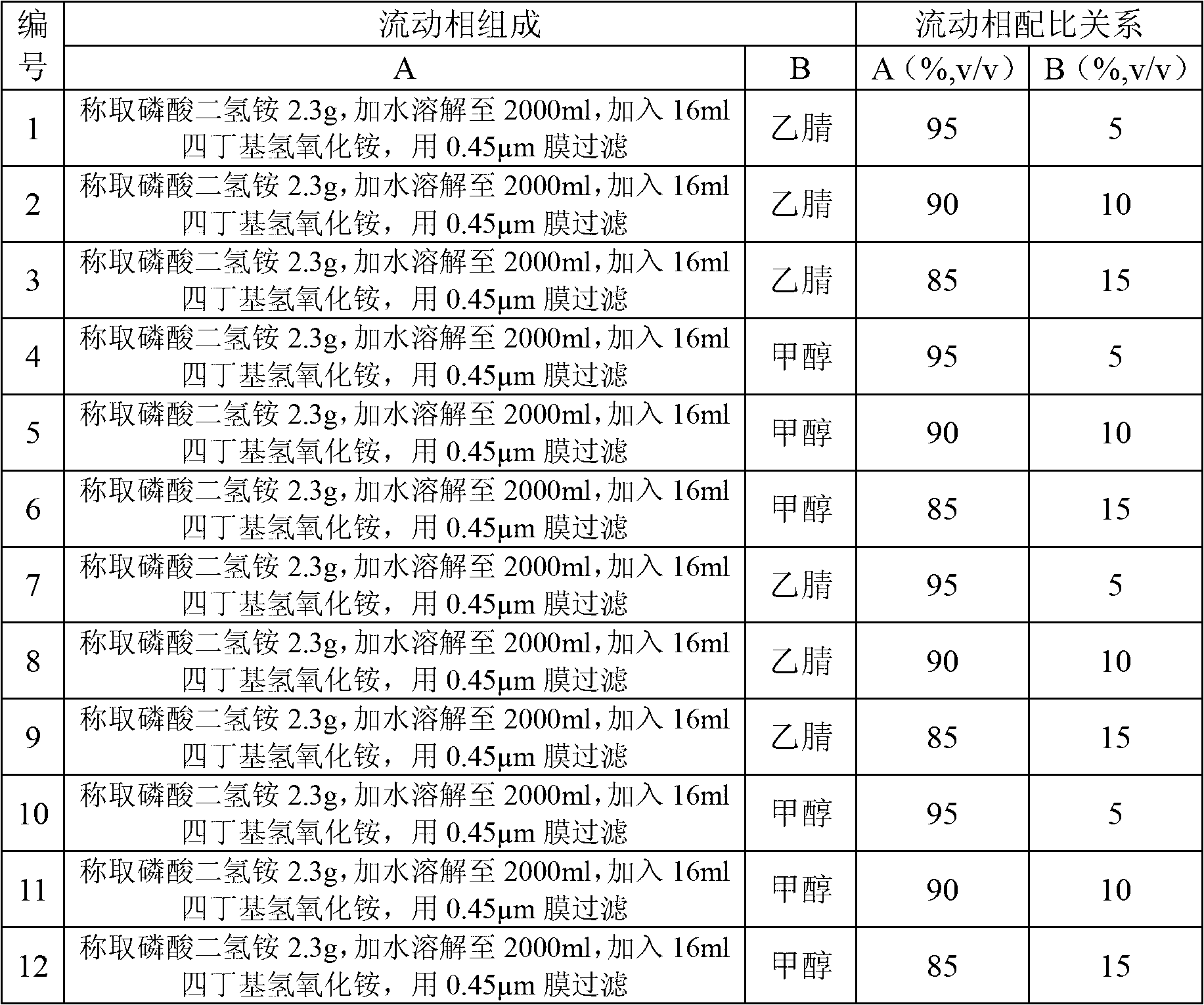
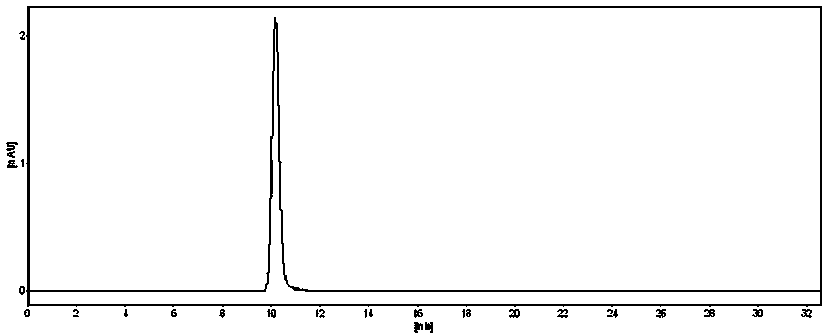
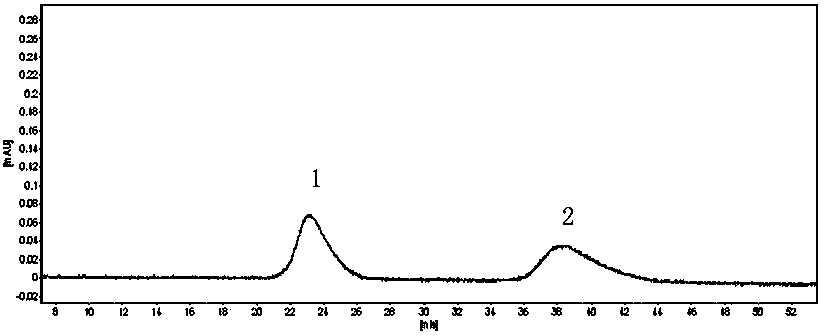
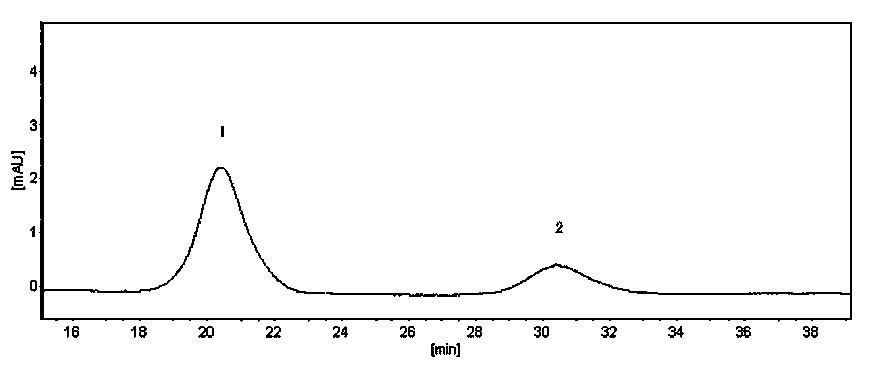
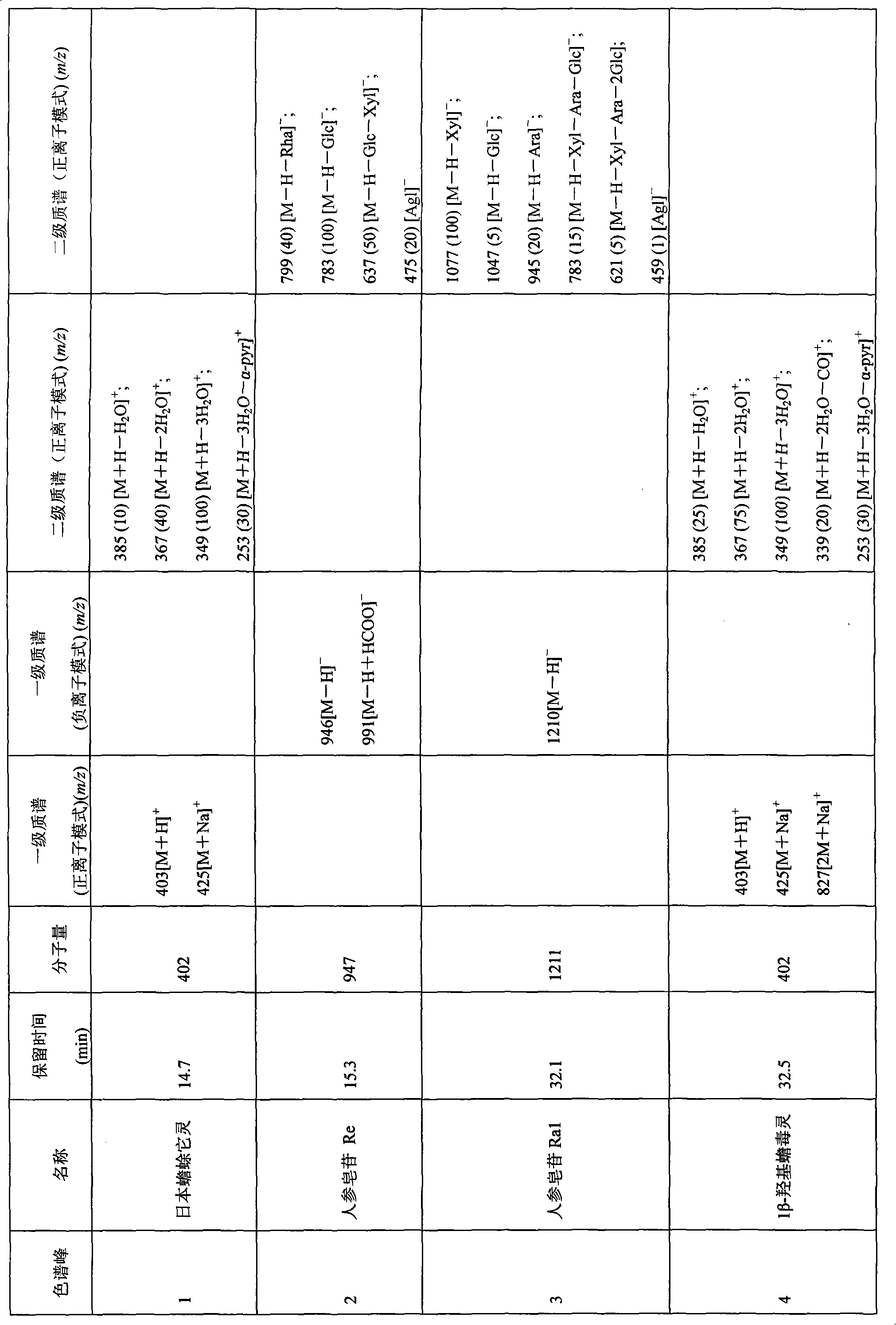
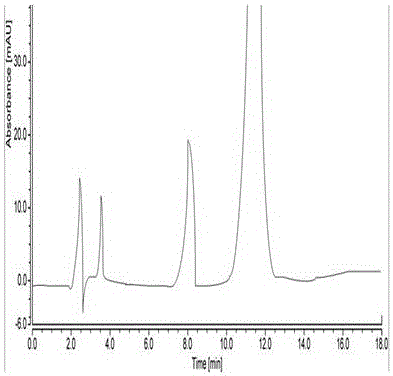
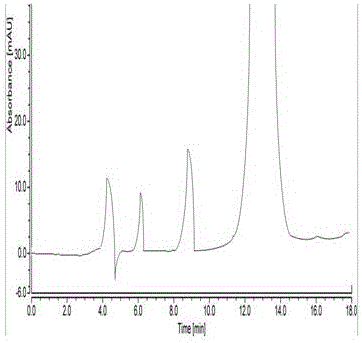
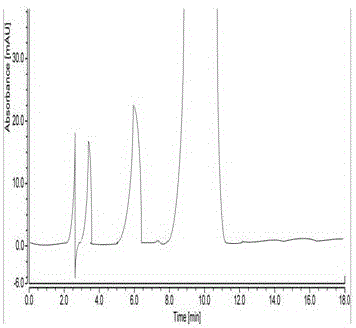
Patents
Literature
595 results about "Drug analysis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Drug analysis is the testing of a suspected controlled substance to determine its composition. For information about forensic toxicology, or the testing of bodily fluids for controlled substances, click here.
