Angiogenesis inhibiting fusion protein and its use
A technology of fusion protein and protein, which is applied in the field of gene recombinant protein, can solve the problems of large side effects, no further development, instability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: Construction of fusion protein and its plasmid.
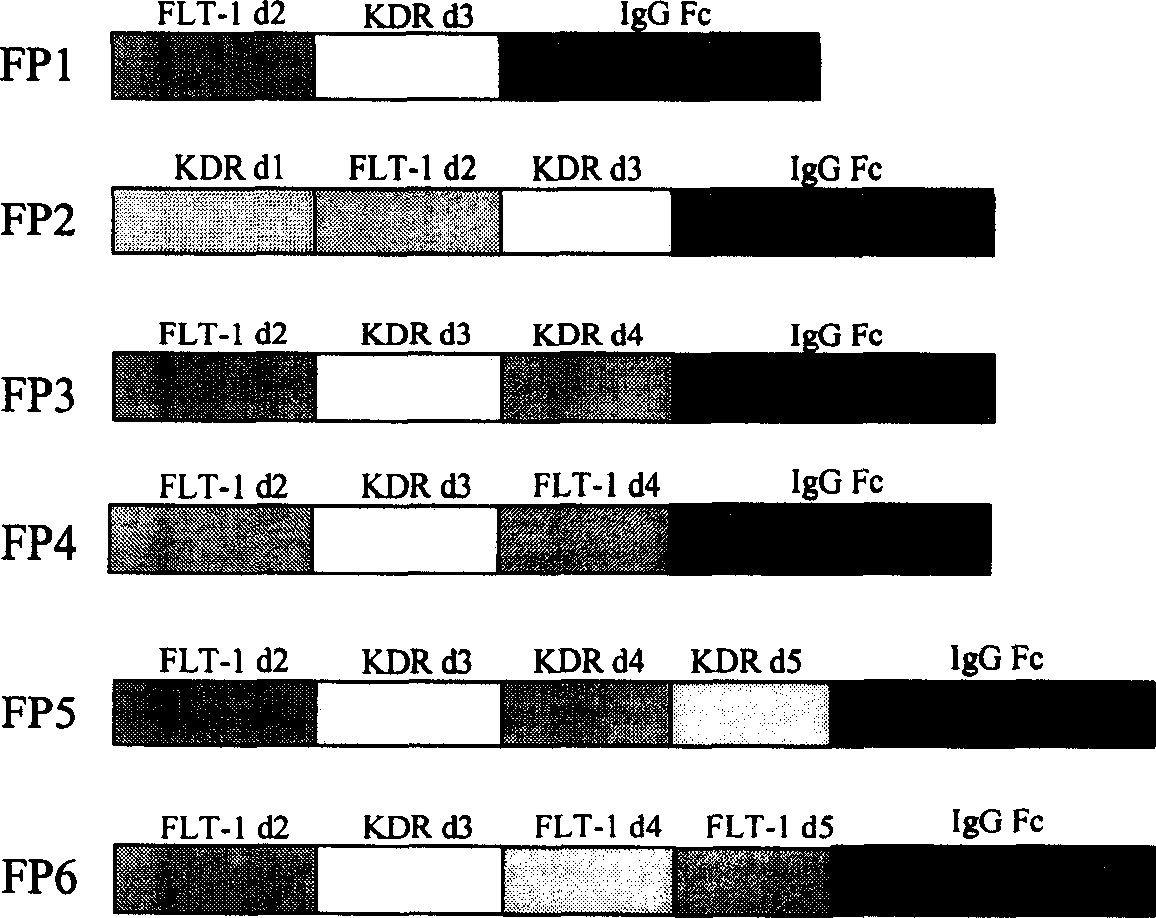
[0043] Except for the immunoglobulin Fc fragment, the original sequences for constructing various fusion proteins in the present invention come from the corresponding cDNAs of FLT-1 and KDR. Since the expression of FLT-1 and KDR is mainly found in vascular endothelial cells, the present invention extracts total RNA from human umbilical vein vascular endothelial cells (HUVEC) with an RNA purification kit (QIAGEN). cDNA is then synthesized from RNA using reverse transcriptase. Then different primers were used to amplify the required FLT-1 and KDR fragments by polymerase chain reaction (PCR). Finally, PCR was used to fuse sequences from FLT-1, KDR, and human immunoglobulin Fc (IgG1 Fc) to construct DNA sequences of different fusion proteins. The structures of the six fusion proteins are shown in figure 1 .
