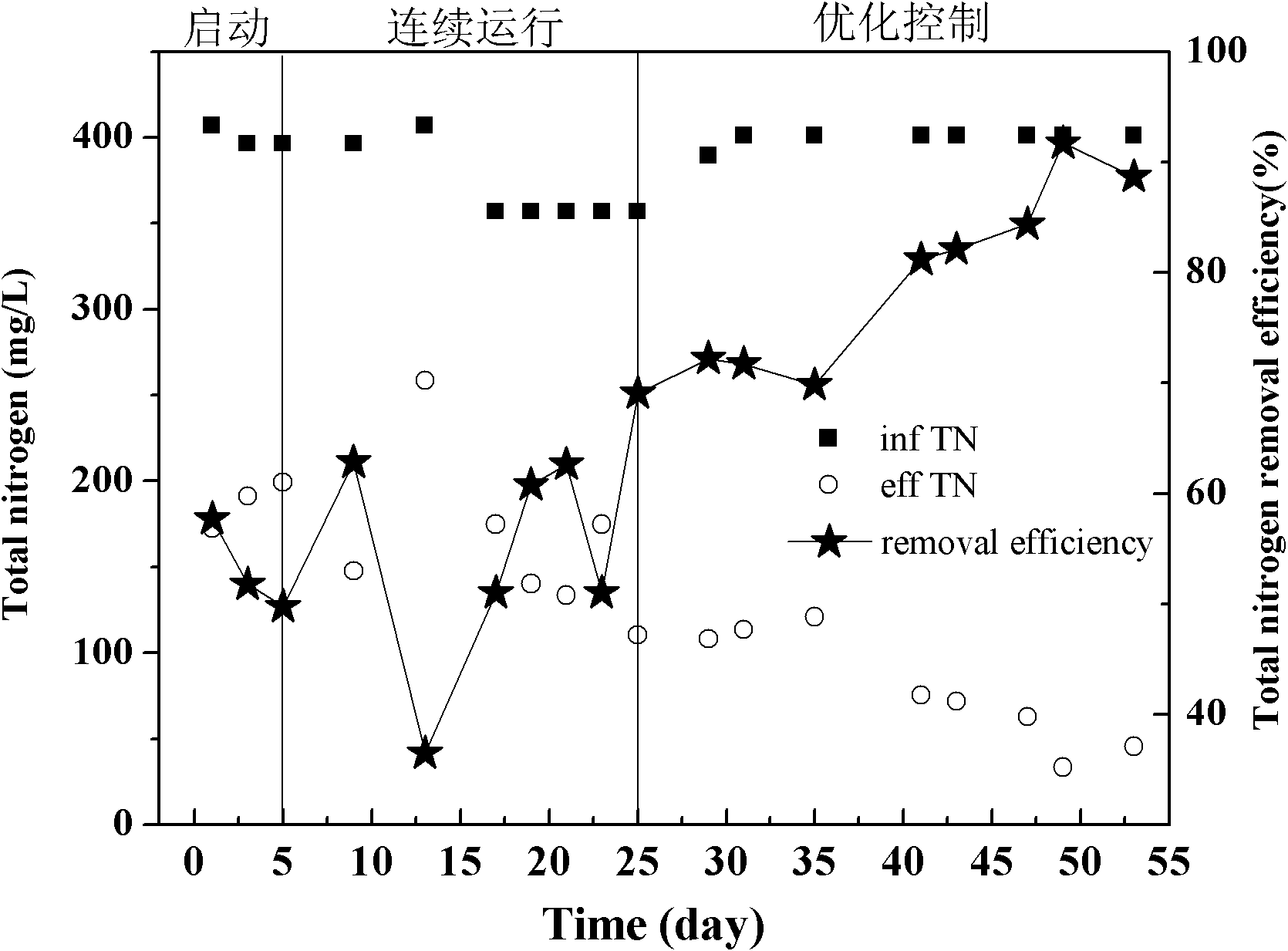
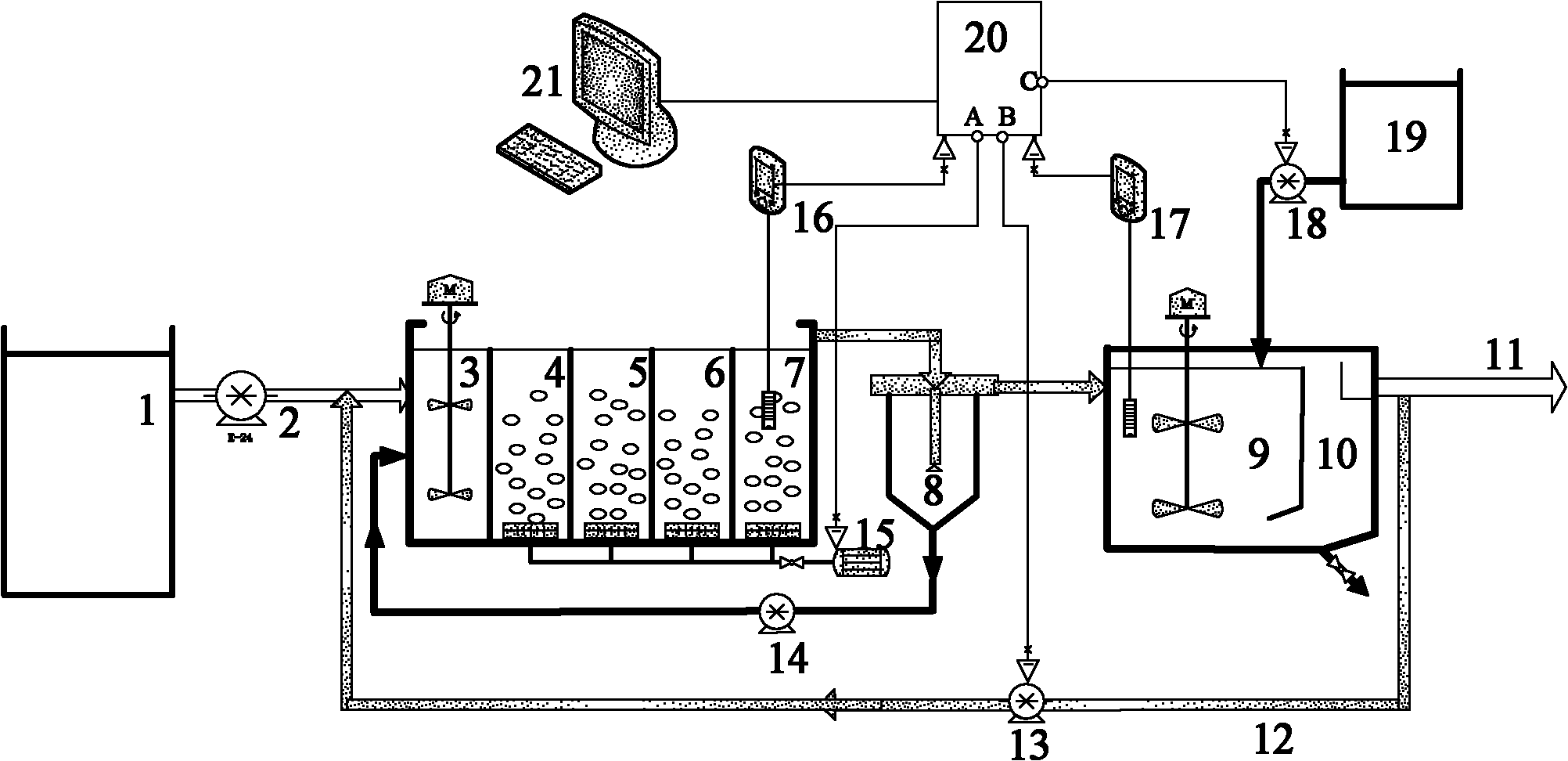
Control method and method for treating sludge digestion liquid through sludge-fermentation-coupled denitrification
ActiveCN102101745AAvoid incomplete oxidationImprove utilization efficiencyMultistage water/sewage treatmentDigestionDose Frequency
The invention discloses a control method and a control method for treating sludge digestion liquid through sludge-fermentation-coupled denitrification, and belongs to the technical fields of high-ammonia-nitrogenous wastewater treatment and primary sludge biochemical treatment. The device is provided with a raw water tank, a sludge-fermentation-coupled denitrification reactor, a sedimentation tank and a sludge storage tank; and through oxide resume power (ORP) / pH sensors arranged in an aerobic reaction area and a fermentation-coupled denitrification reaction area, the aeration amount supply, the effluent backflow ratio and the dosing frequency and quality of primary sludge are optimally controlled. The invention is suitable for optimally controlling the treatment of the sludge digestion liquid by dosing the primary sludge serving as a denitrification carbon source, and can save carbon sources and improve denitrification efficiency. In addition, the control strategy has the advantages of simple operation and obvious effect.