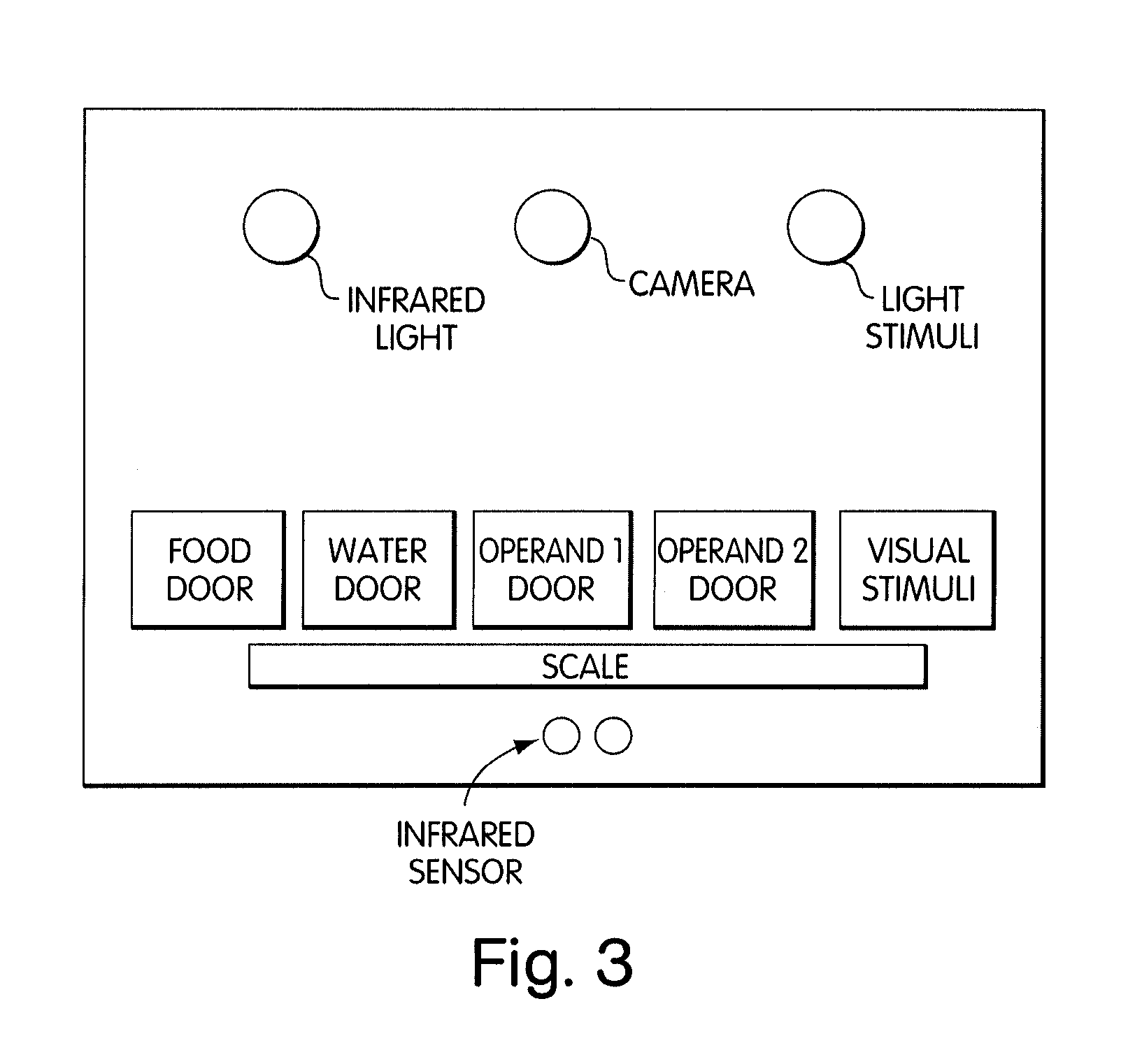
Systems and methods for monitoring behavior informatics
InactiveUS20030100998A2Improve statistics performanceExpand selectionDrug and medicationsBiostatisticsMulti dimensionalOrganism
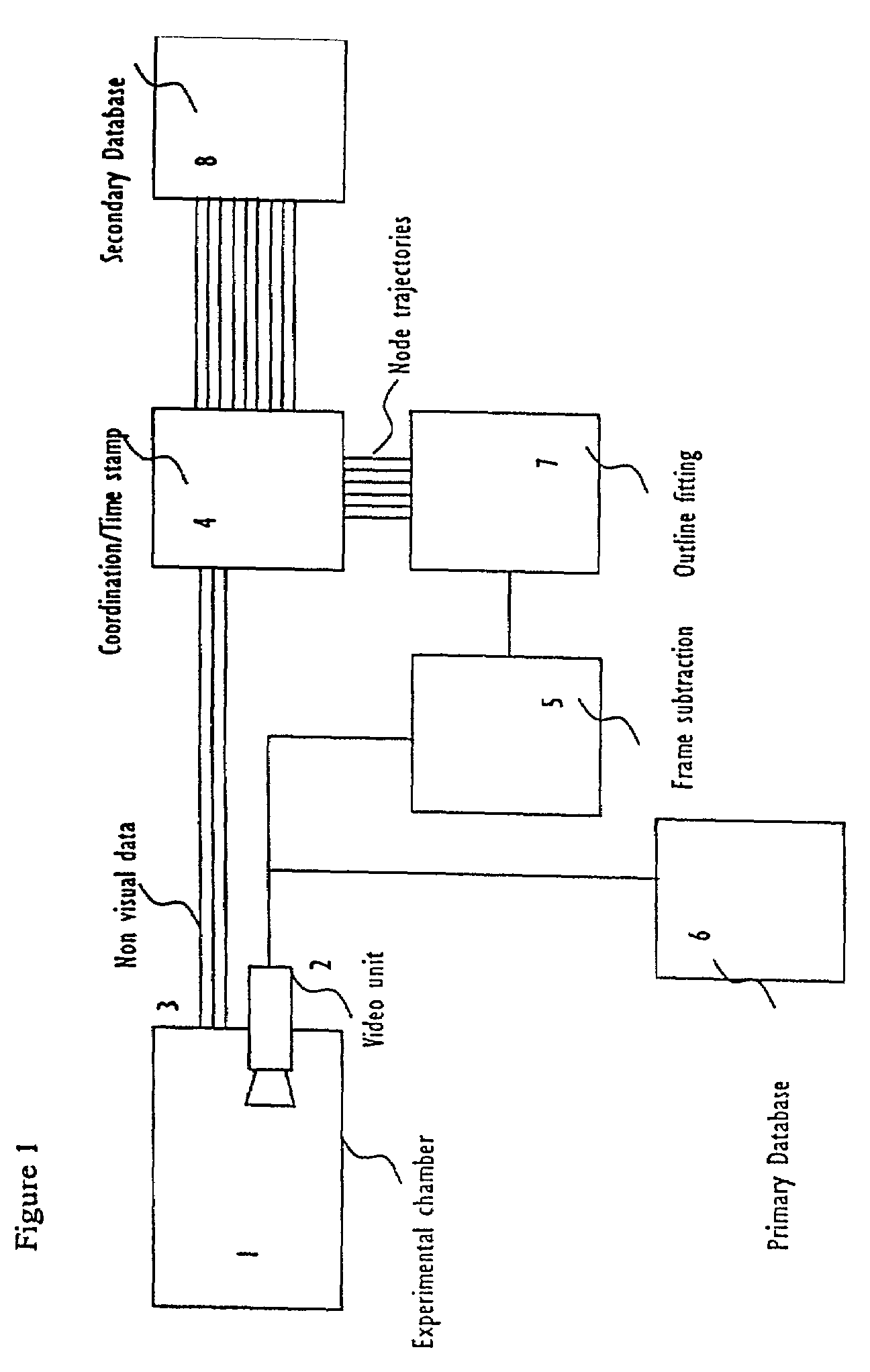
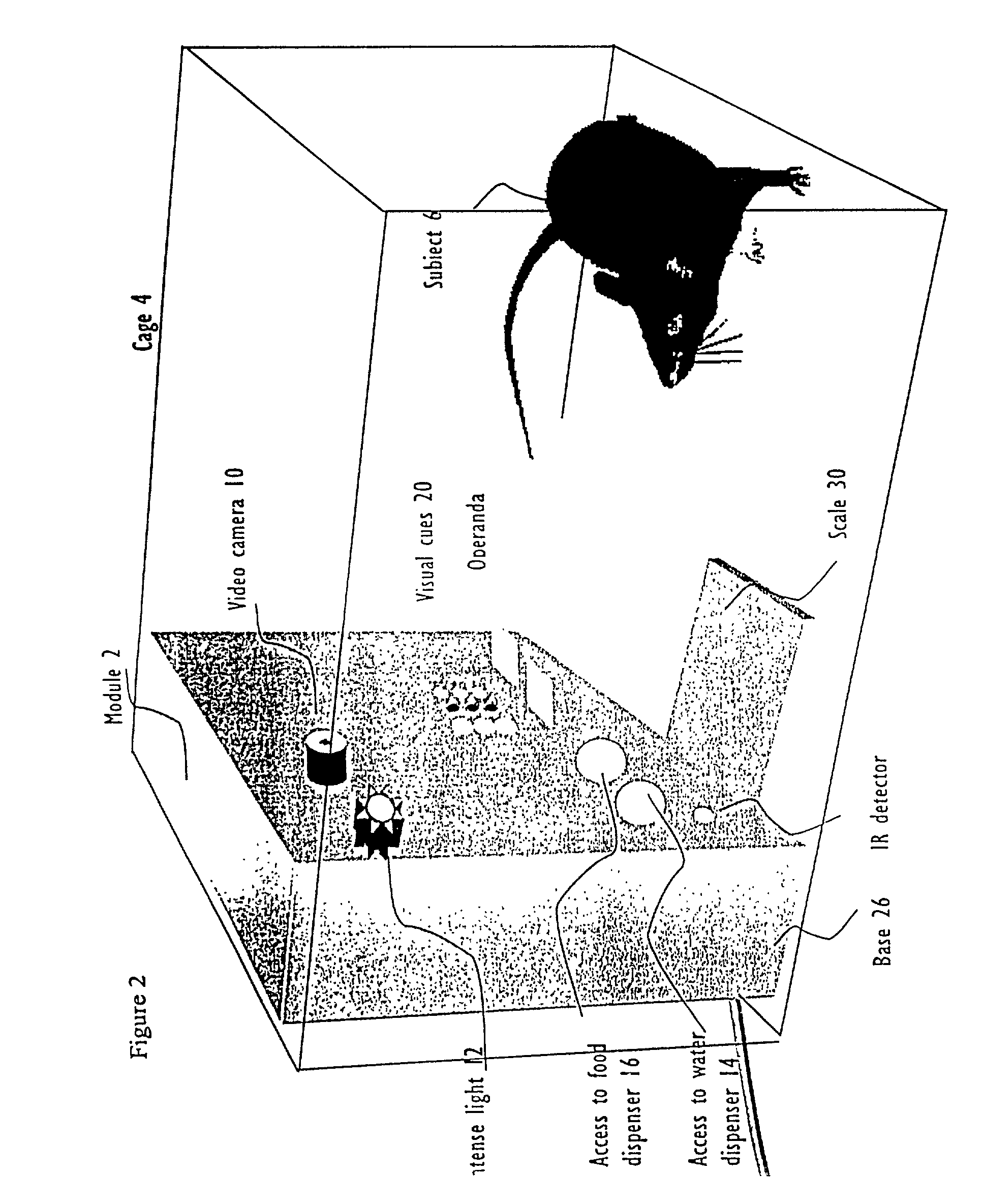
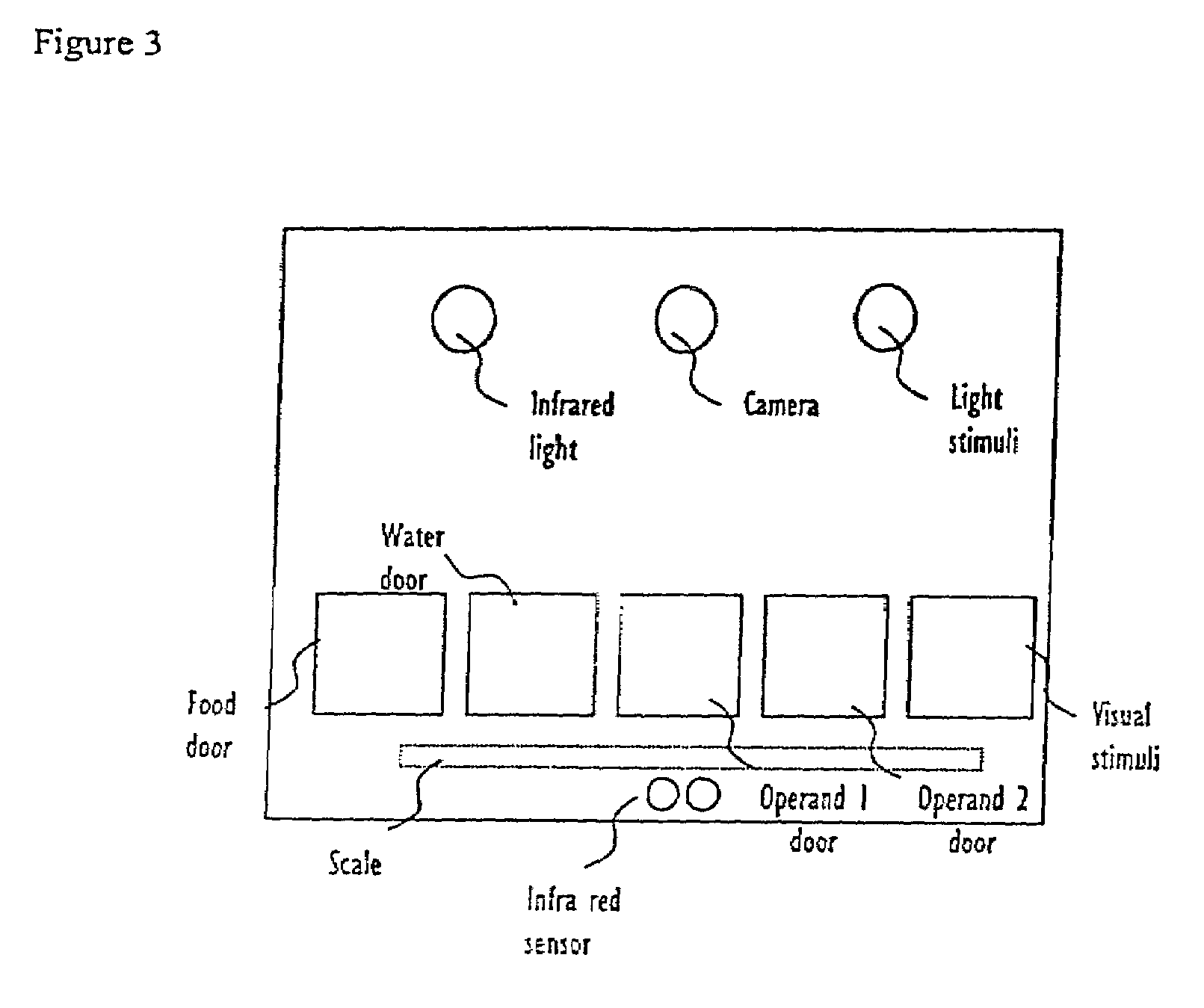
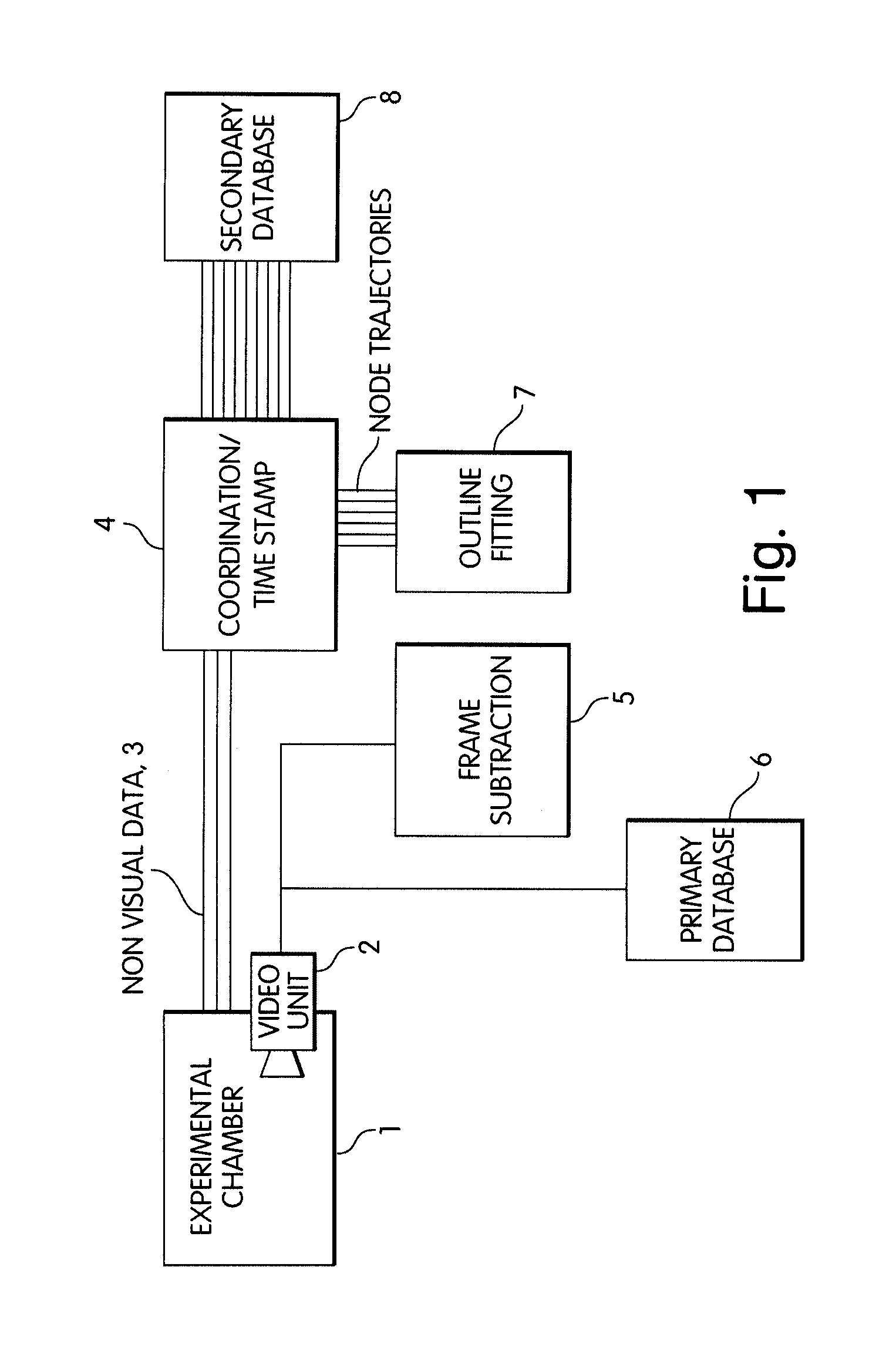
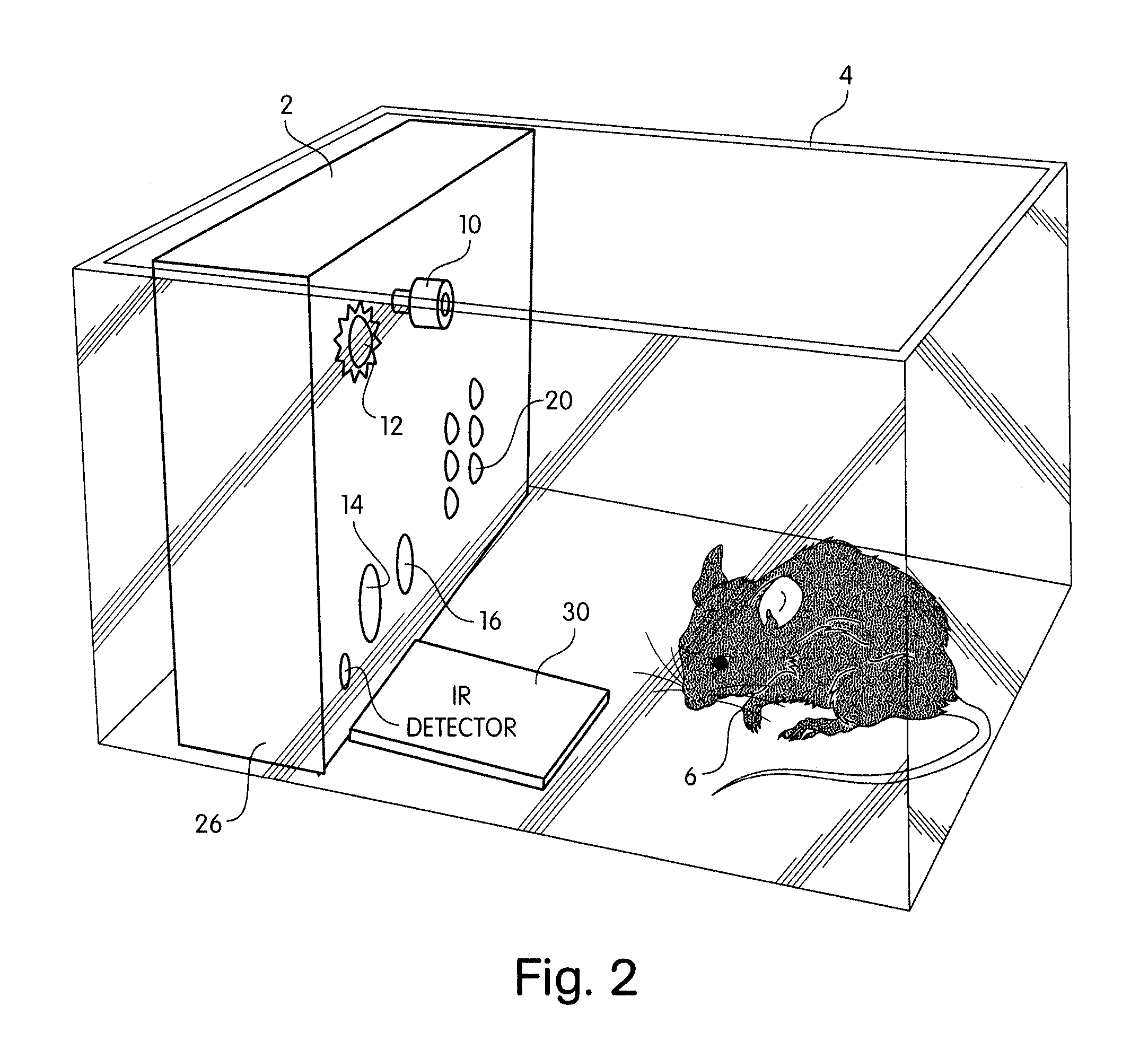
Abstract of Disclosure A system and method used to assess animal behavior includes a module having sensors that collects a variety of physical and biological data from a test subject. Interpretation of the data is provided to assess the test subject's behavior, neurology, biochemistry and physiology. The module is useful in observing the effects of a drug on the test animal and providing information on the drug's signature. Another advantage is module's portability that allows it to be used in standard laboratory cages. This portability allows the animal to be tested in its own habitat, that can reduce any erroneous data due to stressing the animal when removed to a test cage. Additionally, the module's design allows for parallel data collection and interpretation from several laboratory animals undergoing different experiments. Multi-dimensional modeling of the test subject based the system's interpretation of the data allows pattern recognition of the drug signature, and predictive drug analysis.
Owner:CARNEGIE MELLON UNIV +1
Systems and methods for monitoring behavior informatics
InactiveUS7269516B2Improve abilitiesIncrease powerDrug and medicationsCharacter and pattern recognitionBehavioral neurologyDiagnosis laboratory
A system and method used to assess animal behavior includes a module having sensors that collects a variety of physical and biological data from a test subject. Interpretation of the data is provided to assess the test subject's behavior, neurology, biochemistry and physiology. The module is useful in observing the effects of a drug on the test animal and providing information on the drug's signature. Another advantage is the module's portability that allows it to be used in standard laboratory cages. This portability allows the animal to be tested in its own habitat, that can reduce any erroneous data due to stressing the animal when removed to a test cage. Additionally, the module's design allows for parallel data collection and interpretation from several laboratory animals undergoing different experiments. Multi-dimensional modeling of the test subject based the system's interpretation of the data allows pattern recognition of the drug signature, and predictive drug analysis.
