Adriamycin liposome temperature-sensitive gel for tumor local injection
A technology of temperature-sensitive gel and doxorubicin, which is applied in the field of medicine, can solve the problems of temperature-sensitive gel influence and poor water solubility of chitosan, and achieve the effects of reducing the number of administrations, stabilizing properties, and reducing systemic side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
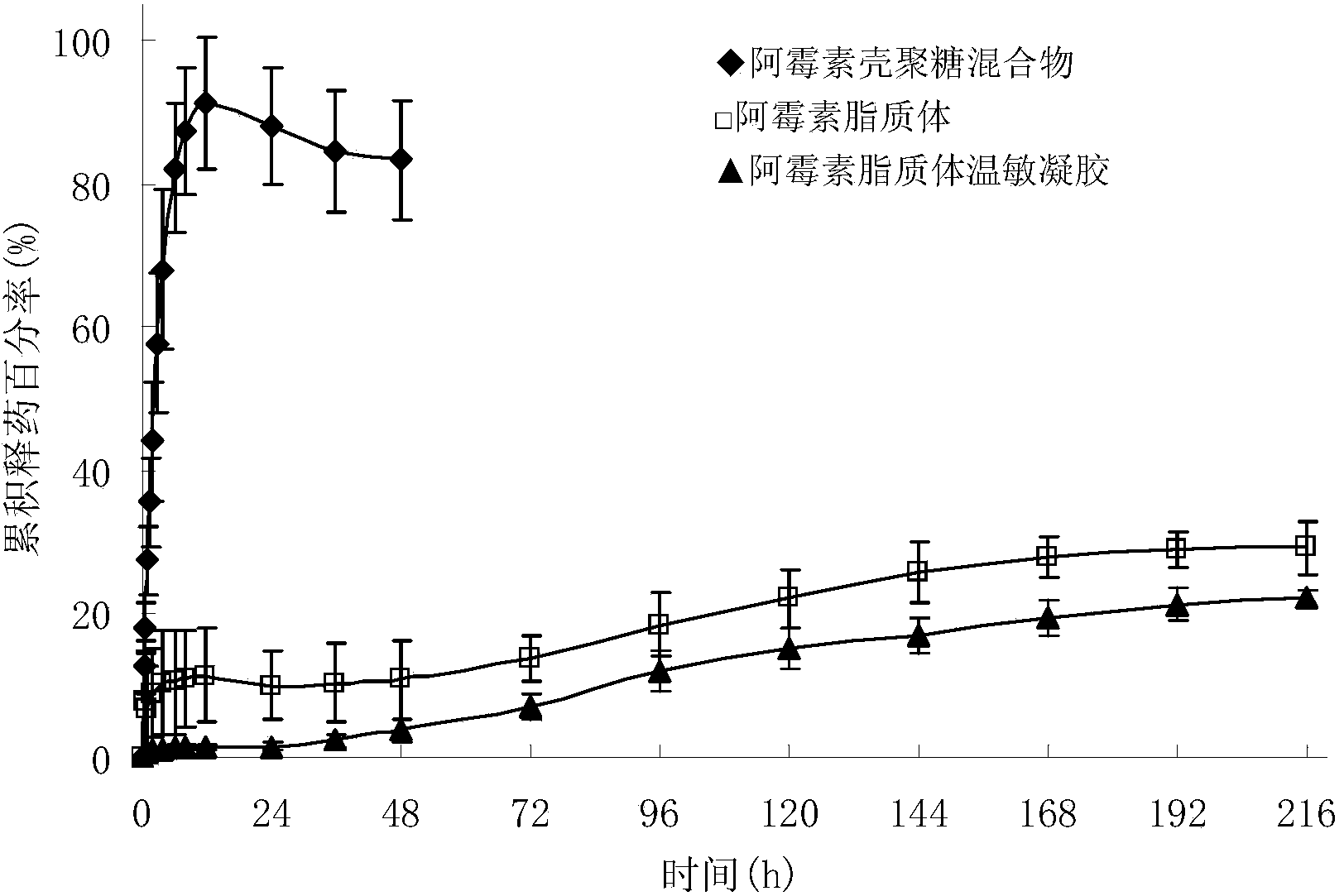
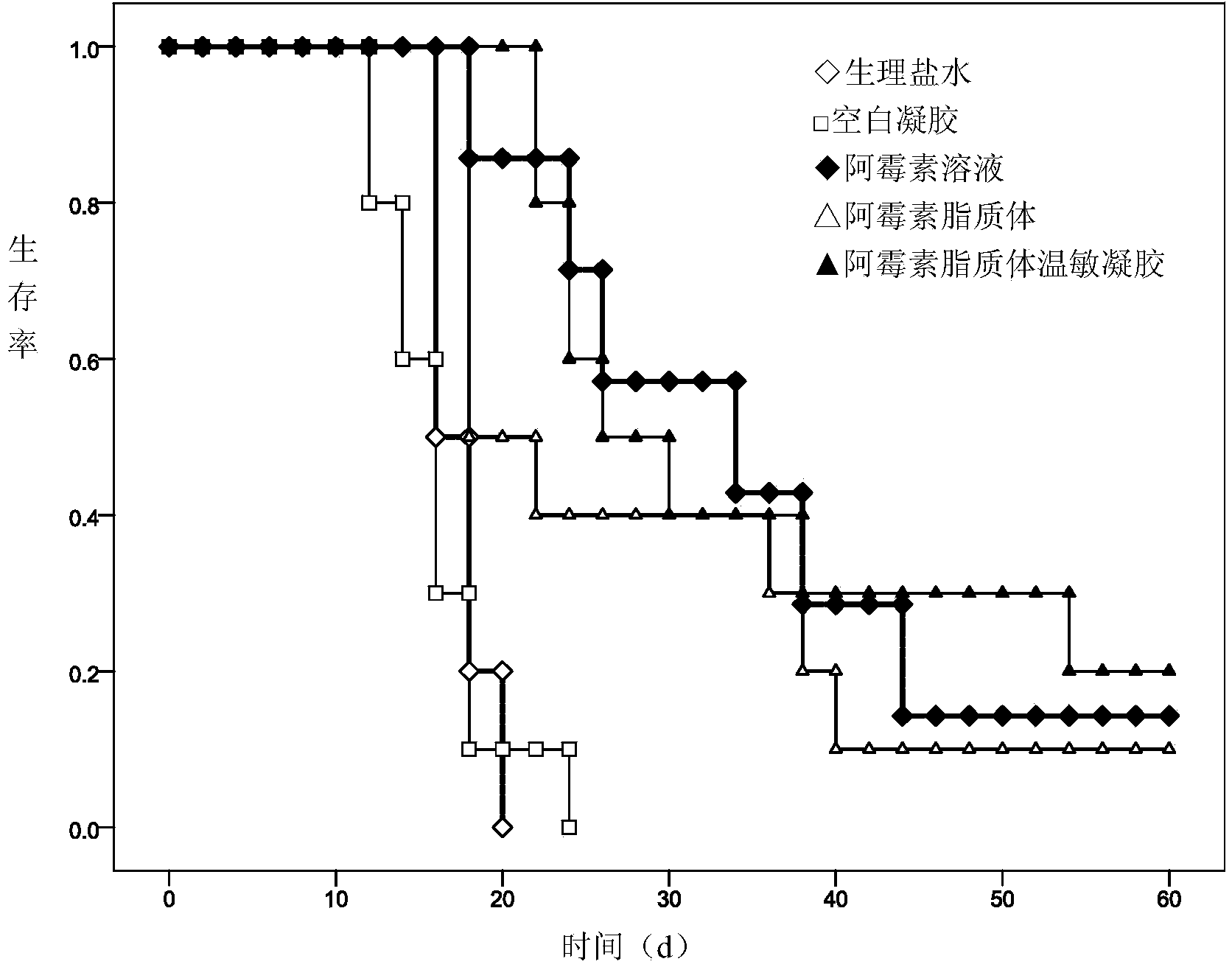
Image
Examples
Embodiment 1
[0025] The preparation of embodiment 1 doxorubicin liposome gel (preparation of liposome by film dispersion method)
[0026] (1) Preparation of blank liposomes
[0027] The preparation of liposomes adopts the ammonium sulfate gradient method: take 500 mg of soybean lecithin and 125 mg of cholesterol, dissolve them in 30 mL of absolute ethanol, place them on a rotary evaporator at 37 °C to remove ethanol by rotary evaporation under reduced pressure, add 8 mL of 0.3 mol L -1The ammonium sulfate aqueous solution was hydrated for 2 hours, and the probe was ultrasonicated 90 times at a power of 400w (working 5s, intermittent 5s). Put the prepared liposomes in a dialysis bag with a molecular weight cut-off of 14000Da, dialyze in 1000 mL of 0.9 wt% sodium chloride for 24 hours, take out the liposomes, add 0.9 wt% sodium chloride solution to dilute to 10 mL , to obtain blank liposomes.
[0028] (2) preparation of doxorubicin liposome
[0029] Doxorubicin hydrochloride was dissolved...
Embodiment 2
[0033] Embodiment 2: Preparation of doxorubicin liposome gel (preparation of liposomes by ethanol injection method)
[0034] (1) Preparation of blank liposomes