Owner:彭永臻
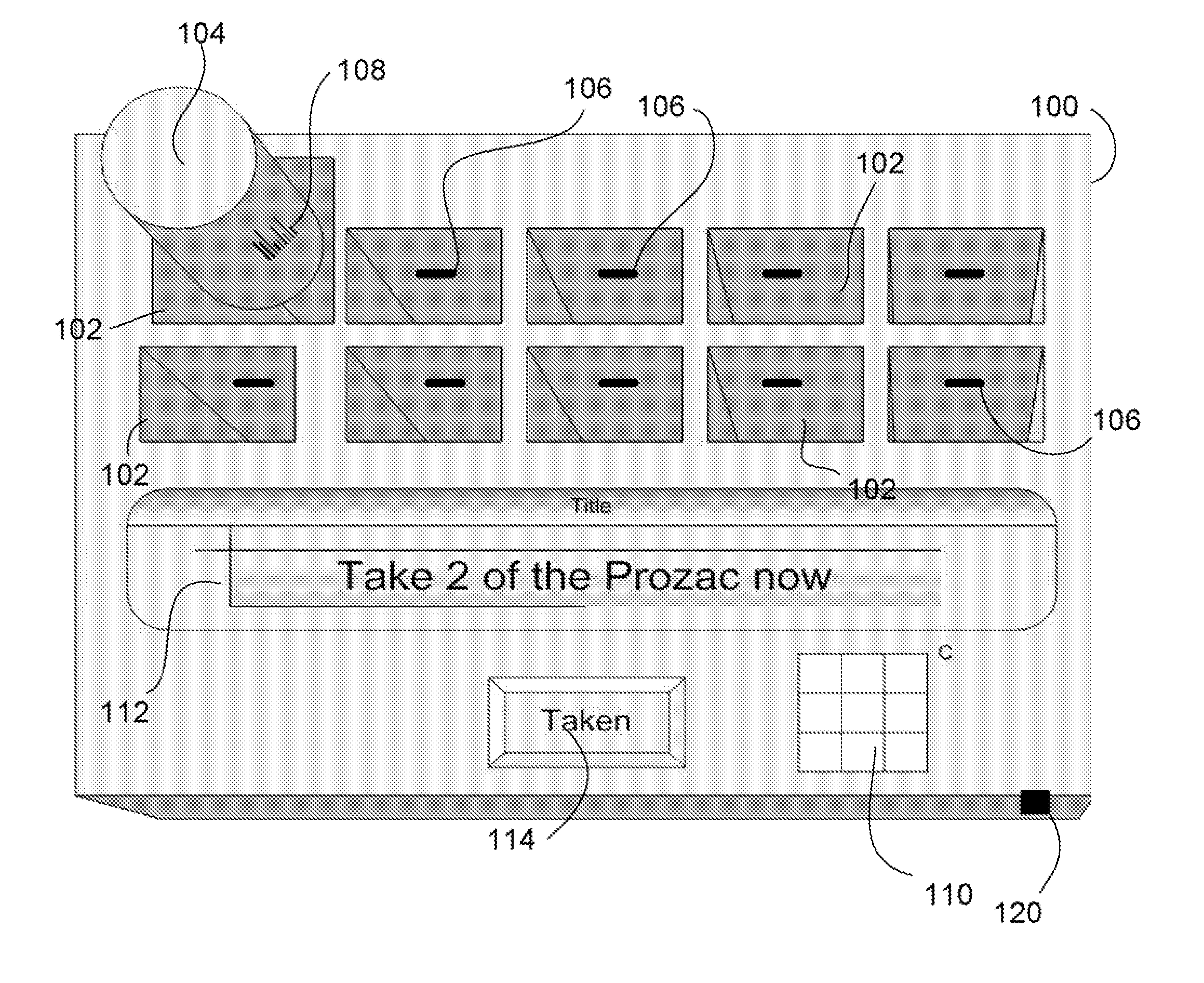
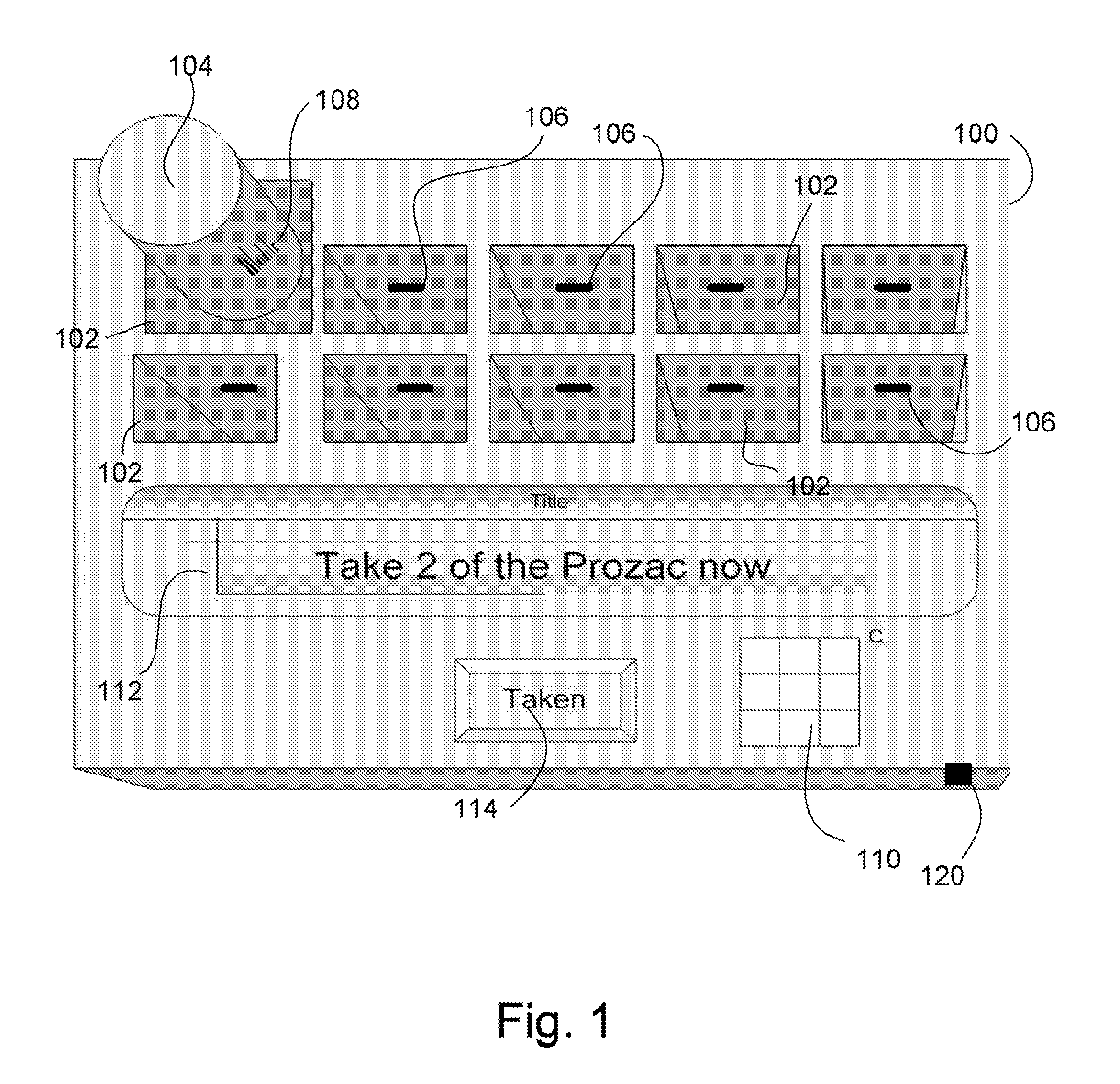
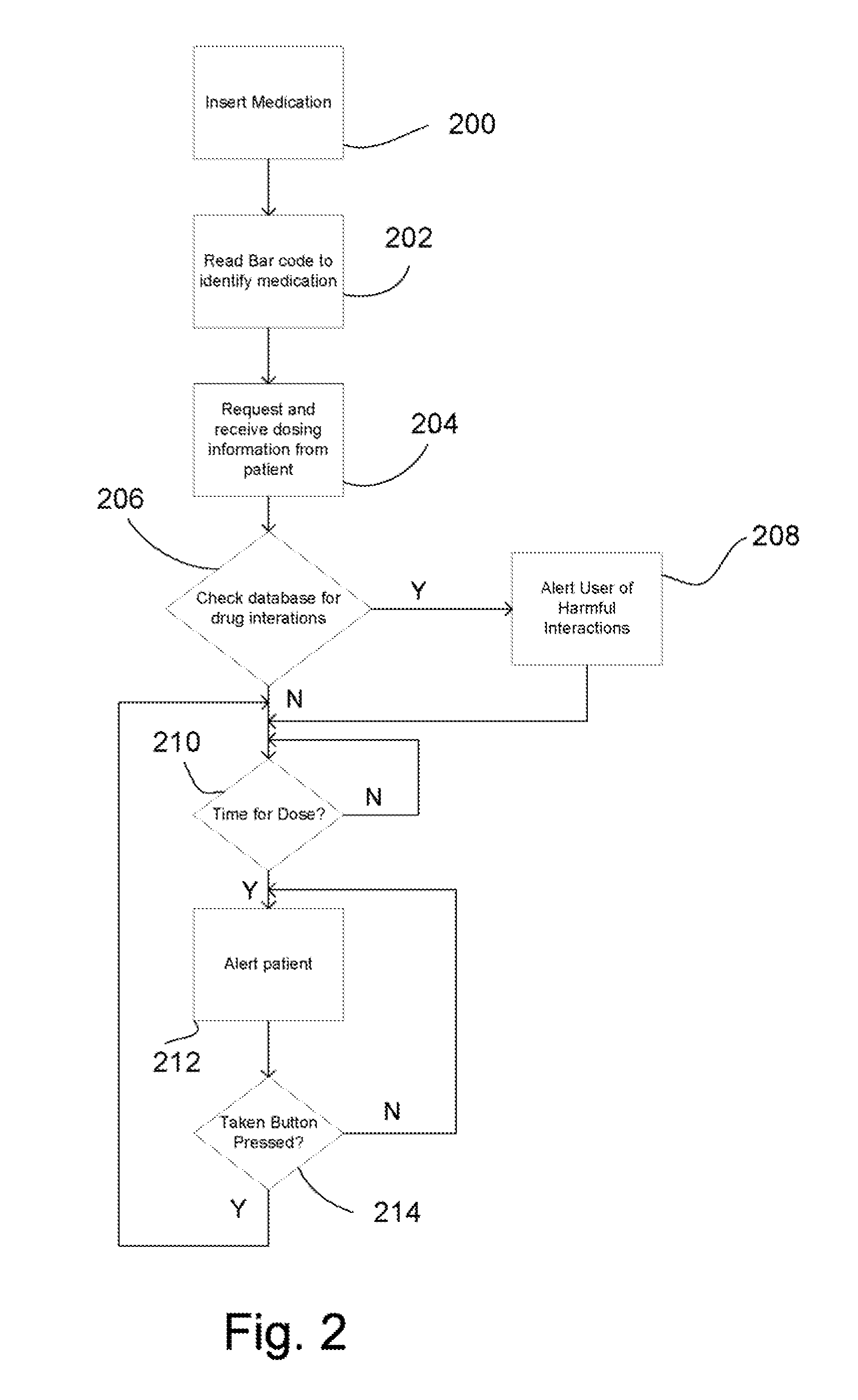
Intelligent medication tracker
InactiveUS20090001093A1Managing their medications regimenImprove patient safety in the hospital settingDrug and medicationsCoin-freed apparatus detailsPharmacyMedication Dispenser
A medication dispenser comprises slots that may be sized to accommodate medication containers of various sizes. Each slot has an associated barcode reader. A user inserts the pharmacy-provided pill or medication container into one slot. The barcode on the container is read. From the barcode the medication in the container is identified. The device contains a simple user interface that allows the user to enter the dosing frequency prescribed, how many pills or cc's are in the container, how many refills and a starting time. The dispenser alerts users to possible drug interactions with medications in other slots as well as alerts them to dosing times and other pertinent information.
Owner:INTEL GE CARE INNOVATIONS
Pesticide film forming agent used for controlling diseases and insect pests of forest trees, and its preparation method
InactiveCN103960228ASolve difficultySolve technical problems such as easy loss of drug effectBiocideFungicidesDiseaseAcute toxicity testing
The invention provides a pesticide film forming agent used for controlling diseases and insect pests of forest trees, and its preparation method. The pesticide film forming agent comprises 0.5-10% of an active component, 0.5-10% of a film forming aid, 5-15% of a solvent, 5-15% of an aid and 50-80% of water; and the active component is one of or a mixture comprising several of tebuconazole, difenoconazole, propiconazole and emamectin benzoate. The drug film forming agent has a good control effect on the diseases and insect pests of the forest trees, has a low price, is convenient to use, and has a good control effect on the dry rot diseases of hickory trees, the canker of various trees, stalk longhorn beetles and bark beetles, leaf aphids, red spider mites and the like especially; the drug film forming agent reduces the active component volatilization and loss possibility and changes the release performance, so the residual period is prolonged, the drug dose and the dosing frequency are reduced; and the drug film forming agent reduces the toxicity of highly toxic pesticides, reduces the acute toxicity of pesticides, reduces the drift of the pesticides, and mitigates the pollution to the environment and the hazard to crops.