Owner:CARNEGIE MELLON UNIV +1
Systems and methods for monitoring behavior informatics
InactiveUS20030083822A2Improve statistics performanceExpand selectionComputer-assisted medical data acquisitionAnimal housingTest objectOrganism
Abstract of Disclosure A system and method used to assess animal behavior includes a module having sensors that collects a variety of physical and biological data from a test subject. Interpretation of the data is provided to assess the test subject's behavior, neurology, biochemistry and physiology. The module is useful in observing the effects of a drug on the test animal and providing information on the drug's signature. Another advantage is module's portability that allows it to be used in standard laboratory cages. This portability allows the animal to be tested in its own habitat, that can reduce any erroneous data due to stressing the animal when removed to a test cage. Additionally, the module's design allows for parallel data collection and interpretation from several laboratory animals undergoing different experiments. Multi-dimensional modeling of the test subject based the system's interpretation of the data allows pattern recognition of the drug signature, and predictive drug analysis.
Owner:PSYCHOGENICS
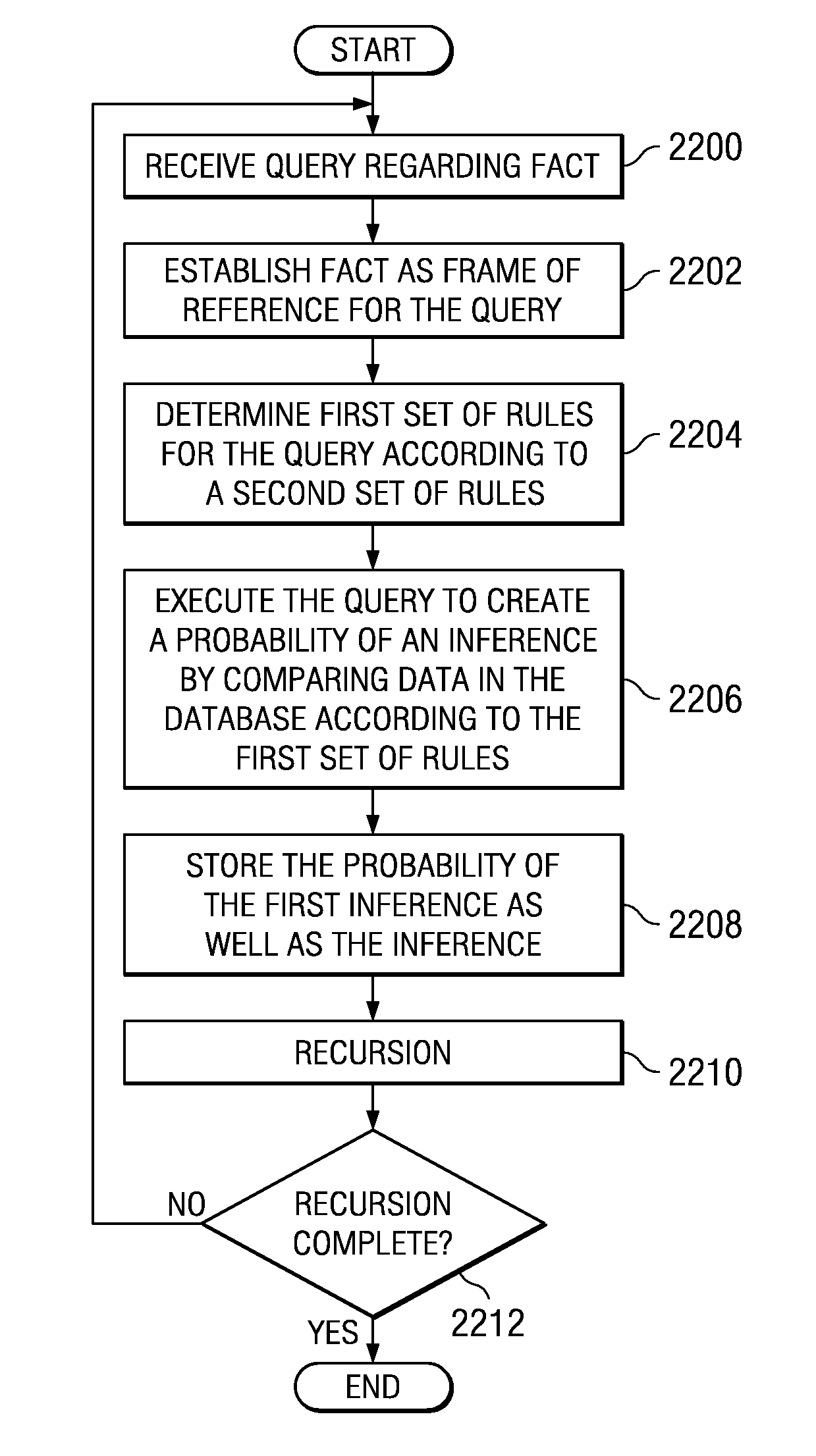
System and method for deriving a hierarchical event based database optimized for pharmaceutical analysis
InactiveUS7970759B2Digital data information retrievalDigital data processing detailsData sourceDependability
A computer implemented method, apparatus, and computer usable program code for inferring a probability of a first inference absent from a database at which a query regarding the inference is received. Each datum of the database is conformed to the dimensions of the database. Each datum of the plurality of data has associated metadata and an associated key. The associated metadata includes data regarding cohorts associated with the corresponding datum, data regarding hierarchies associated with the corresponding datum, data regarding a corresponding source of the datum, and data regarding probabilities associated with integrity, reliability, and importance of each associated datum. The query is used as a frame of reference for the search. The database returns a probability of the correctness of the first inference based on the query and on the data.
Owner:SERVICENOW INC
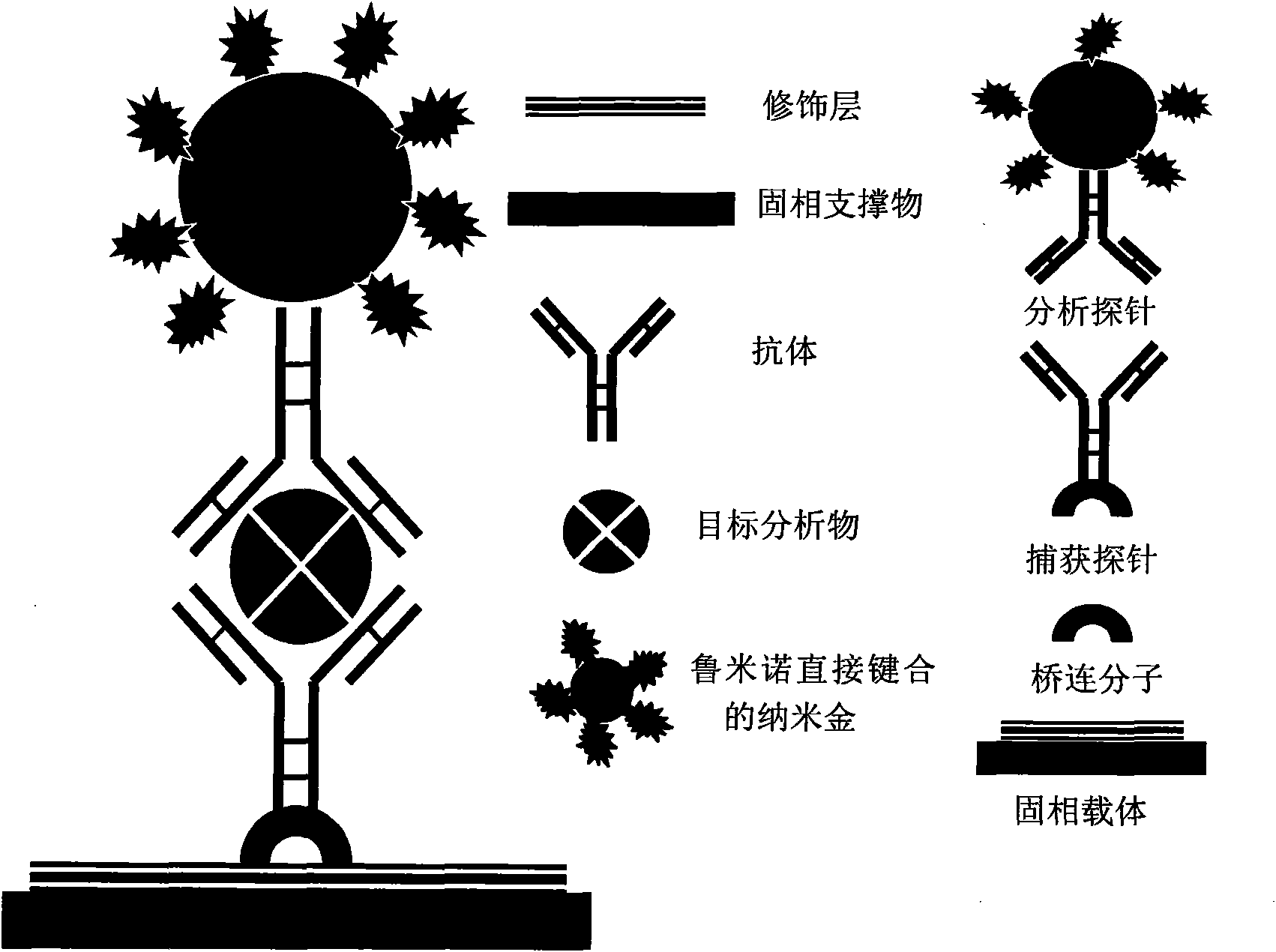
