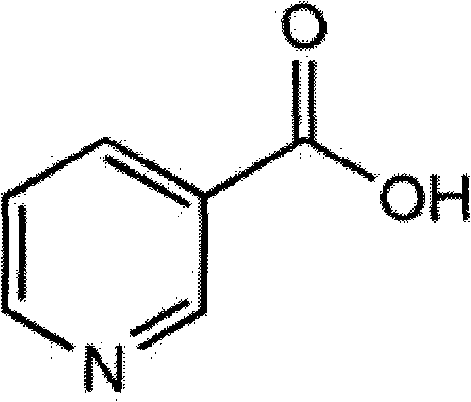
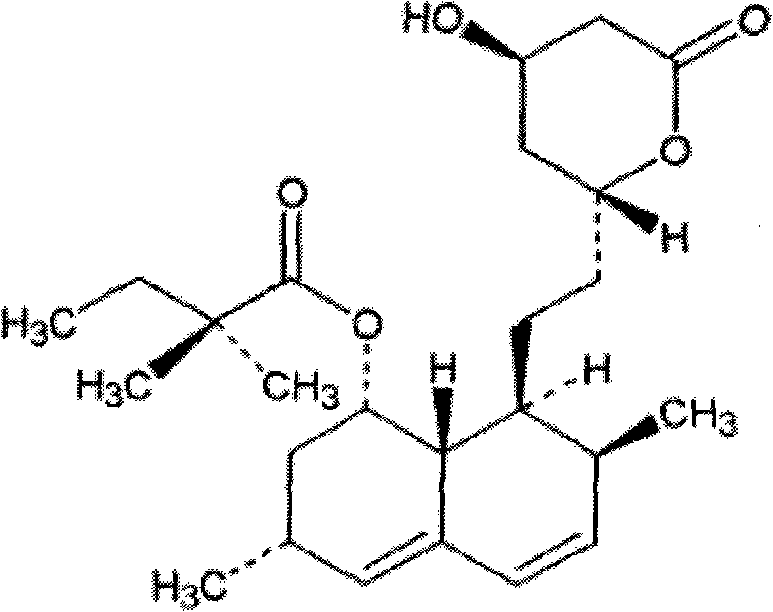
Compound simvastatin niacin sustained release tablet and preparation method thereof
A technology for simvastatin and sustained-release tablets, which is applied in pharmaceutical formulations, medical preparations containing active ingredients, and pill delivery, etc. It can solve the problems of niacin adverse reactions, poor patient compliance, skin flushing, etc., and reduce the number of medications , reduce toxic and side effects, reduce the effect of fluctuations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Chip:
[0035] Niacin 500mg
[0036] Hypromellose 2208 200mg
[0037] Stearic acid 7mg
[0038] Isolation layer:
[0039] Hypromellose 2910 10mg
[0040] Macrogol 400 1mg
[0041] Simvastatin layer:
[0042] Simvastatin 10mg
[0043] Hypromellose 2910 50mg
[0044] Macrogol 400 5mg
[0045] tert-butylhydroxyanisole 0.3mg
[0046] Protective film coating layer:
[0047] Hypromellose 2910 20mg
[0048] Macrogol 400 2mg
[0049] Titanium Dioxide 2mg
[0050] The preparation method is as follows:
[0051] Preparation of tablet cores: Mix niacin and hypromellose 2208 evenly, make granules with suitable equipment such as a strong mixer, dry and granulate, add stearic acid, mix well, and press into tablets to obtain the product.
[0052] Coating isolation layer: make hypromellose 2910 and polyethylene glycol 400 into a coating solution, place the prepared tablet core in a high-efficiency coating machine or other coating equipment, spray the coating solution, and ...
Embodiment 2
[0056] Chip:
[0057] Niacin 500mg
[0058] Hypromellose 2208 200mg
[0059] Stearic acid 14mg
[0060] Isolation layer:
[0061] Hypromellose 2910 10mg
[0062] Macrogol 400 1mg
[0063] Simvastatin layer:
[0064] Simvastatin 10mg
[0065] Hypromellose 2910 50mg
[0066] Macrogol 400 5mg
[0067] Propyl gallate 0.3mg
[0068] Protective film coating layer:
[0069] Hypromellose 2910 20mg
[0070] Macrogol 400 2mg
[0071] Yellow Iron Oxide 2mg
[0072] The preparation method is as follows:
[0073] Preparation of tablet cores: Mix niacin and hypromellose 2208 evenly, make granules with suitable equipment such as a strong mixer, dry and granulate, add stearic acid, mix well, and press into tablets to obtain the product.
[0074] Coating isolation layer: make hypromellose 2910 and polyethylene glycol 400 into a coating solution, place the prepared tablet core in a high-efficiency coating machine or other coating equipment, spray the coating solution, and blow Ho...
Embodiment 3
[0078] Chip:
[0079] Niacin 500mg
[0080] Hypromellose 2208 200mg
[0081] Stearic acid 7mg
[0082] Povidone 35mg
[0083] Isolation layer:
[0084] Hypromellose 2910 10mg
[0085] Macrogol 400 1mg
[0086] Polysorbate 80 0.25mg
[0087] Simvastatin layer:
[0088] Simvastatin 10mg
[0089] Hypromellose 2910 50mg
[0090] Macrogol 400 5mg
[0091] Polysorbate 80 1.25mg
[0092] Citric acid 0.1mg
[0093] Protective film coating layer:
[0094] Hypromellose 2910 20mg
[0095] Macrogol 400 2mg
[0096] Polysorbate 80 0.5mg
[0097] Tartrazine 2mg
[0098] The preparation method is as follows:
[0099] Preparation of tablet cores: Mix nicotinic acid, hypromellose 2208, and povidone evenly, make granules with suitable equipment such as a strong mixer, dry and granulate, add stearic acid, mix well, and press into tablets to obtain the product.
[0100] Coating isolation layer: make hypromellose 2910, polyethylene glycol 400, and polysorbate 80 into a coating li...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com