Owner:ZHEJIANG FORESTRY UNIVERSITY
Method for preparing abamectin microcapsules by complex coacervation
ActiveCN102893985AImproves the property of being easy to decompose when exposed to lightReduce releaseBiocideAnimal repellantsWater bathsDose Frequency
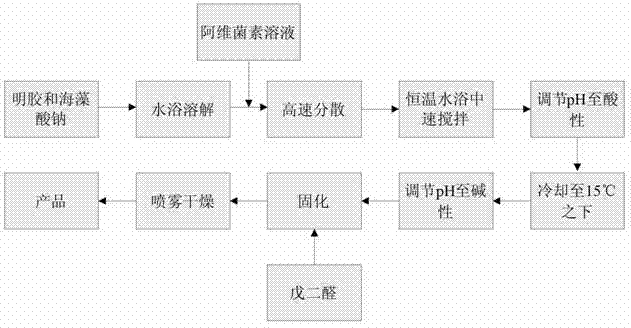
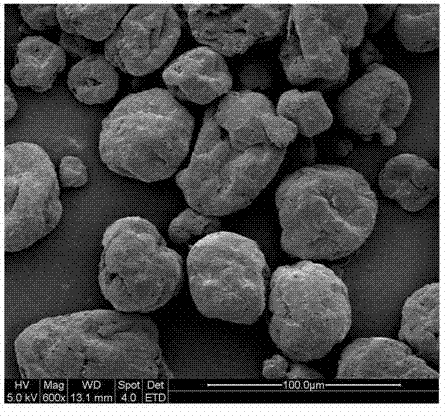
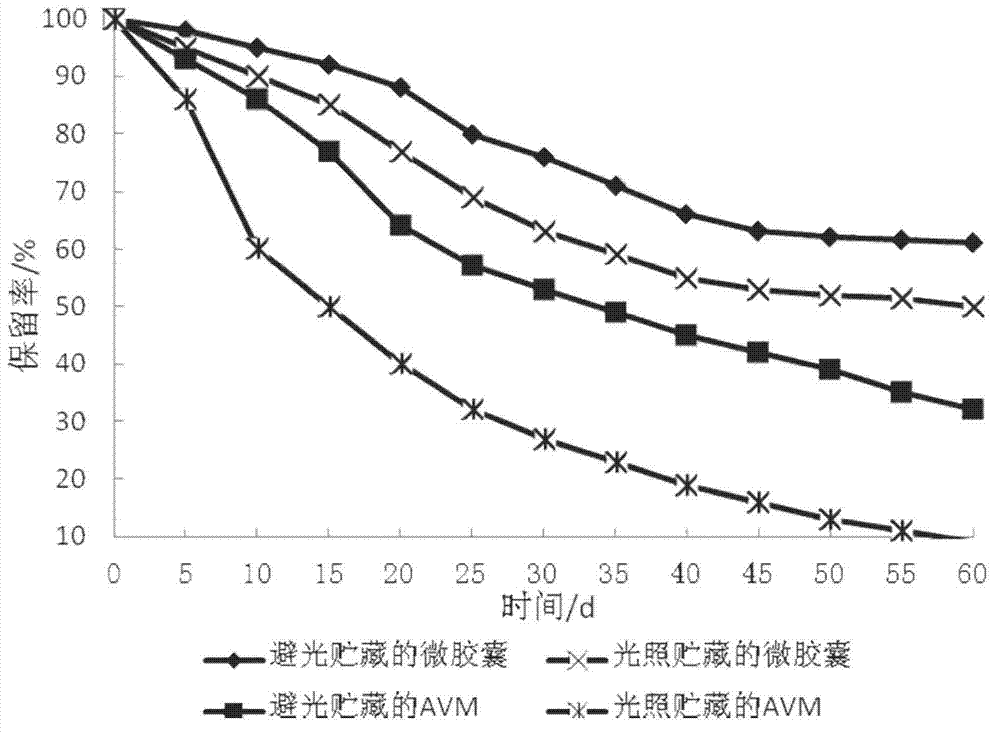
The invention relates to a method for preparing abamectin microcapsules by complex coacervation, belonging to the technical field of preparation of microcapsules. The invention aims to provide a preparation method of novel abamectin microcapsules. The method comprises the following steps: taking sodium alginate as wall materials instead of combination of Arabic gums and gelatins and preparing the novel abamectin microcapsules by water bath solution, high-speed cutting and dispersing, acid regulating, cooling, reducing, solidifying and drying. The method has the advantages of low cost, small pollution, good film-forming property and so on; microencapsulation products have the embedding rate not less than 85%, the drug loading capacity not less than 75%, the lasting period increased by 3-8 times and the retention rate more than 70% after storing at room temperature for three months; the method effectively control the slow release effect of abamectins while improving the light stability of the abamectins and achieves the purposes of reducing the dosing frequency, the dosage and the cost and increasing a control effect.
Owner:JIANGNAN UNIV
Long-acting growth hormone and methods of producing same
InactiveCN104010650AIncrease weightPeptide/protein ingredientsMetabolism disorderNucleotidePolynucleotide
Use of a growth hormone protein and polynucleotides encoding the same comprising an amino-terminal carboxy-terminal peptide (CTP) of chorionic gonadotrophin and two carboxy-terminal chorionic gonadotrophin CTPs attached to the growth hormone in methods of inducing weight loss or body fat reduction, methods of increasing insulin-like growth factor (IGF-1) levels, and methods of reducing the dosing frequency of a growth hormone in a human subject are disclosed. Pharmaceutical compositions comprising the growth hormone and polynucleotides encoding the growth hormone of the invention and methods of using same are also disclosed.
Owner:OPKO BIOLOGICS
Temperature sensitive biogel preparation and application thereof
ActiveCN109820816AEasy to storeConvenient for clinical operationAerosol deliveryOintment deliveryDosing FrequencyMesenchymal stem cell
The invention relates to a temperature sensitive biogel preparation comprising, by mass, 20-30 parts of mesenchymal stem cell exosome, 20-28 parts poloxamer 407, 5-10 parts of poloxamer 188, 0.9-2.4 parts of polymer stabilizer for adjusting gelation temperature and drug release characteristics and 32-54 parts of water. The biocompatible degradable polymer is used as a drug-loading matrix of the mesenchymal stem cell exosome, and a polymer stabilizer is added to adjust the gelation temperature and the drug release characteristics, so that the loaded MSCs exosome can be released continuously toreduce the dosing frequency, prevent leakage of liquid medicine, improve the bioavailability and provide the safe effective preparation convenient to apply clinically for treatment of IUA.
Owner:江苏拓弘生物科技有限公司
Long-acting viscose dispersing type transdermal patch and preparing process thereof
InactiveCN105250243AHigh transdermal efficiencyModerate viscosityOrganic active ingredientsAntipyreticTransdermal patchTG - Triglyceride
The invention discloses a long-acting viscose dispersing type transdermal patch and a preparing process thereof and belongs to the medical technical field. The transdermal patch is mainly prepared from bulk pharmaceutical chemicals (such as non-steroid anti-inflammatory drug ibuprofen and salt form thereof, naproxen and salt form thereof, ketoprofen, indometacin and salt form thereof), a penetration enhancer (menthol, oleic acid, medium chain triglyceride, propylene glycol monolaurate, azone, propylene glycol and the like), a dispersing solvent (water, acetone, ethanol, carbinol, ethyl acetate and the like), a polyacrylate pressure-sensitive adhesive (crylic acid, butyl acrylate, crylic acid 2-ethylhexyl ester and the like), a backing layer and a release liner. The long-acting viscose dispersing type transdermal patch and the preparing process thereof have the advantages that drugs can be released from a matrix continuously for 12-48 h, the number of drug residues is low, transdermic absorption property is excellent, and dosing frequency and dosing amount can be reduced; skin irritation is avoided, and adhesion and compliance are high; main drugs and additives are stable in a viscose mechanism; preparing technology is simple, and pollution is avoided.
Owner:CHINA PHARM UNIV
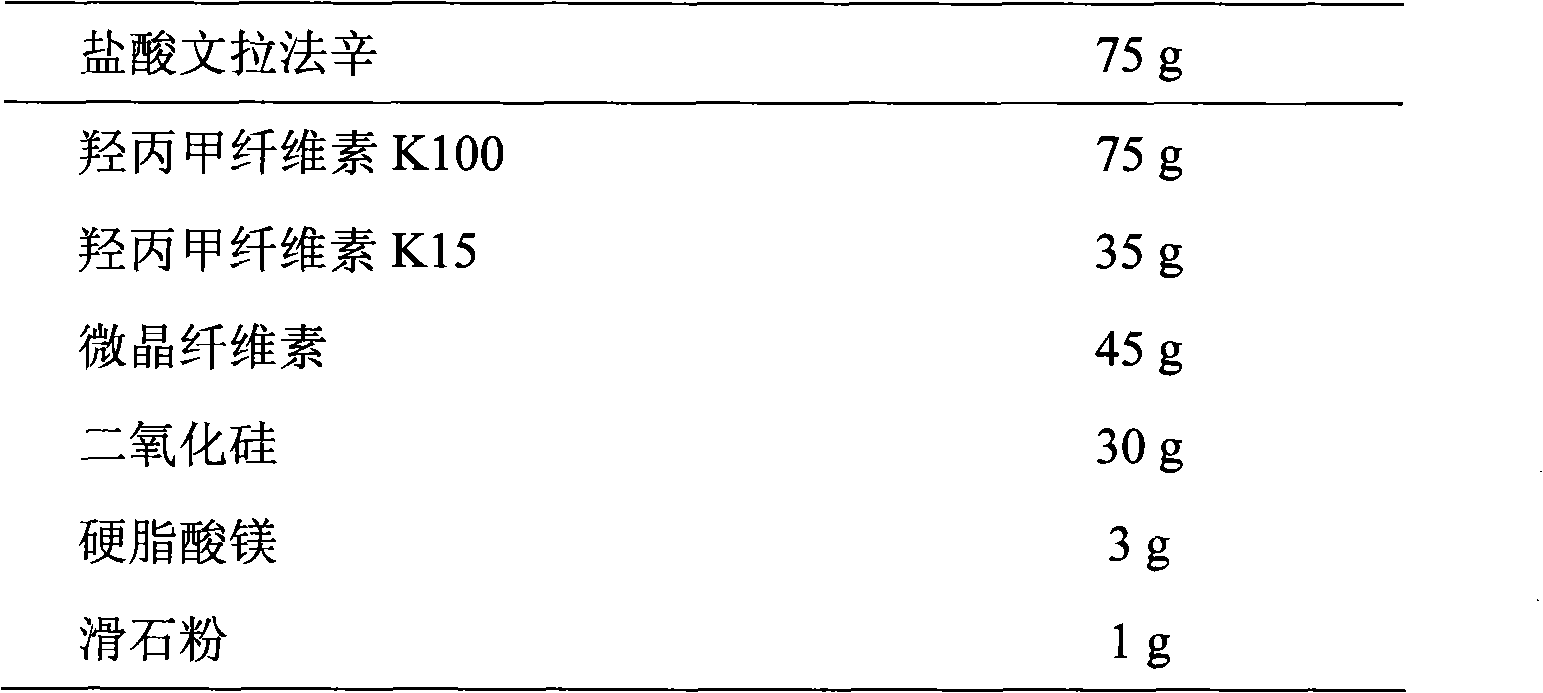
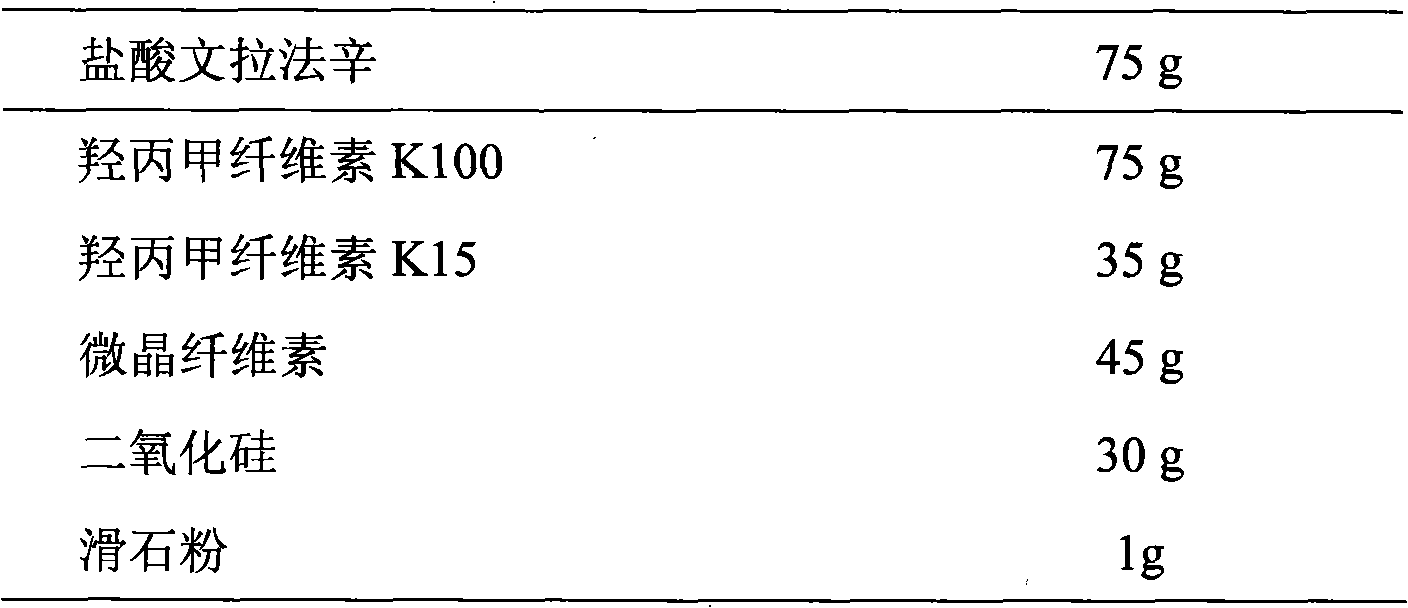
Venlafaxine hydrochloride sustained-release tablet preparation and preparation method thereof
InactiveCN101584674AGood sustained release effectOrganic active ingredientsNervous disorderSustained Release TabletAdhesive
The invention discloses a venlafaxine hydrochloride sustained-release tablet preparation which comprises venlafaxine hydrochloride, framework material, diluent, lubricant, adhesive and coating material. The experiments demonstrate that the venlafaxine hydrochloride sustained-release tablet achieves better sustained-release effect, can reduce the dosing frequency and is convenient for the patients to use. The invention also provides a preparation method thereof.