[0035] Take soybean lecithin 300mg, cholesterol 75mg, dissolve in 10mL absolute ethanol. Another 0.1mol L -1 Heat 20 mL of ammonium sulfate aqueous solution to 60°C, slowly inject the lipid ethanol solution under magnetic stirring, stir at constant temperature for 2 hours, evaporate the ethanol, and sonicate the probe 180 times under 400w power (working 5s, intermittent 5s), to obtain liposomes. Load the liposomes on a Sephadex G50 Sephadex column, elute with 10 wt% sucrose, collect the liposome eluate, add 10 wt% sucrose to dilute to 10 mL, and obtain blank liposomes.
[0036] (2) preparation of doxorubicin liposome
[0037] The resulting blank liposomes were mixed with 1 mL of 30 mg·mL doxorubicin aqueous solution, shaken evenly, and incubated at 40°C for 30 min to obtain doxorubicin liposomes.
[0038] (3) Prep...
Embodiment 3
[0040] Embodiment 3 thermosensitive property investigation
[0041] The thermosensitivity of doxorubicin lipid thermosensitivity gel system was determined by test tube tilting method. Add the doxorubicin lipid body temperature-sensitive gel solution prepared in Example 1 above into a glass test tube, place it in a constant temperature water bath at 37°C, insert a fixed thermometer into the glass tube, directly measure the temperature of the mixed system, and observe the gelation situation . When the temperature in the thermometer rises to 35°C, start counting, tilt the test tube 90°, and stop counting if the liquid level in the test tube does not move within 30 seconds, and the recorded time is the gelation time. As a result, it was found that the gelation time of the doxorubicin liposome-sensitive gel system was 4.00 ± 0.18min, compared with the gelation time (3.16±0.29min) of the blank liposome-sensitive gel system and the blank without liposomes. There was no significant ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com