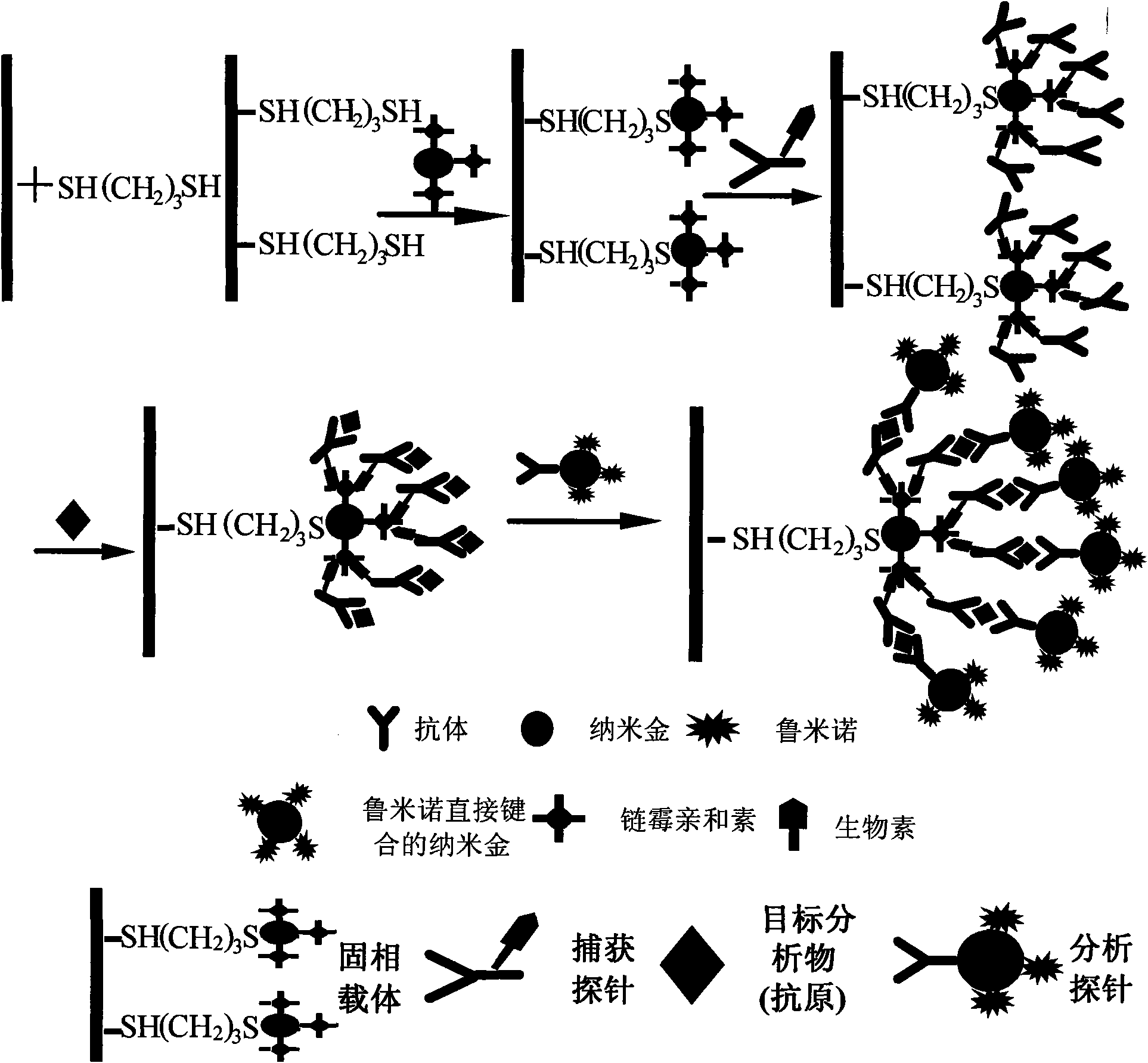
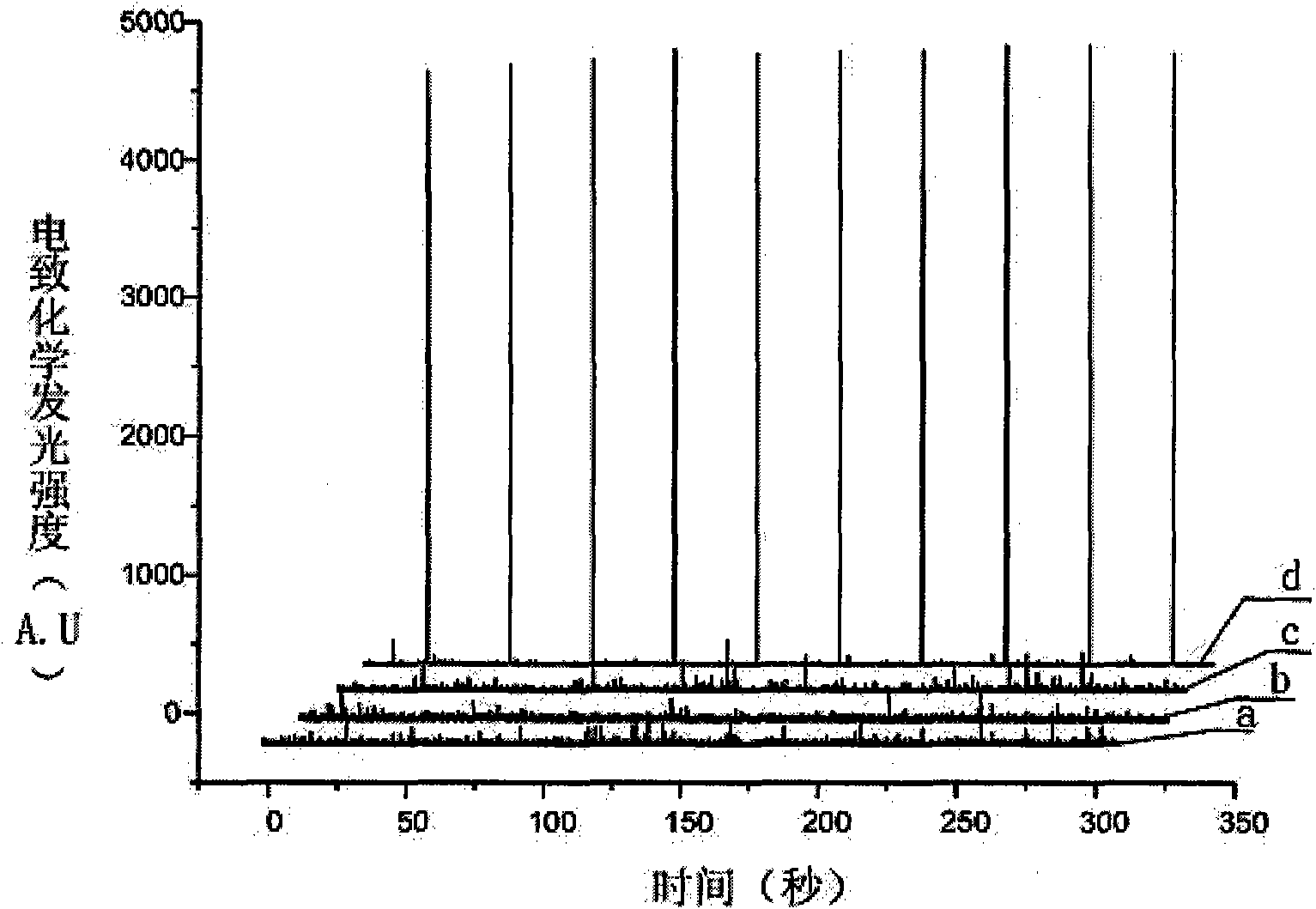
Application of nano-gold directly bonded with luminol in immunoassay
ActiveCN101900723ASimple methodFast wayAnalysis by electrical excitationBiological testingMulti analyteLinearity
The invention discloses an application of nano-gold directly bonded with luminol in an immunoassay. The invention is characterized in that the immunoassay probe of a nano-gold directly bonded with luminol comprises antibodies which are labeled by the nano-golds directly bonded with luminol, wherein the nano-gold directly bonded with luminol are prepared through reducing chloroauric acid by the luminol at a single step; a chemiluminescence immunoassay method is based on the immunoassay probe of the nano-gold directly bonded with luminol; and a kit is used for carrying out the immunoassay method. The chemiluminescence immunoassay method of the invention has the advantages of high sensitivity (for instance, the detection limit can reach 1.0pg / mL for detecting human IgG), wide range of linearity, good repeatability, simple operation, low cost and the like, can be applied to detect multi-analyte in various samples and has key application prospect in fields, such as clinical diagnosis and cure, drug analysis, food security detection, environmental monitoring and the like.
Owner:UNIV OF SCI & TECH OF CHINA
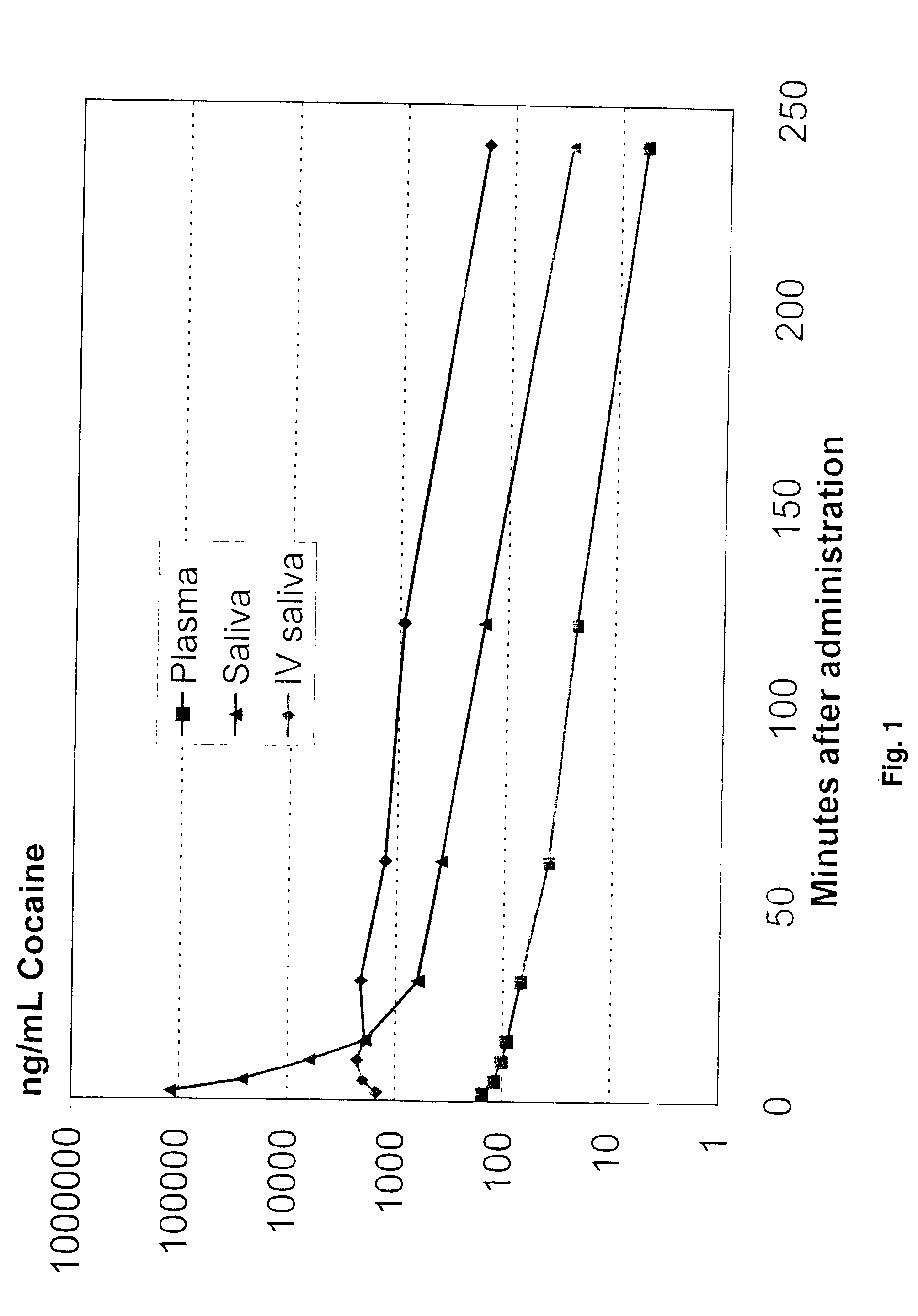
Ion selective electrodes for direct organic drug analysis in saliva, sweat, and surface wipes
InactiveUS20030121779A1Immobilised enzymesBioreactor/fermenter combinationsHydrophobic polymerMedication monitoring
A hand-held portable drug monitoring system to detect and quantitate cocaine and other organic drugs in saliva, sweat, and surface wipes by using an ion selective electrode or an array of ion selective electrodes. The ion selective electrode has a cast membrane reference electrode and a sensing electrode with a hydrophobic polymer, a plasticizer, and an ionophore selective for the organic drug to be tested. The ion selective electrode can be connected to a converter that coverts a voltage reading from the ion selective electrode to a quantitative drug concentration level. Also disclosed is the related method of using an ion selective electrode to detect an organic drug in saliva, sweat, and surface wipes, the method of testing electrical contact in an ion selective electrode, and the method of making a cast membrane reference electrode.