Owner:上海医药科技发展有限公司
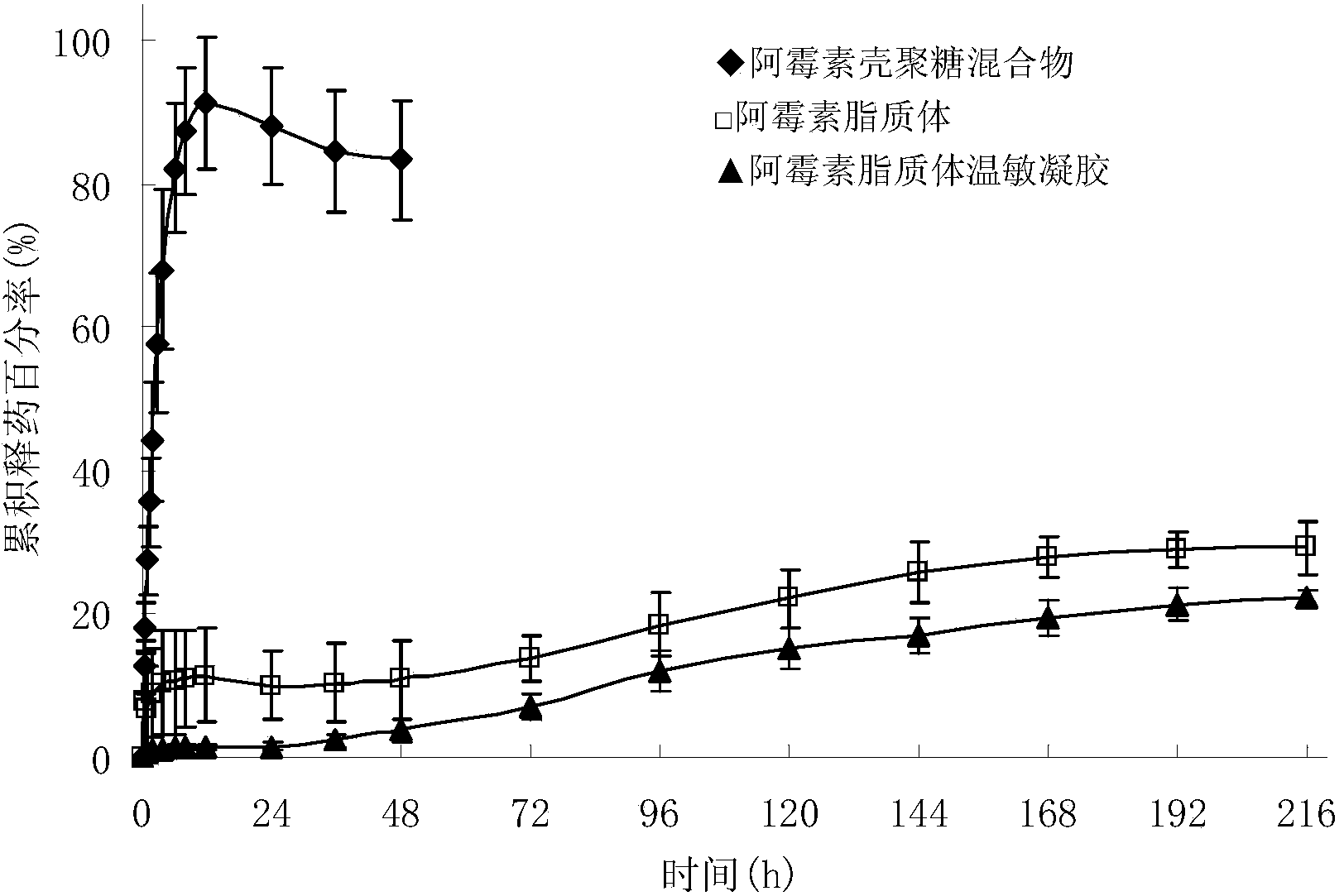
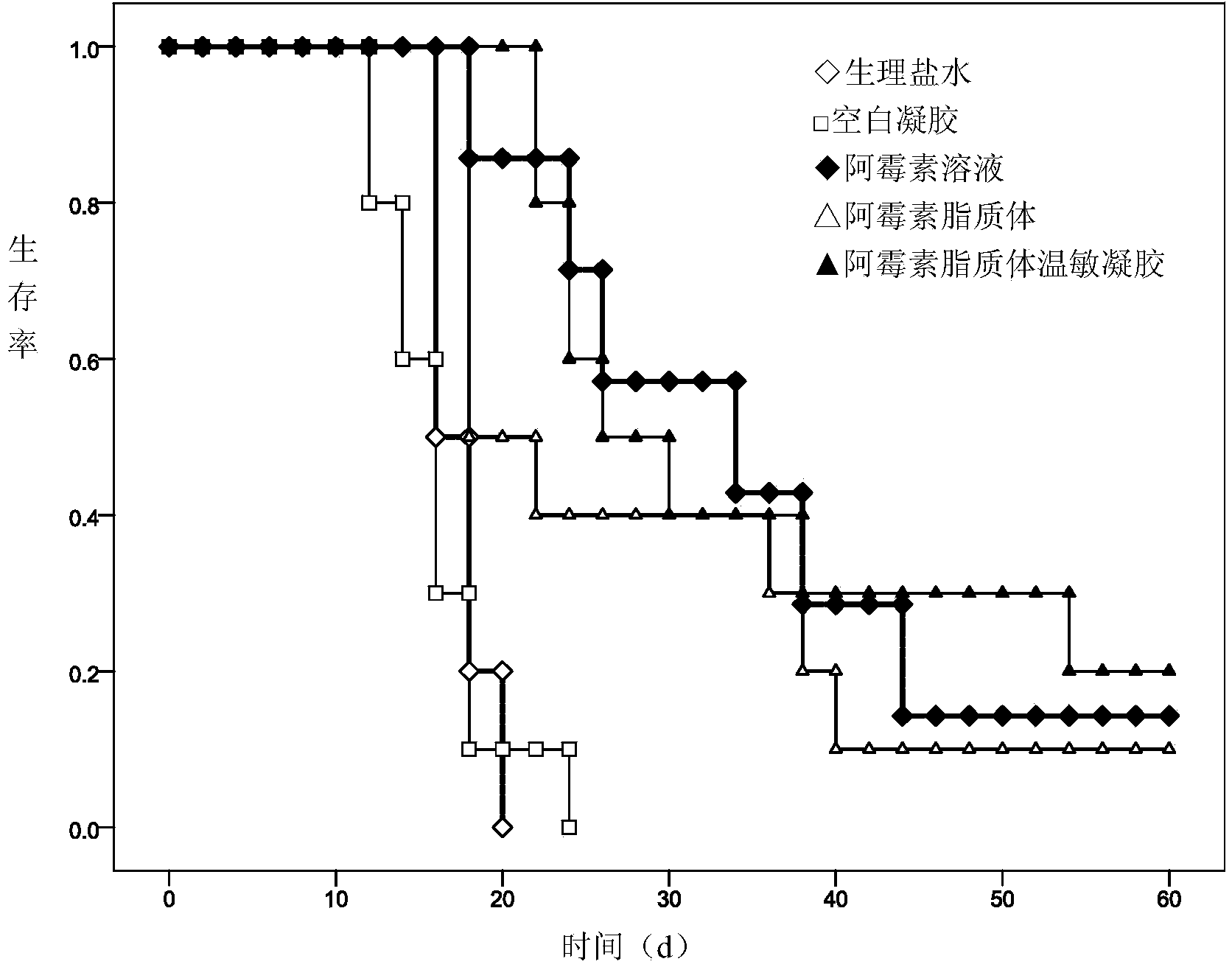
Adriamycin liposome temperature-sensitive gel for tumor local injection
ActiveCN104382918AImprove anti-tumor effectReduce the number of dosesOrganic active ingredientsOintment deliverySide effectAdriamycin Hydrochloride
The invention discloses adriamycin liposome temperature-sensitive gel and a preparation method thereof. The adriamycin liposome temperature-sensitive gel comprises the following components in parts by weight: 1-3 parts of adriamycin or adriamycin hydrochloride, 10-30 parts of soyabean lecithin, 2-8 parts of cholesterol, 5-20 parts of ammonium sulfate, 10-40 parts of 2-hydroxypropyl trimethyl ammonium chloride chitosan, 40-120 parts of sodium glycerophosphate and 500-1500 parts of menstruum. When the adriamycin liposome temperature-sensitive gel is applied to the tumor local injection, the anti-tumor effect of an adriamycin preparation is improved, the dosing frequency is reduced, and the systemic side effects are reduced; the nature of the temperature-sensitive gel is relatively stable and is unlikely to be affected by the environmental pH and the ionic strength.