[0044] Construction of FP3 gene:
[0045] Human umbilical vein vascular endothelial cells (HUVEC cel...
Embodiment 2
[0056] Example 2: Expression of fusion protein in cells.
[0057] One of the components of the present invention is the expression of the constructed fusion protein in cells. After completing the construction of each plasmid, high-purity plasmid DNA was extracted with a plasmid DNA purification kit (QIAGEN). Then, the plasmid DNA was introduced into 293 cells by using the FUGEN6 plasmid transfection kit (ROCHE Company). According to the amount of required protein, two different plasmid transfection methods were used to express the fusion protein.
[0058]The first method is the transient transfection method, by which a small amount of fusion protein can be obtained. Firstly, 293 cells were cultured in a cell culture dish with DMEM complete medium containing 10% fetal bovine serum. When the cells grow to cover 60-80% area, the complex of plasmid DNA and FUGEN6 reagent is added to the cell culture medium. The next day, replace the medium with serum-free DMEM medium. The cul...
Embodiment 3
[0061] Example 3: Binding experiment of fusion polypeptide and VEGF.
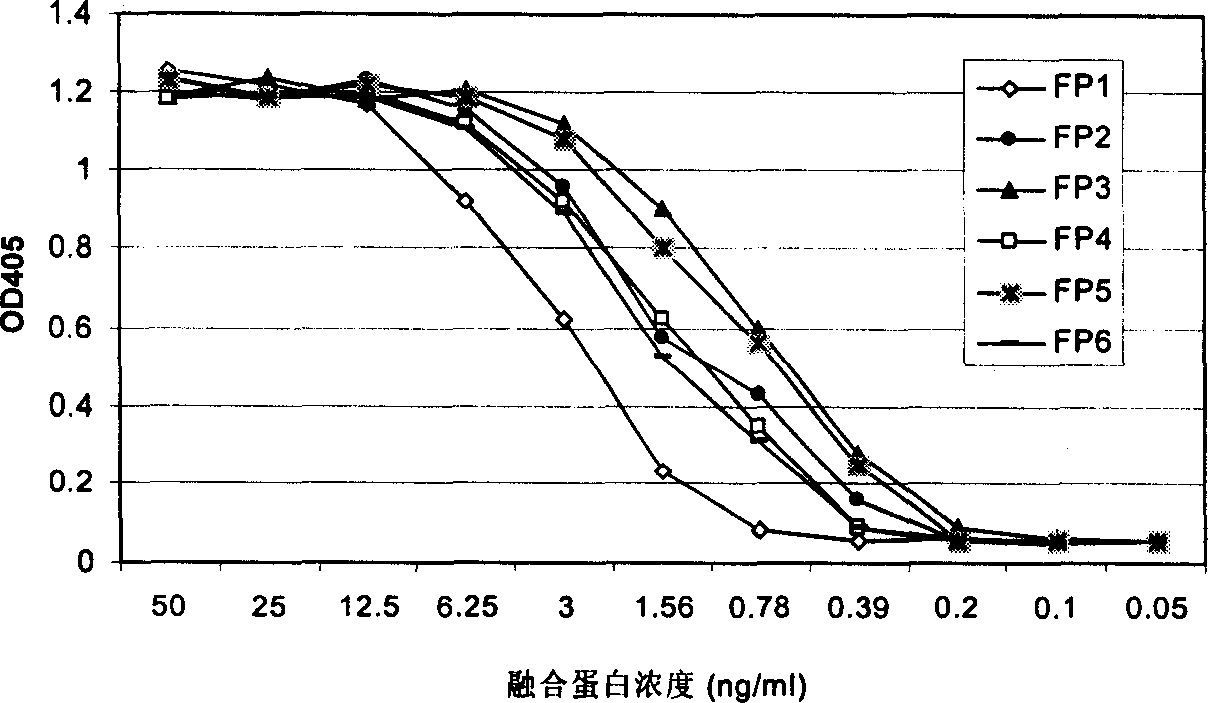
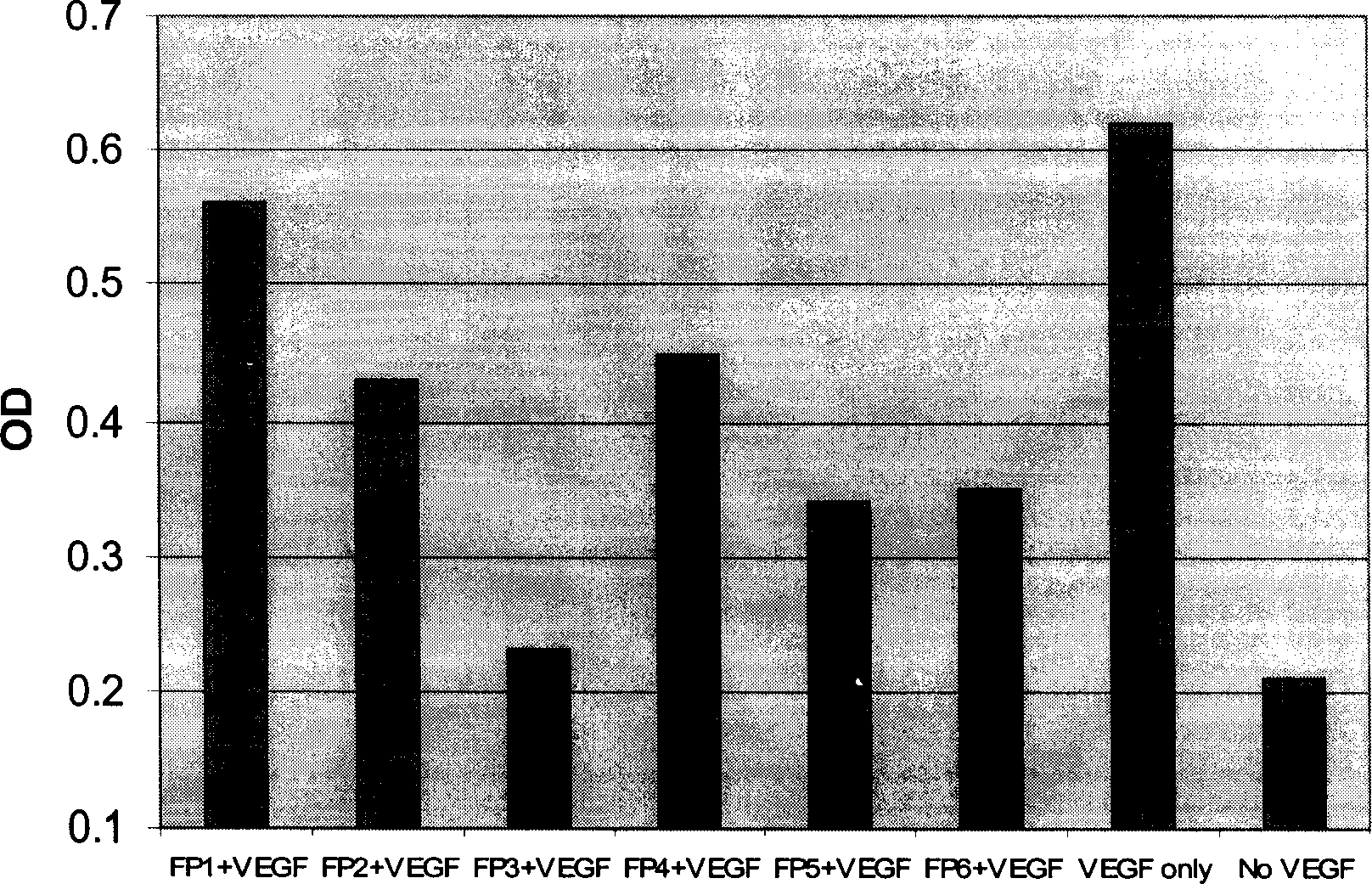
[0062] The present invention uses a VEGF binding test to measure the binding ability of each fusion polypeptide to VEGF. In this assay, recombinant VEGF (Chemicom) protein was first coated on a 96-well ELISA plate. Non-specific protein binding sites were then blocked with a 5% milk powder solution. Add various fusion proteins containing different concentrations to each well, and incubate at 37 degrees for two hours. After washing, rabbit anti-human Ig antibody-HRP was added. Finally, the color is developed with a peroxidase substrate. The OD value of each well of the 96-well plate was measured with an ELISA reader. High OD values represent the binding signal of the fusion protein to VEGF.
[0063] Such as figure 2 As shown, the six fusion proteins constructed and expressed in the present invention all have the ability to combine with VEGF. Their binding signal to VEGF can be detected at a concentra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com