Owner:THE UNITED STATES OF AMERICA AS REPRESENTED BY THE SECRETARY OF THE NAVY
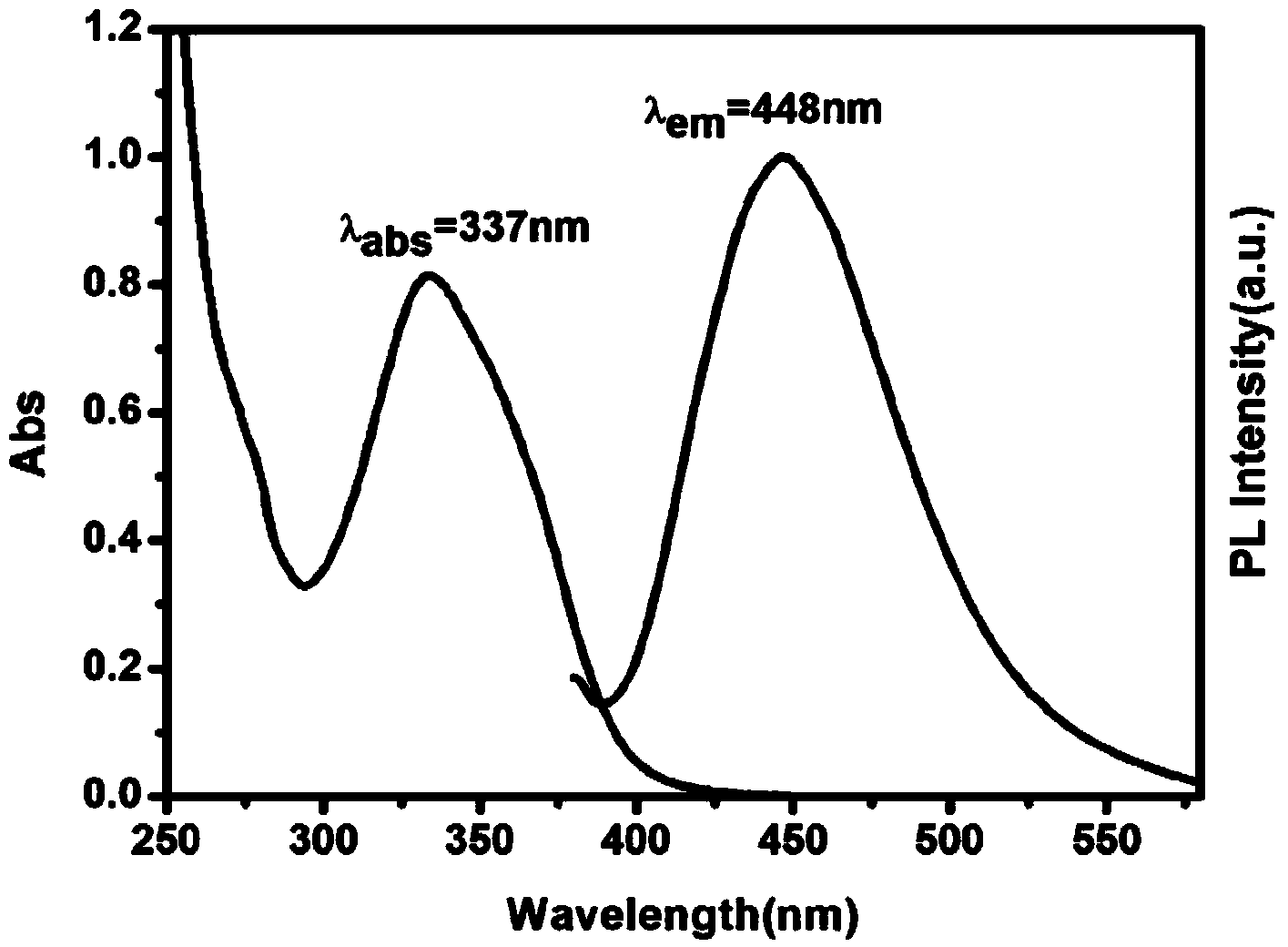
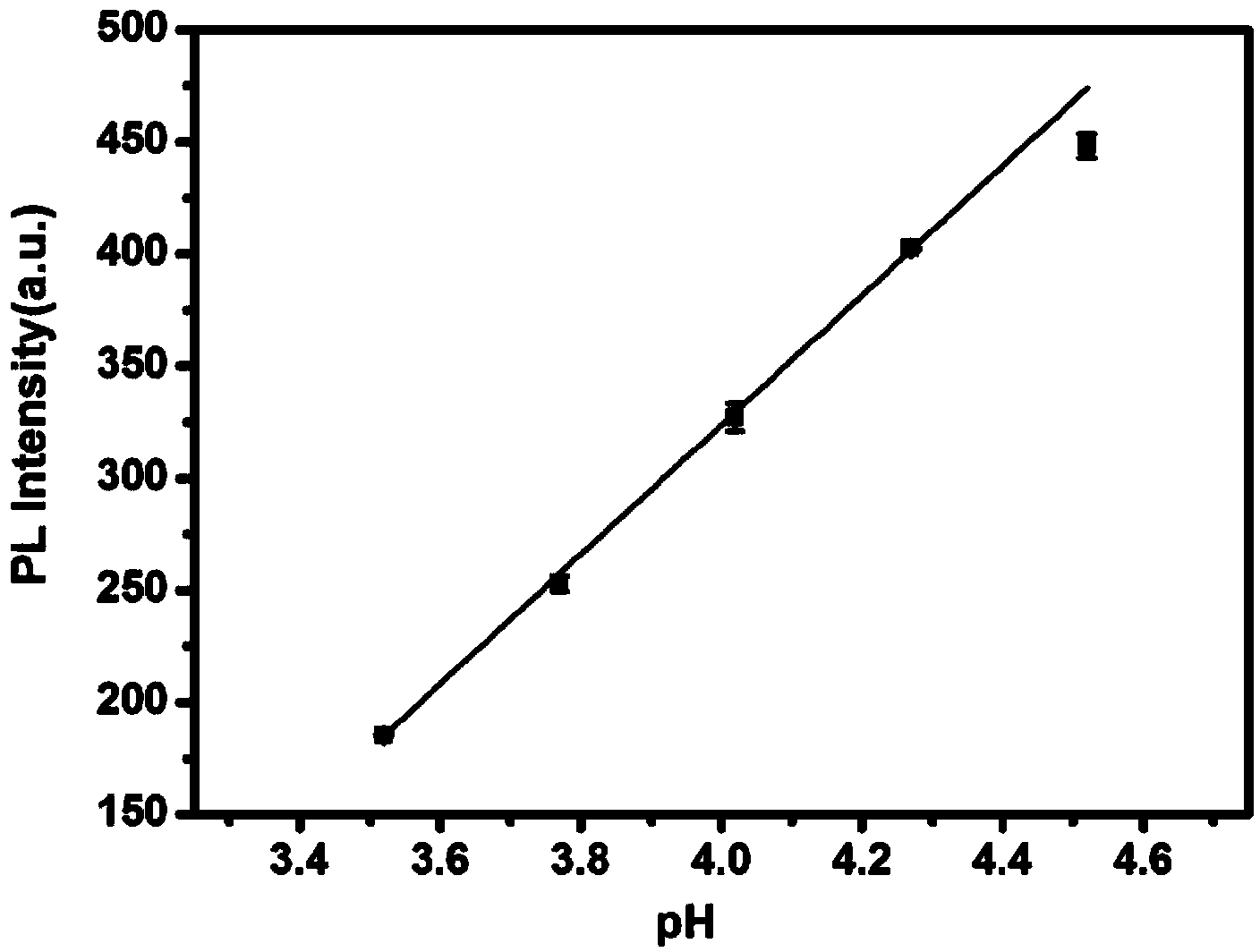
Method for preparing water-soluble fluorescent silicon quantum dots by using hydrothermal process
ActiveCN103896271ASynthetic operation is simpleGood reversibilityNanoopticsSilicon compoundsQuantum yieldSilanes
The invention belongs to the technical field of the preparation methods of the nanomaterials and specifically discloses a method for preparing water-soluble fluorescent silicon quantum dots by using a hydrothermal process. The method comprises the steps of firstly introducing an amino silane and a reducing agent into a solvent in which nitrogen is introduced and mixing, continuing introducing the nitrogen for protection for a while, next, transferring to a hydrothermal reaction kettle, and heating for reacting for a while, thereby obtaining the fluorescent silicon quantum dots having excellent chemical properties. The method has the advantages of low raw material cost due to the adoption of the amino silane as a silicon source, simple operation steps, direction synthesis in water phase, easy large-scale production, and being green and environment; the obtained quantum dots have high quantum yield and excellent chemical characteristics, and are small in particle size, evenly in distribution, non-toxic, good in biocompatibility and acid resistance; besides, the quantum dots have pH sensitive characteristic in a certain range, and can be widely applied to the biochemical and biomedical sensing fields such as biochemical detection, drug analysis, cell and living imaging and targeting tracing and also can be used as photovoltaic conversion and light-emitting display materials.
Owner:WUHAN UNIV
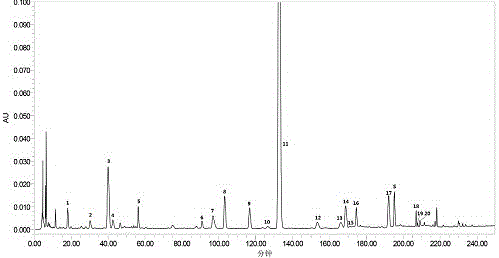
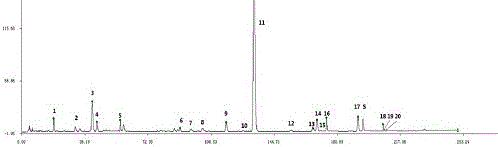
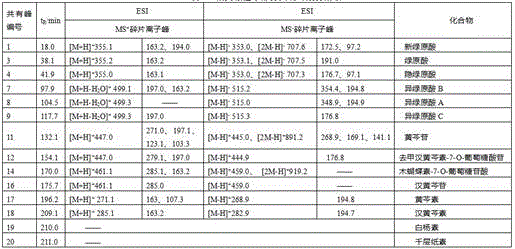
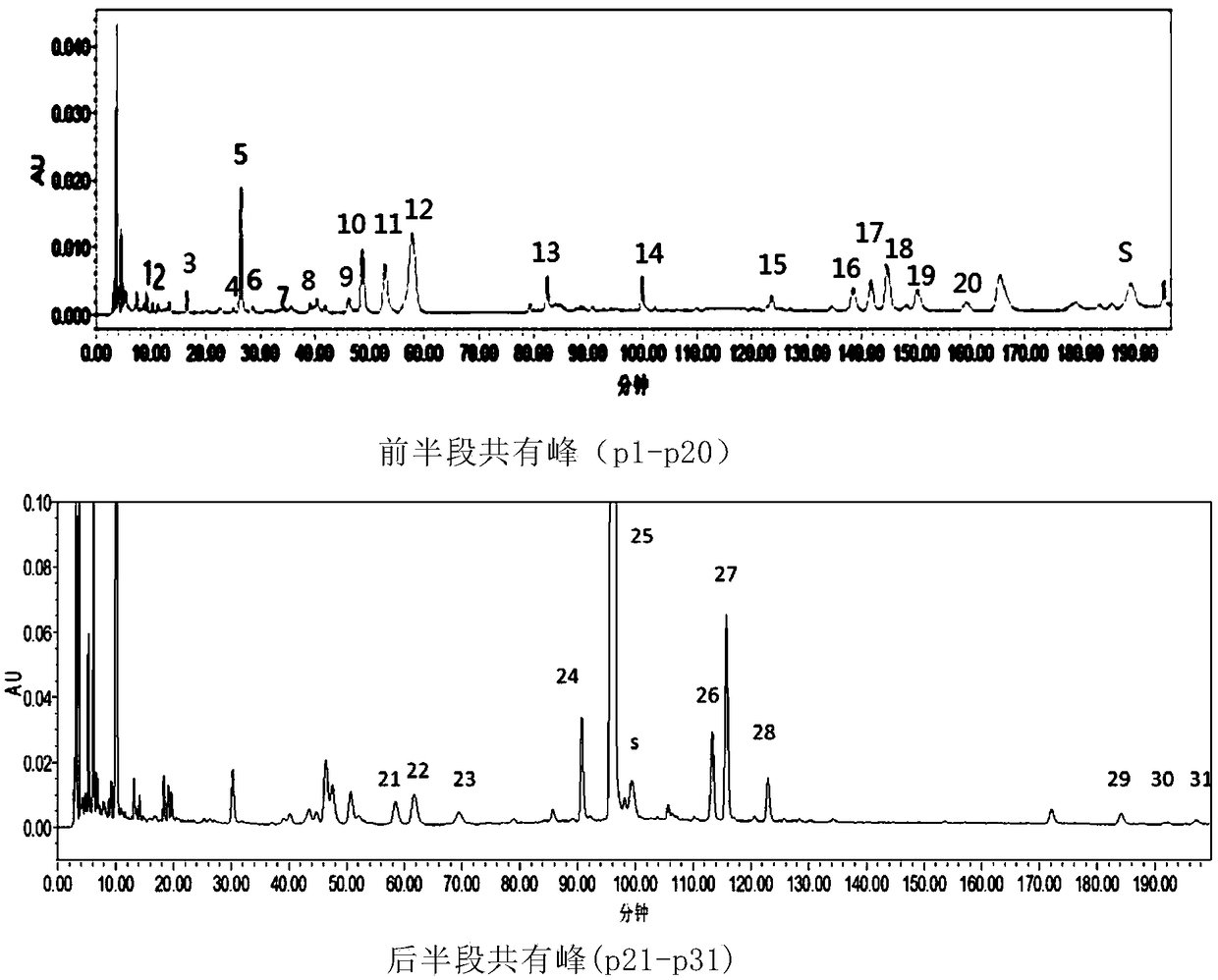
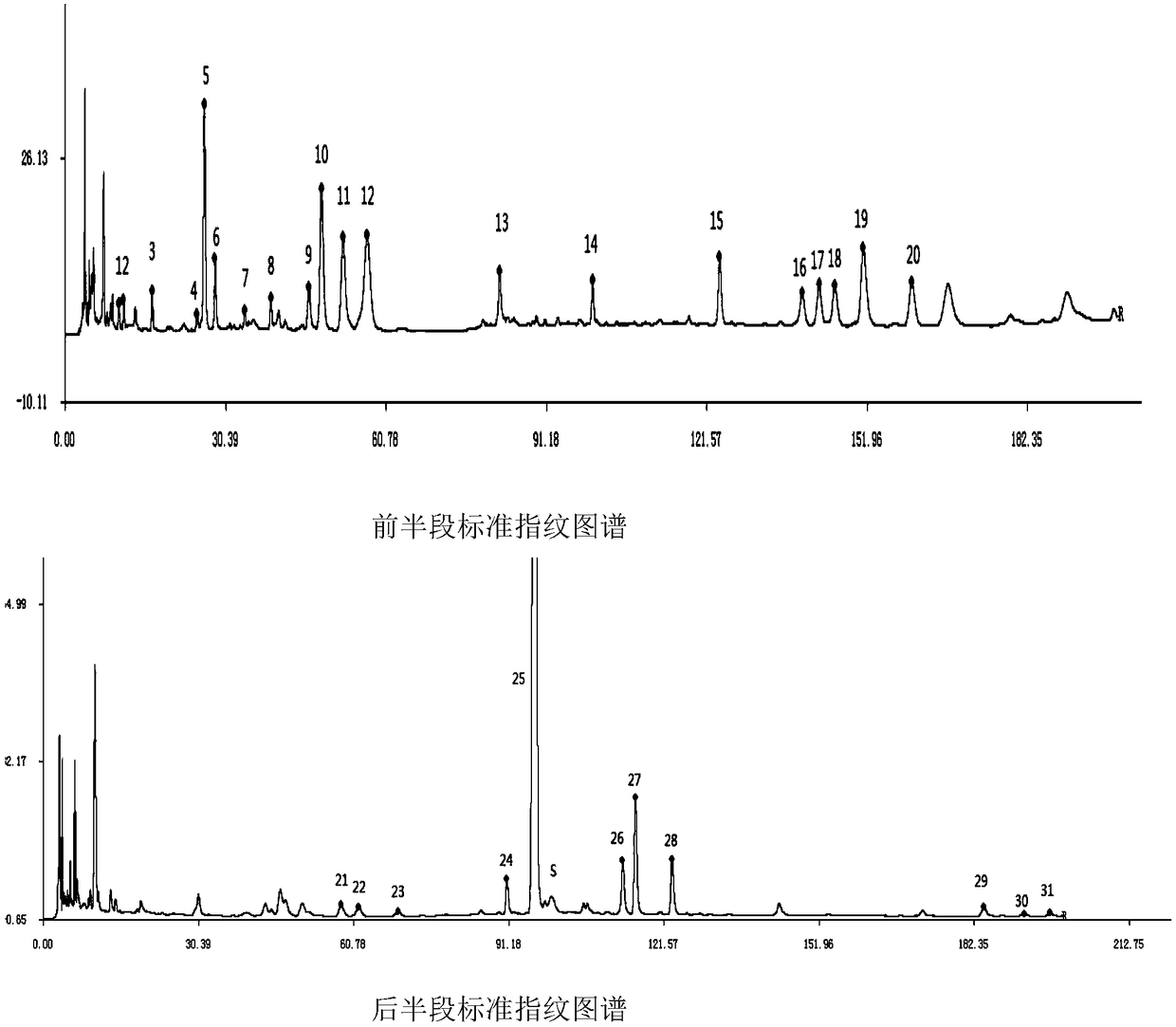
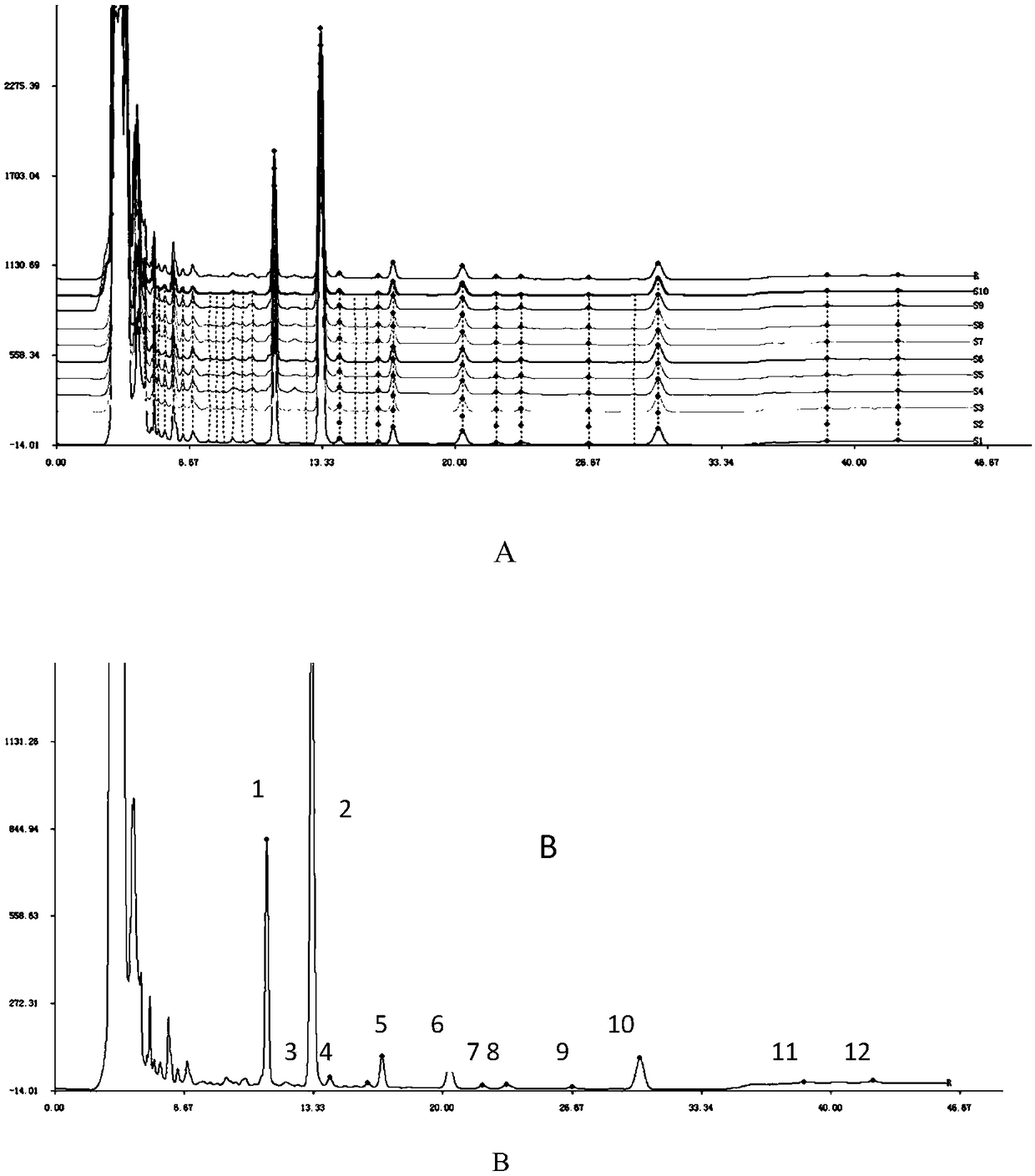
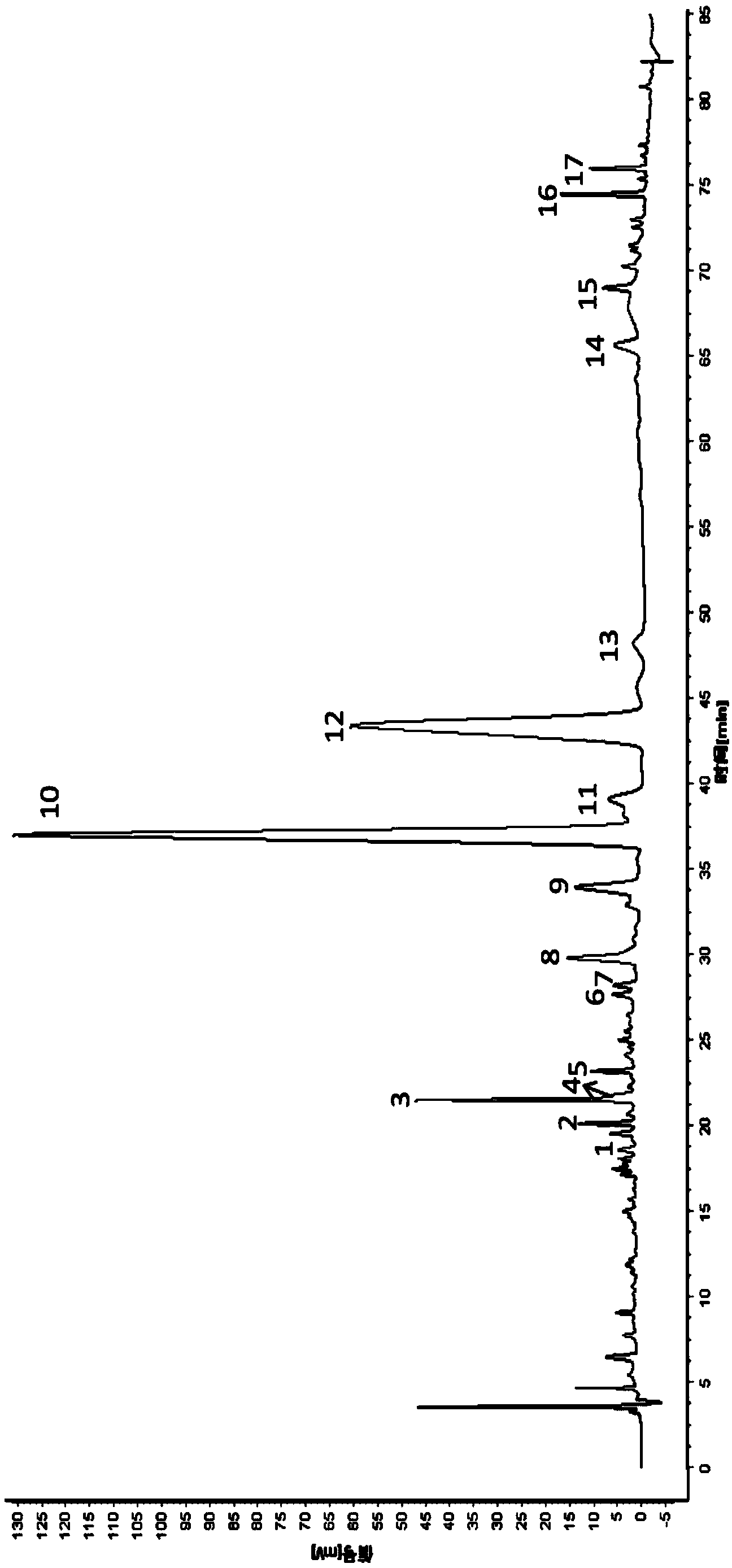
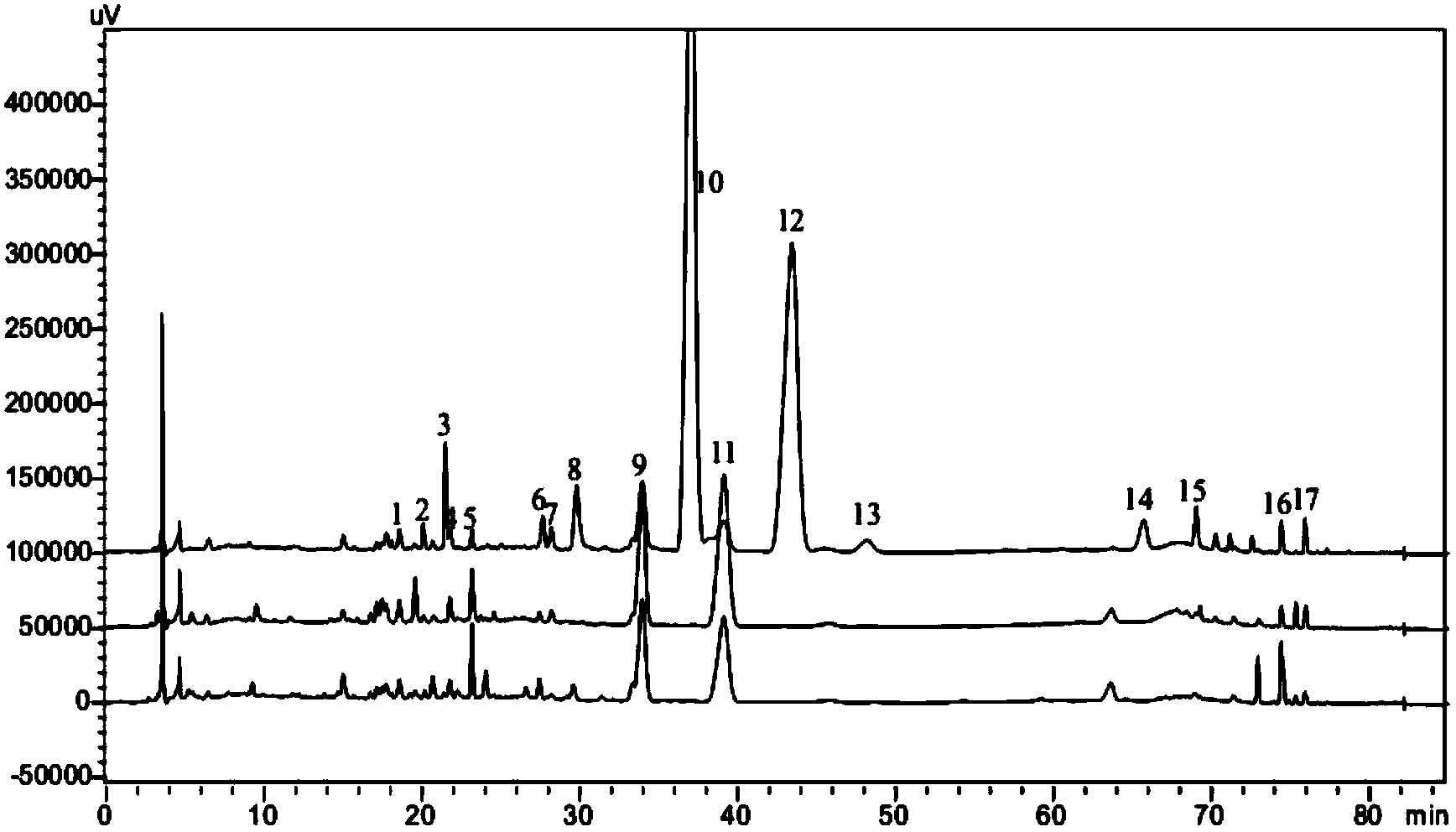
Measurement method of honeysuckle and radix scutellariae granule fingerprint spectrum
InactiveCN105911186AEasy to distinguishEasy to separateComponent separationChemical constituentsHplc mass spectrometry