Owner:ZHEJIANG UNIV OF TECH
Preparation method of anti-wrinkle skin care product composition containing acetyl hexapeptide-8
InactiveCN110269814AImprove permeabilityPromote repairCosmetic preparationsToilet preparationsPersonal careArginine
The invention belongs to the technical field of skin care product composition preparation, and discloses a preparation method of an anti-wrinkle skin care product composition containing acetyl hexapeptide-8. The anti-wrinkle skin care product composition containing the acetyl hexapeptide-8 is prepared from materials in parts by weight: 0.5-6 parts of the acetyl hexapeptide-8, 0.1-0.5 part of sodium hyaluronate, 0.5-2 parts of palmitoyl tripeptide-5, 2 parts of butanediol, 0.5 part of [water, oligopeptide-1,dipotassium phosphate, sodium chloride and glycerinum], 90 parts of water and 0.1 part of arginine. According to the preparation method of the anti-wrinkle skin care product composition containing the acetyl hexapeptide-8, a percutaneous drug delivery penetrating mode of the acetyl hexapeptide-8 can be affected by individual metabolic rate and use dose frequency, and nursing is carried out according to static wrinkles and dynamic wrinkles so as to reach an anti-wrinkle personal care function. The preparation method of the anti-wrinkle skin care product composition containing the acetyl hexapeptide-8 can improve permeability of the acetyl hexapeptide-8 and repair of the static wrinkles, can be applied to addition of efficacy matters of facial mask essence, essence, emulsion and cream and milk for repairing skin in skin care products, and the product stability is protected.
Owner:广州市拉凯尔干细胞研究所
Method for treating multiple sclerosis
InactiveUS20100172869A1Maximize efficacyReduce adverse side effectsNervous disorderPeptide/protein ingredientsDosing FrequencyInterferon alpha
Methods for treating multiple sclerosis (MS) and clinically isolated syndromes suggestive of MS are provided. The methods comprise administering a therapeutically effective dose of interferon-beta (IFN-beta) to a subject in need thereof, where the dose is administered intramuscularly with a dosing frequency of two- to three-times per week.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
Application of conjugate of polyethylene glycol and local anesthetic to non-narcotic analgesia
ActiveCN107789628AReduce clinical adverse reactionsReduce the number of dosesAntipyreticAnalgesicsPolyethylene glycolDosing Frequency
The invention discloses application of a conjugate of polyethylene glycol and local anesthetic to non-narcotic analgesia. The local anesthetic is made into a prodrug or a sustained release preparation, wherein high-molecular polymers such as polyethylene glycol in the prodrug are covalently bonded with local anesthetic, and auxiliary materials with a sustained release function in the sustained release preparation are covalently bonded with the local anesthetic. After application of the conjugate, anesthesia and analgesia effects are not achieved until release of free local anesthetic, and an analgesia effect is achieved after release of the free local anesthetic. Due to low release speed of the prodrug or the sustained release preparation of the local anesthetic, drug concentration can bestably and enduringly kept in an effective concentration range of non-narcotic analgesia, long-acting non-narcotic analgesia effects can be achieved while remarkable reduction of clinical untoward effects of the local anesthetic and reduction of dosing frequency are realized, drug effectiveness is improved, and a clinical application range of the local anesthetic is expanded.
Owner:JENKEM TECH CO LTD (LIAONING)
Compound doxycycline-hydrochloride florfenicol sustained-release microsphere suspension injection for veterinary use
ActiveCN105287607AImprove clinical efficacyReduce clinical dosageAntibacterial agentsTetracycline active ingredientsSuspending AgentsVeterinary Drugs
The invention belongs to the technical field of veterinary drug preparation, and relates to a compound doxycycline-hydrochloride florfenicol sustained-release microsphere suspension injection for veterinary use. The suspension injection is produced through a preparation technology combining an inclusion technology, a microcapsule technology and a high-pressure homogenization technology. The suspension injection comprises the following ingredients according to W / V: 10-30% of an inclusion material, 5-20% of doxycycline hydrochloride, 5-20% of florfenicol, 2.5-7.5% of a high-molecular capsule material, 0.2-1% of a suspending agent, 0.25-1% of an anti-oxidant, 0.05-0.2% of a metal chelator, 0.1-0.4% of an antiseptic, and the balance injection water. The active ingredients in the injection possess synergic antibacterial effects and obvious sustained release effect, clinic dosing frequency is reduced, the injection does not contain organic solvents, does not stimulate target animals, is small in toxic and side effects, and is capable of controlling respiratory diseases caused by streptococcus suis, actinobacillus pleuropneumoniae, pasteurella multocida, haemophilus parasuis, mycoplasma and the like.
Owner:HUAZHONG AGRI UNIV
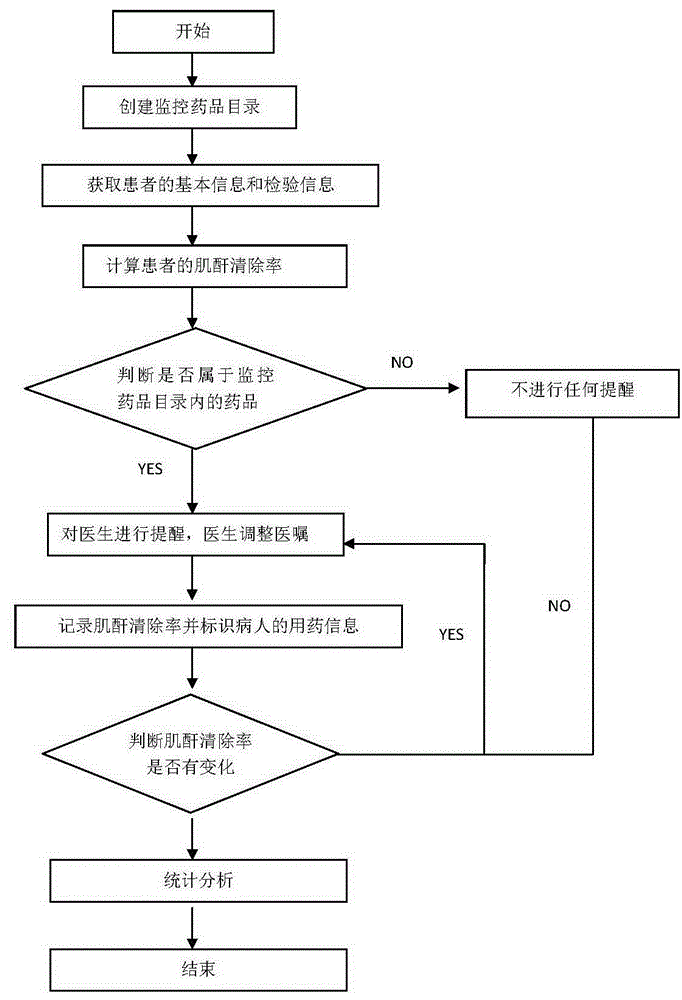
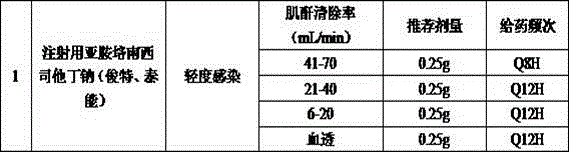
Method for monitoring medication based on renal function level of patient
InactiveCN106599554AGuaranteed timelinessEnsure safetyHealth-index calculationComputer-assisted medicine prescription/deliveryMedication informationDose Frequency
The invention discloses a method for monitoring medication based on the renal function level of a patient. The method comprises the following steps: S1, creating a monitored medicament directory, and setting a monitoring creatinine clearance rate value and a corresponding recommended medicament dose, a dosing frequency or a forbidden identifier; S2, acquiring basic information and checking information of the patient, including information of the gender, the age, the height and the serum creatinine value; S3, calculating the creatinine clearance rate of the patient according to the acquired checking information of the patient; S4, judging whether a medicament order of a doctor belongs to medicaments in the range of the monitored medicament directory or not; and S5, when the medicament order of the doctor belongs to the medicaments in the range of the monitored medicament directory, prompting the doctor according to the calculated creatinine clearance rate, recording the creatinine clearance rate and identifying medication information of the patient. Through adoption of the method, the problems of low manual intervention efficiency and incapability of prompting immediately at present are solved, and the timeliness and security of clinical medication are ensured. Moreover, the method has a pre-prompting function, an in-process monitoring function, and a post statistical analysis function.
Owner:GUANGDONG HOSPITAL OF TRADITIONAL CHINESE MEDICINE
Compound simvastatin niacin sustained release tablet and preparation method thereof
InactiveCN101574345ASmall toxicityReduce the number of dosesOrganic active ingredientsMetabolism disorderDyslipidemiaSide effect
The invention discloses a compound simvastatin niacin sustained release tablet and a preparation method thereof. The compound simvastatin niacin sustained release tablet consists of the following four components: (1) a tablet core containing niacin; (2) an isolation layer; (3) a simvastatin layer; and (4) a protective film-coat layer. In the compound preparation, the niacin serves as a sustained release component and the simvastatin serves as a quick release component, thus all components play a synergistic action in vivo to comprehensively adjust the components of blood fat so as to play the drug action to the utmost extent, reduce the toxic side effects of the medicaments, reduce the fluctuation of the blood concentration in vivo, and reduce the dosing frequency of a patient; and the compound simvastatin niacin sustained release tablet is applicable to treating hypercholesteremia and mixed dyslipidemia.
Owner:WUXI DINGFU PHARMA
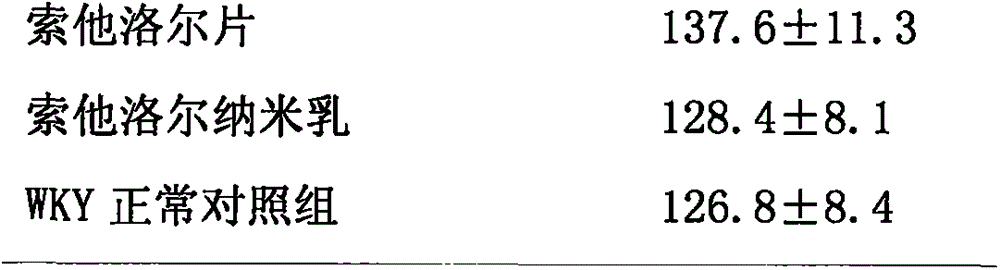
Oil-in-water type sotalol nanoemulsion antihypertensive drug
InactiveCN105997874AEvenly distributedSystem transparencyPharmaceutical non-active ingredientsAmide active ingredientsHalf-lifeWater soluble drug
The invention discloses an oil-in-water type sotalol nanoemulsion antihypertensive drug, the raw materials and the mass percentages of each raw material are: 1%-18% of sotalol, 15%-40% of surfactant, Co-surfactant is 0-25%, the rest is distilled water, and the sum of the mass percentages of the above-mentioned raw materials is 100%. The nano-emulsion emulsion has small particles, uniform distribution, low viscosity and good fluidity. After the water-soluble drug sotalol is prepared into nanoemulsion form, the stability and drug efficacy of sotalol are increased, the antihypertensive effect is obviously increased, the half-life of the drug is prolonged, and the number of administrations is reduced.
Owner:张鸿利
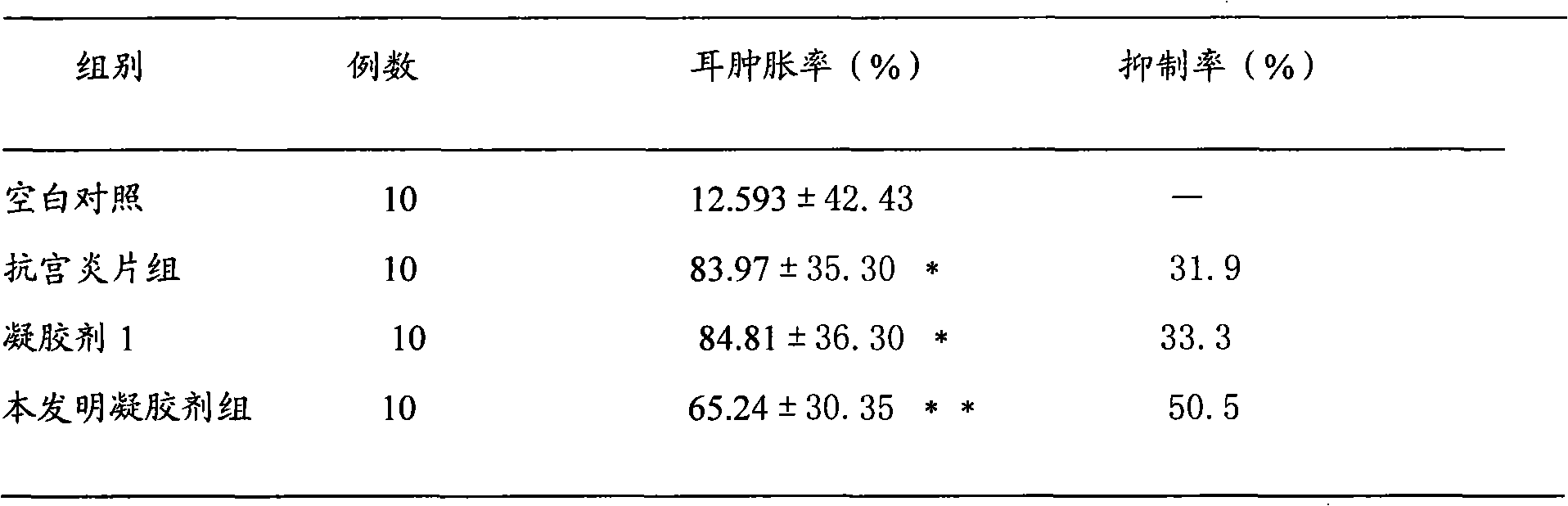
Gynecological disease resisting gel for treating gynecological disease and preparation method thereof
InactiveCN102028794AAvoid first pass effectAvoid stimulationAerosol deliveryOintment deliveryDiseaseRed bean
The invention discloses a gynecological disease resisting gel for treating gynecological disease and a preparation method. The gynecological disease resisting gel comprises the following components in parts by weight: 225-275 parts of radix sophorae flavescentis, 225-275 parts of polygonum perfoliatum, 135-165 parts of amur corktree bark, 45-55 parts of fructus forsythiae, 27-33 parts of motherwort herb, 27-33 parts of red bean, 27-33 parts of folium artemisiae argyi, 27-33 parts of Chinese angelica, and 27-33 parts of combined spicebush root. The preparation method of the gynecological disease resisting gel is as follows: crushing 160 parts of the total amount of the radix sophorae flavescentis, 90 parts of the total amount of the amur corktree bark, 15 parts of the total amount of the fructus forsythiae and 15 parts of the total amount of the red bean into fine powder for later use; adding water into nine medicine materials such as the rest of radix sophorae flavescentis, polygonum perfoliatum and the like, decocting for two times with the first time for 2 hours and the second time for 1 hour; combining decocted liquid, filtering to obtain filtered liquid, concentrating the filtered liquid to thick paste with the relative density of 1.31-1.35 at the temperature of 60-80 DEG C, adding the fine powder, and mixing uniformly; taking a medium, adding water for swelling, and preparing into 0.1-0.5% glue solution; and adding 2.5-5 parts of medicinal thick paste into 2.5-5 parts of glue solution, adding water under stirring, preparing into 1-4 parts of 30% wetting agent solution, and stirring uniformly, thus obtaining the gynecological disease resisting gel. By utilizing the invention, the dosing frequency can be greatly reduced, the treatment cycle is short, and the efficiency is high.
Owner:贵州远程制药有限责任公司
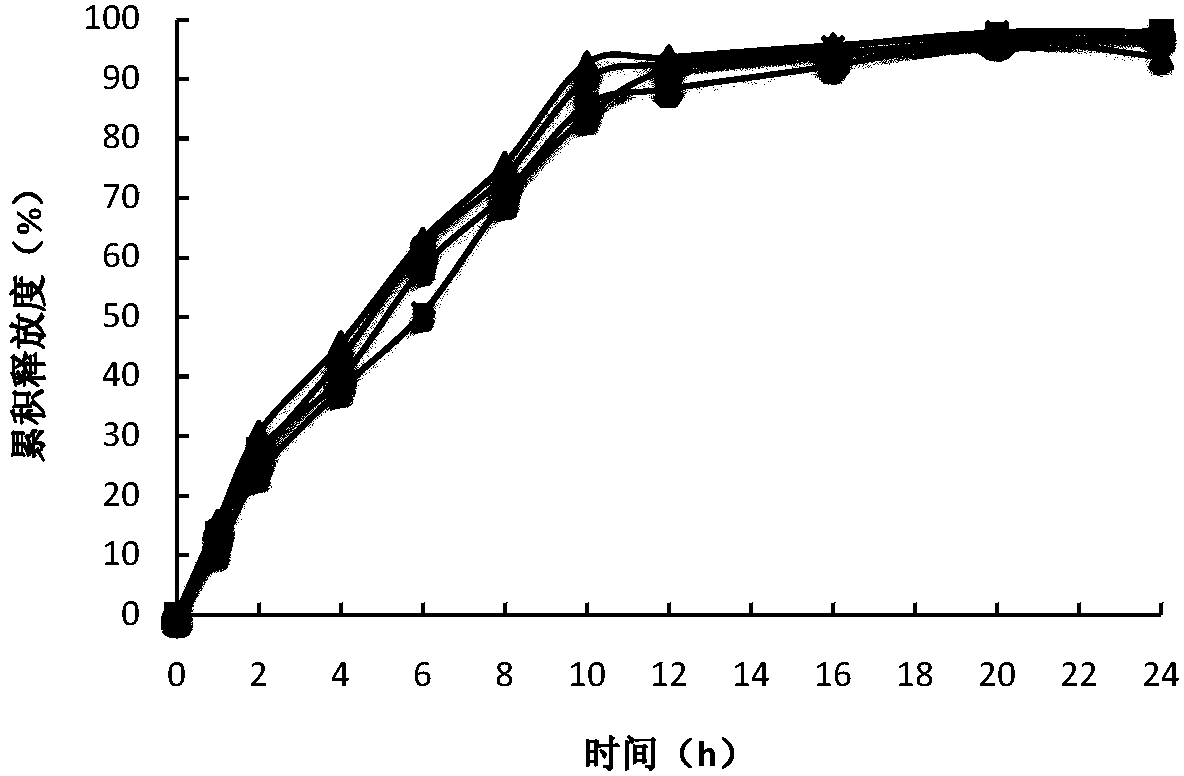
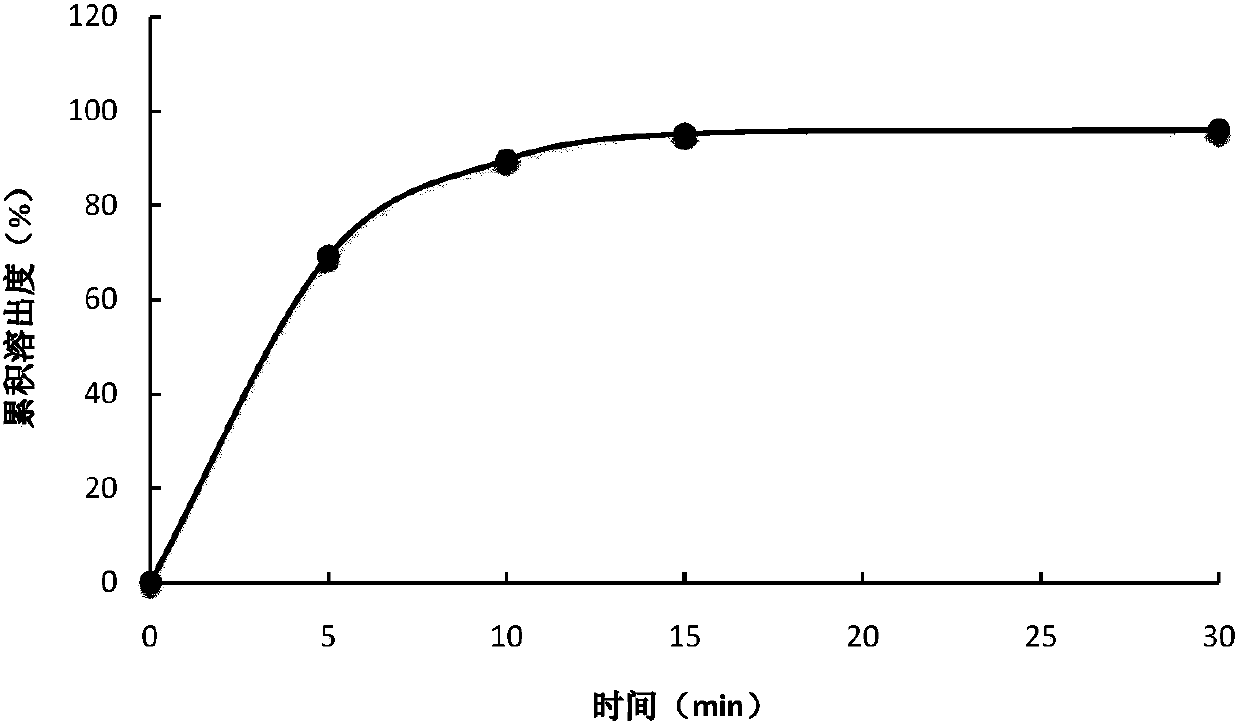
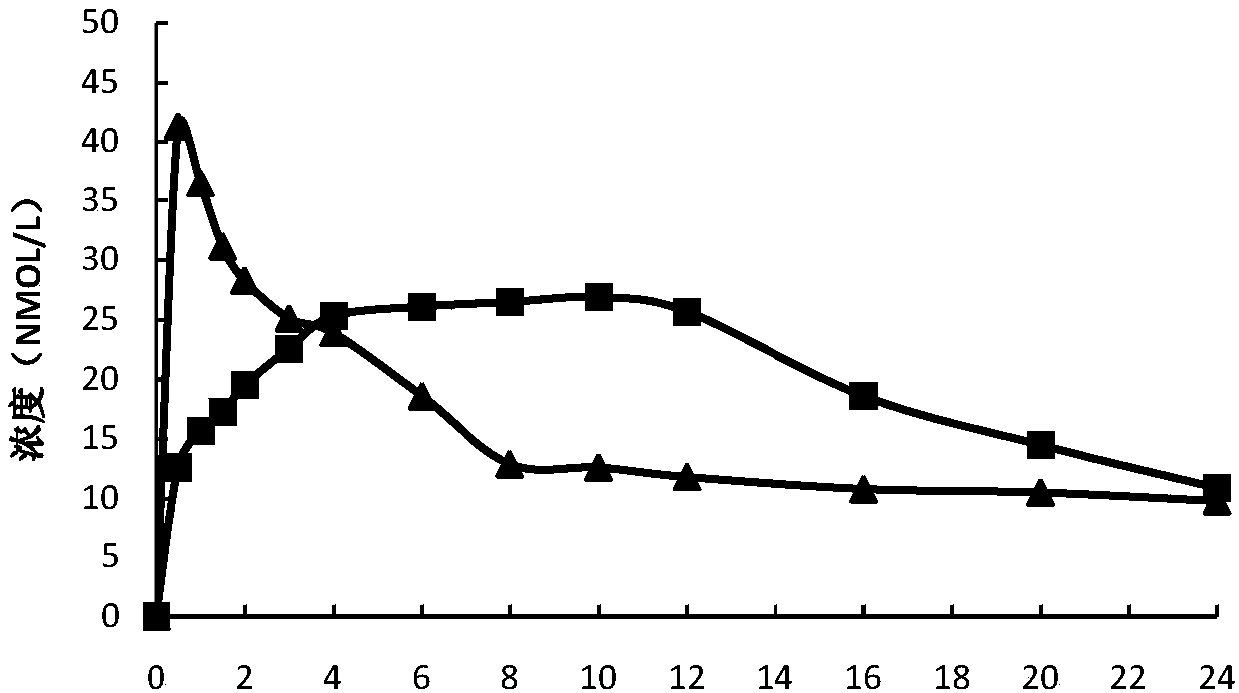
Controlled release preparation containing 5-methyltetrahydrofolate
ActiveCN109939077AGood curative effectAvoid the "peak" and "trough" phenomenon of blood drug concentrationOrganic active ingredientsPill deliveryDosing FrequencyOsmotic pump
The invention relates to a controlled release preparation containing 5-methyltetrahydrofolate. The controlled release preparation comprises a 5-methyltetrahydrofolate osmotic pump tablet core and a polymeric semi-permeable membrane coating layer. The semi-permeable membrane coating layer accounts for 2-15% of the weight of the tablet core, and the 5-methyltetrahydrofolate osmotic pump tablet corecan be a single-layer tablet or a double-layer tablet. By means of the preparation, 5-methyltetrahydrofolate can be slowly and stably released for a long time, the dosing frequency is reduced, and themedication compliance of a patient is improved.