The invention relates to a quality control method for honeysuckle and radix scutellariae granules, particularly relates to a measurement method of a honeysuckle and radix scutellariae granule fingerprint spectrum, and belongs to the technical field of medicine analysis. The method includes the steps of: 1) preparing a reference substance solution; 2) preparing an internal standard solution; 3) preparing a sample solution; and 4) performing analysis and measurement with high performance liquid chromatography-mass spectrum. Through the HPLC-MS method, chemical components of a common peak of the honeysuckle and radix scutellariae granules are accurately measured. The method is simple and accurate, has high sensitivity and good repeatability, can monitor qualities of raw material medicines, semi-products and finish products more comprehensively, and can also monitor stability of a production process.
Owner:SHANDONG ACAD OF CHINESE MEDICINE
Method for determining fingerprint of Shuanghuanglian oral preparation
ActiveCN108760903AEasy to separateReflect absolute changeComponent separationAdditive ingredientPeak area
The invention relates to a method for determining fingerprint of a Shuanghuanglian oral preparation and belongs to the technical field of drug analysis. The method comprises: preparing a reference solution and a test solution and carrying out analysis detection through high performance liquid chromatography-mass spectrometry. The HPLC detection is carried out under different elution conditions sothat good separation effects are obtained. Through an external internal standard method, the test solution containing the internal standard substance is detected in the fingerprint. The relative peakarea ratio of the common peak and the internal standard peak is used as the mathematical expression of the fingerprint so that the method can reflect the absolute and relative changes of common peak contents better than the method for calculating the relative peak area of each common peak through traditional Chinese medicine ingredients.
Owner:SHANDONG UNIV OF TRADITIONAL CHINESE MEDICINE
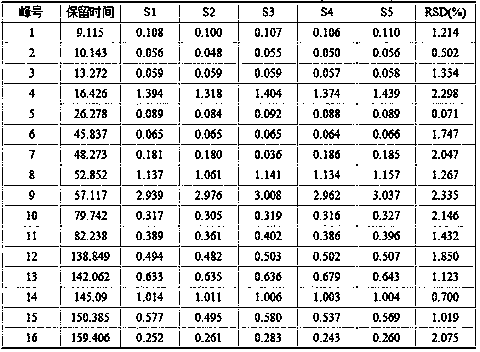
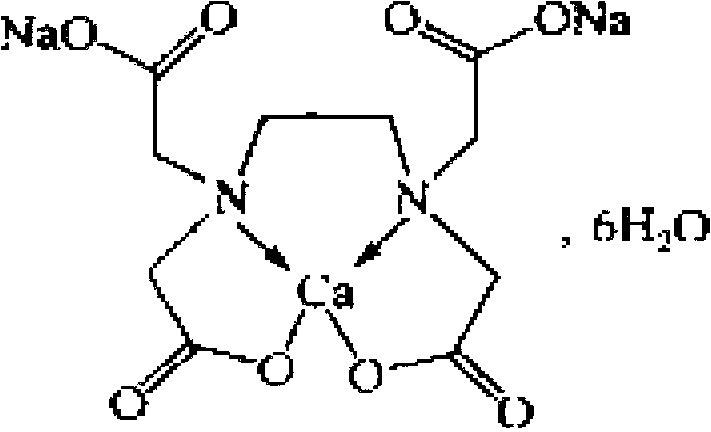
Detection method and content determining method of sodium calcium edetate in pantoprazole sodium for injecting