Owner:SHENZHEN AUSA PHARM CO LTD +1
Low-toxicity nematode pesticide
The invention relates to a low-toxicity nematode pesticide comprising the following components: 10-20 parts of cypermethrin, 5-8 parts of dibutyl citrate calcium naphthalene sulfonate, 2-7 parts of xylene, 5-20 parts of common threewingnut root, 10-25 parts of Indian quassawood extract liquid, 12-22 parts of aromatic hydrocarbon liquid, 5-10 parts of ferrous sulfate, 20-25 parts of kerosene, and 20-40 parts of water. The pesticide also comprises 2-10 parts of tetramethrin. The pesticide comprises 15-20 parts of the Indian quassawood extract liquid. The low-toxicity nematode pesticide provided by the invention is suitable for various vegetable, melon, and fruit crops. With the pesticide, dosing frequency can be reduced, yield can be increased, melon and fruit sweetness can be improved, and cost can be saved. The pesticide has good practical value.
Owner:QINGDAO AIHUALONG BIOTECH
Method for establishing animal model for senile dementia, special liquid medicine and dosing device
InactiveCN103284980ATypical behaviorTypical pathological symptomsHydroxy compound active ingredientsMedical devicesSterile environmentSenile dementia
Owner:KUNMING INST OF ZOOLOGY CHINESE ACAD OF SCI
Rice field weeding composition and preparations therefor
InactiveCN103355338ADelay drug resistanceSynergisticBiocideAnimal repellantsDosing FrequencyDrug resistance
The invention relates to the pesticide preparation field, and discloses a rice field weeding composition and preparations therefor. The weeding composition is composed of azimsulfuron and benzobicylon. The weeding composition and preparations therefor take compound azimsulfuron and benzobicylon as effective constituents. Azimsulfuron and benzobicylon complement each other's advantages and have synergistic effects. The composition can delay generation of drug resistance of weeds, has synergistic effects on rice field weeds, reduces dosage and dosing frequency greatly, has advantages of safety, high efficiency, environmental protection and the like, and can be widely used for control of rice field grass damage.
Owner:LIANBAO CROP TECH
Pramipexole dihydrochloride sustained release tablet and preparation method thereof
InactiveCN104146979AContinuous and stable releaseFacilitated releaseOrganic active ingredientsNervous disorderTolerabilitySide effect
The invention provides a pramipexole dihydrochloride sustained release tablet and a preparation method thereof. The pramipexole dihydrochloride sustained release tablet comprises a pramipexole dihydrochloride tablet core and a coating, and one side or two sides of the tablet are provided with holes. The tablet core comprises at least a water-soluble filler, or comprises at least one cationic polymer, which is not dissolved in almost neutral (pH 5-7) environment and is rapidly dissolved in a medium with the pH less than 5. Due to the cationic polymer, the final tablet can release in the medium with the pH less than 5 faster than in the medium with the pH of 5-7. The pramipexole dihydrochloride sustained release tablet can constantly and stably release in a body, is not affected by the change of pH of the gastrointestinal tract, only needs to be taken once a day, is easy to take, has small side effects, stable curative effect and good tolerance and compliance, is beneficial to treatment of patients of Parkinsonism, can be used for maintaining the blood concentration in effective therapeutic concentration range for long and reducing the dosing frequency, and has greater clinical application value. The preparation method is simple and is applicable to industrial production.
Owner:SHANGHAI SUNTECH PHARMA
Drug information management device and drug information management method
ActiveCN103827915AContinuous quality improvementData processing applicationsDrug and medicationsMedication informationDosing Frequency
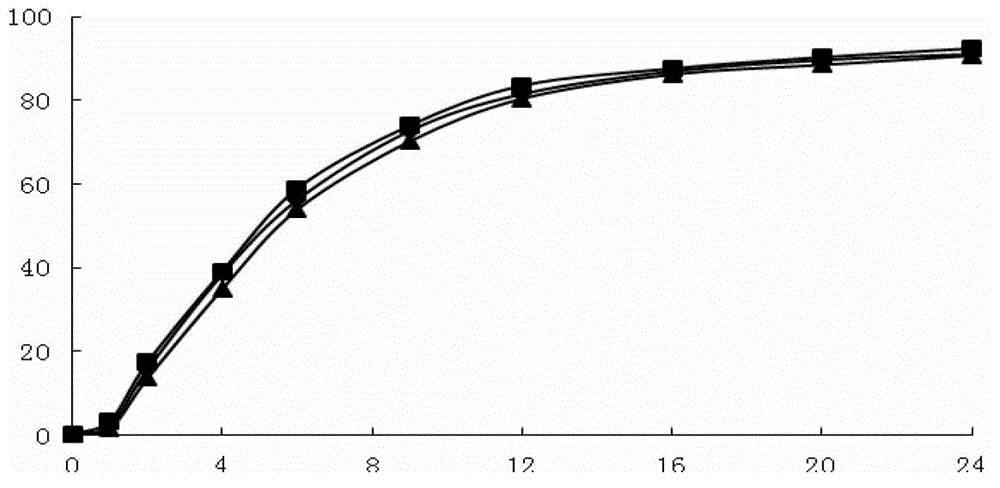
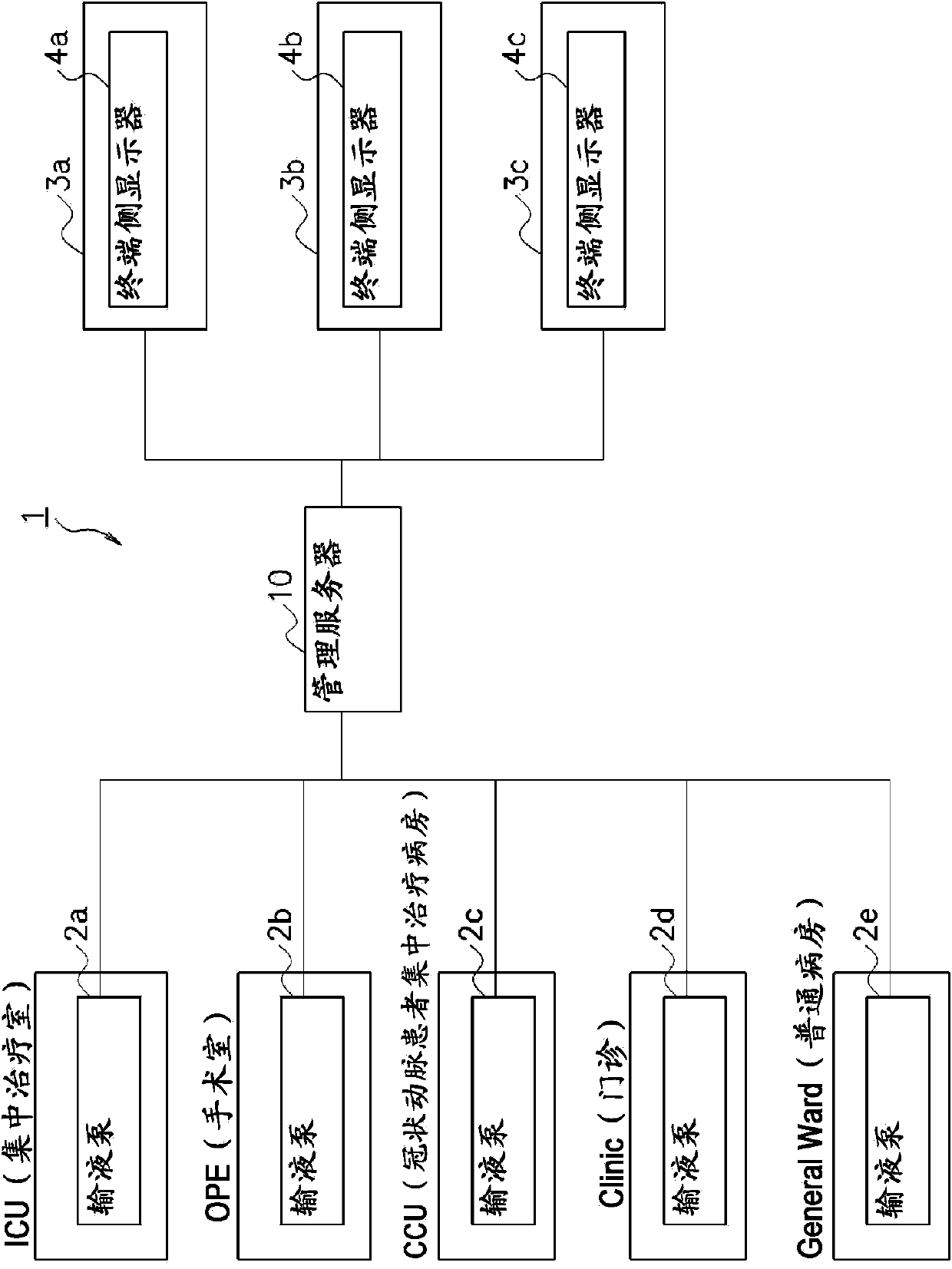
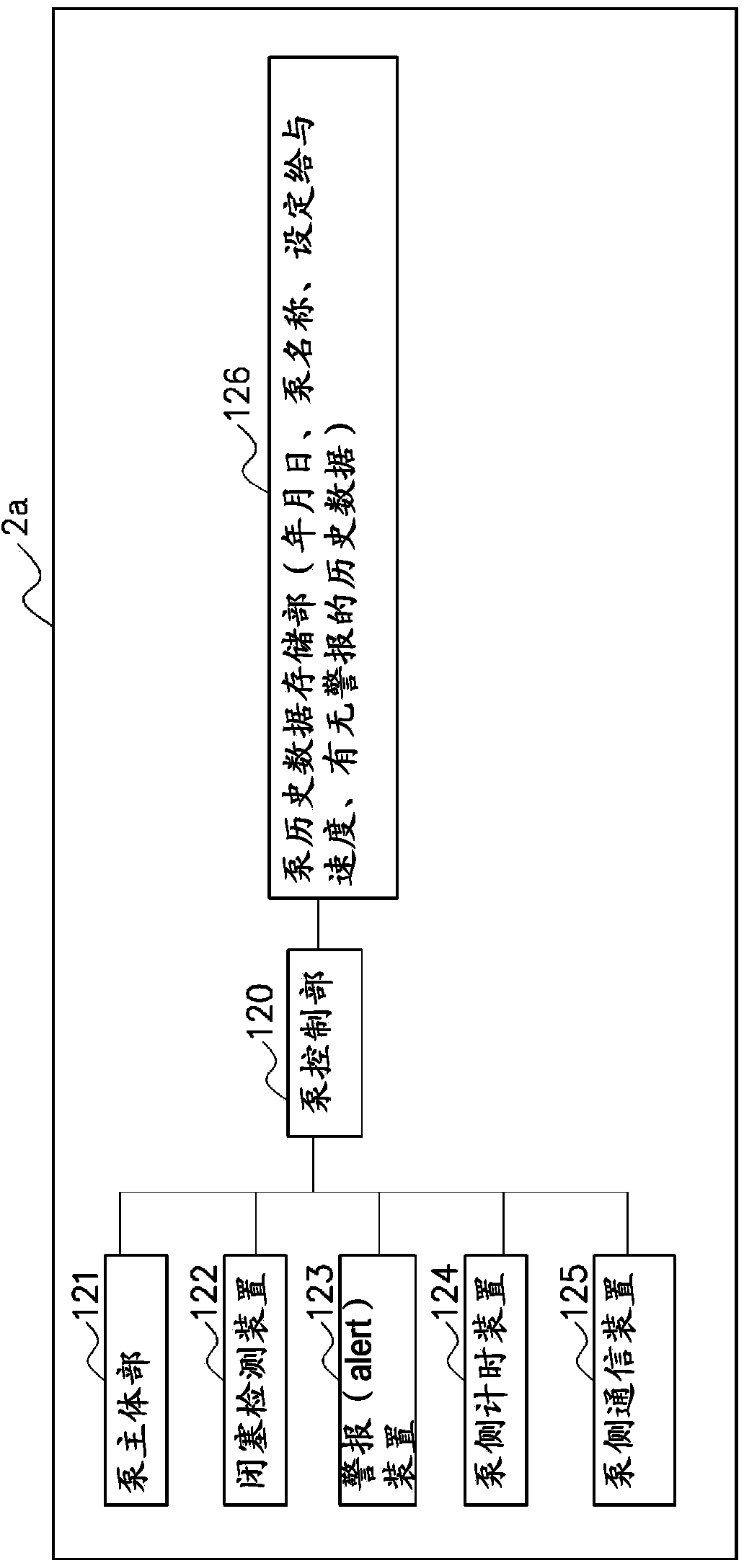
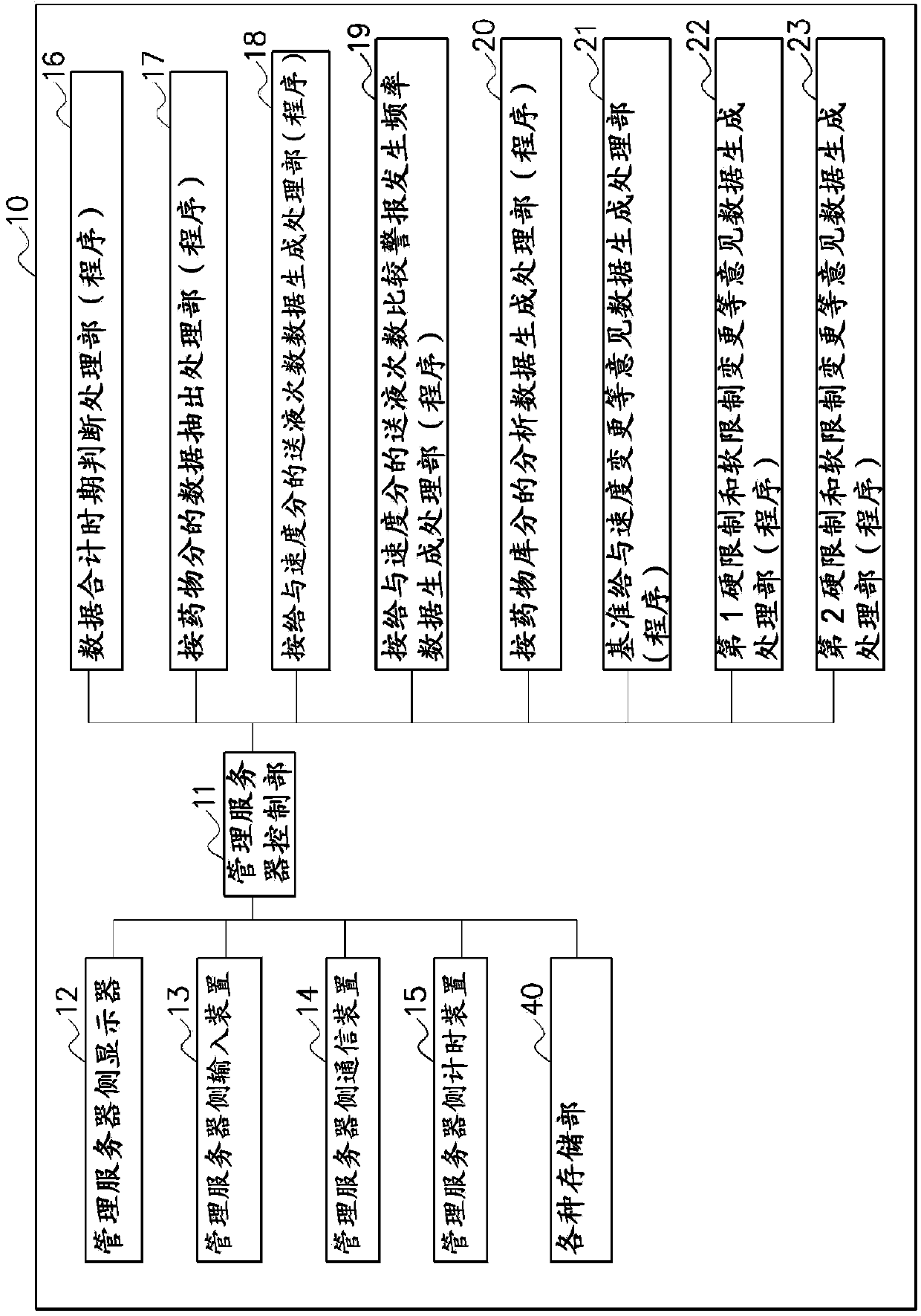
To provide a drug information management device and the like that enables CQI of information such as usage limits for drugs used in medical devices and such. A drug information management device (10) generates and stores dose frequency information related to dose level information, the dose frequency information being actual dose frequency information included in the dose level information; stores drug limit information that is information for limiting drug use in relation to the dose level information; stores, in association with drug information and the dose level information, failure information about a failure that has occurred in actual use of the drug according to the dose level information; generates and stores failure frequency and level information related to dose frequency and level on the basis of frequency information included in failure information and dose frequency information related to dose level information; generates drug analysis information that indicates, as information associated with the dose frequency information related to dose level information, the failure frequency and level information related to dose frequency and level and the drug limit information; and displays this drug analysis information on a display unit (4a) of a subject-side terminal (3a).
Owner:TERUMO KK
Oil-in-water type terazosin nanoemulsion antihypertensive drug
InactiveCN105997873AGood blood pressure effectStable buckOrganic active ingredientsPharmaceutical non-active ingredientsHalf-lifeWater soluble drug
The invention discloses an oil-in-water type terazosin nanoemulsion antihypertensive drug, the particle diameter of the nanoemulsion antihypertensive drug is between 45.3 and 92.4 nm, and the raw materials and the mass percentage of each raw material are: 1%-15% of lazosin, 15%-40% of surfactant, 0-20% of co-surfactant, the rest of the ingredients are distilled water, and the sum of the mass percentages of the above raw materials is 100%. The nano-emulsion emulsion has small particles, uniform distribution, low viscosity and good fluidity. After the water-soluble drug terazosin is prepared into a nanoemulsion type, the stability and drug efficacy of the terazosin are increased, the antihypertensive effect is obviously increased, the half-life of its drug is prolonged, and the number of administrations is reduced.
Owner:张鸿利
ITE for Cancer Intervention and Eradication
ActiveUS20130310429A1Good curative effectInhibit angiogenesisPhotovoltaic supportsBiocideProstate cancerCarrier system
A method of cancer intervention or eradication by administering an effective amount of an endogenous ligand for the aryl hydrocarbon (Ah) receptor (AhR) named ITE or one of its analogs (the active ingredient) to a subject with cancer is disclosed. An effective dose and dosing frequency of the active ingredient are determined by measuring its blood levels of the subject after dosing. The active ingredient formulated with a carrier system is applied topically, enterally, or parenterally to the subject. The formulated drug can also be administered together with one or more of other cancer therapeutic agents. A maintenance dosing is provided after the subject is free of cancer to insure the cancer eradication. Subjects with cancers of prostate, liver, lung, ovarian, and breast are preferably accepted for treatment.
Owner:ARIAGEN INC
Deratting plaster and preparation method therefor
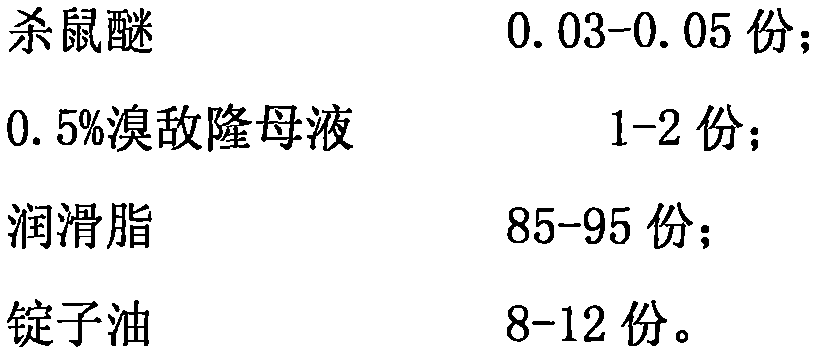
ActiveCN105379709AEasy to killGood effect on killing ratsBiocideDead animal preservationDosing FrequencySpindle oil
The present invention relates to a deratting plaster and a preparation method therefor. The deratting plaster is made up of the following raw materials in parts by weight: 0.03-0.05 part of coumatetralyl; 1-2 parts of bromadiolone mother liquor; 85-95 parts of lubricating grease; and 8-12 parts of spindle oil. The preparation method comprises: after the above raw materials are weighed based on parts by weight, adding absolute ethanol into coumatetralyl with stirring to dissolved, so as to obtain a coumatetralyl solution; and mixing the obtained resulting coumatetralyl solution and 0.5% bromadiolone mother liquor evenly, adding spindle oil and lubricating grease with stirring evenly, so as to obtain the deratting plaster. The deratting plaster of the present invention requires a long time to be air-dried and hardened, less dosing frequency and a long dosing interval, without causing rats to be alert, so that the rats can be effectively eliminated with a low safety risk.
Owner:HEBEI YANHENG ENVIRONMENTAL PROTECTION TECH CO LTD
Arnica long-acting sustained release preparation for treating contusions and preparation method thereof
InactiveCN105412191AImprove stabilityExtension of timeAntipyreticAerosol deliveryRelease timeDosing Frequency
The invention relates to an arnica long-acting sustained release preparation for treating contusions and a preparation method thereof, and belongs to the technical field of traditional Chinese medicine preparations. By means of the arnica long-acting sustained release preparation, long-acting dosing of medicine for external use is achieved, stability of active ingredients is improved, the release time of the arnica long-acting sustained release preparation is prolonged, the dosing frequency is reduced, skin stimulation caused by inconvenience and excessive medicament of multiple times of dosing in each day is avoided, and tolerance of patients is improved to a certain degree. By means of the arnica long-acting sustained release preparation, the aim of prolonging efficacy is achieved through the specific composition ratio and the specific preparation method.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com