The invention belongs to the field of medicament analysis and particularly relates to a detection method and a content determining method for sodium calcium edetate as an accessory in pantoprazole sodium for injecting. The invention aims at solving the technical problem of providing a method with the advantages of simpleness in and convenience for operation, quickness and accuracy for detecting the sodium calcium edetate as the accessory in the pantoprazole sodium for injecting. HPLC (High Performance Liquid Chromatography) detection conditions are as follows: a stationary phase: octadecylsilane bonded silica gel is used as a filling agent; a mobile phase: in terms of volume, an ion pair buffer solution is 85-95 percent, acetonitrile is 5-15 percent and the pH value is adjusted to 2.2-2.6 with phosphoric acid; the flowing speed is 0.8-1.2 ml / min; the column temperature is 30-40DEG C; the detection wavelength is 250-260 nm; and the theoretical plate number is required not to be lower than 2000 calculated in terms of a sodium calcium edetate peak. The detection method has the advantages of simple and convenient operation, accurate and reliable determination result, stronger specificity and shorter detection time; and the retention time of a main peak is about 6 minutes.
Owner:CHENGDU BAIYU JINGELAI PHARMA CO LTD
HPLC (high performance liquid chromatography) analyzing method for 3-aminopiperidine
The invention belongs to the technical field of medicament analysis, and particularly relates to a qualitative and quantitative method of 3-aminopiperidine as well as a detection method of chiral purity of 3-aminopiperidine. According to the invention, excessive benzoyl chloride is adopted as a derivatization reagent to be subjected to rapid bi-derivatization reaction with 3-aminopiperidine so as to generate diphenyl 3-aminopiperidine, and then common phase reversal HPLC-UV(high performance liquid chromatography-ultraviolet) is used for analyzing diphenyl 3-aminopiperidine qualitatively and quantitatively; Chiral HPLC Columns taking glycoprotein as a filling are adopted for determining the enantiomeric excess rate of diphenyl 3-aminopiperidine. The method is simple and rapid to operate, is good in reproducibility, is high in flexibility, and is easy for standardized operation.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
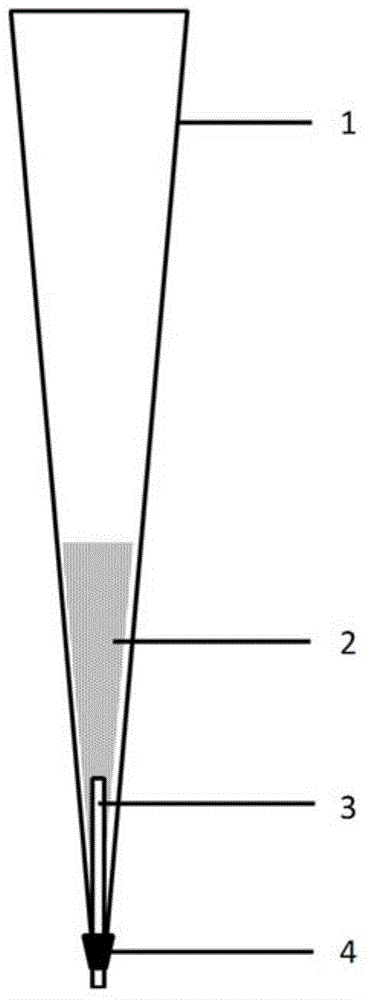
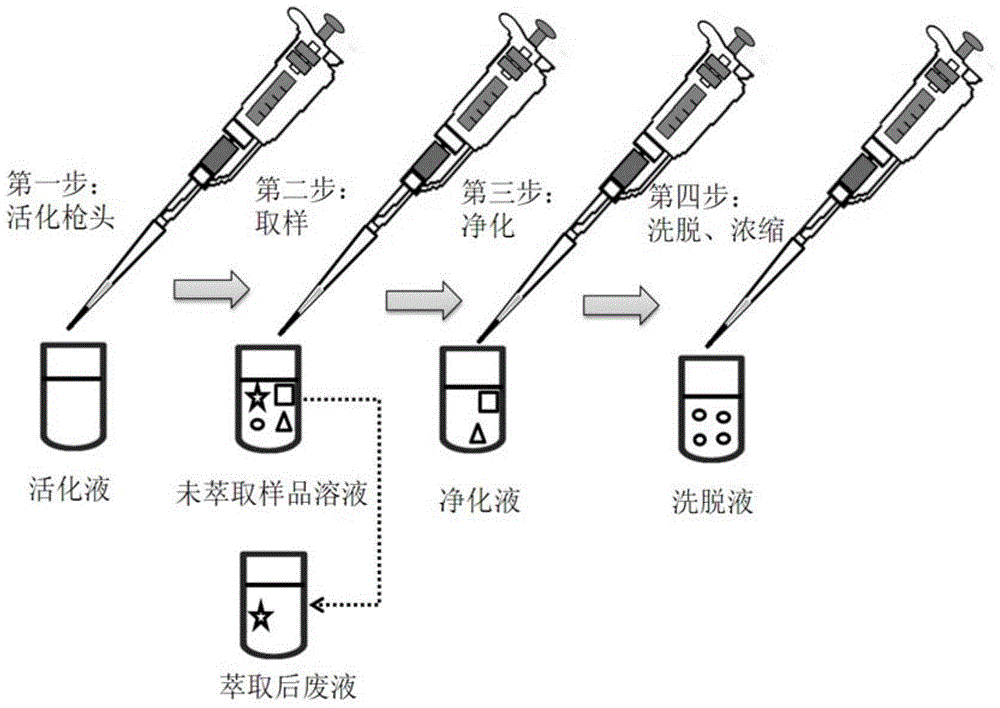
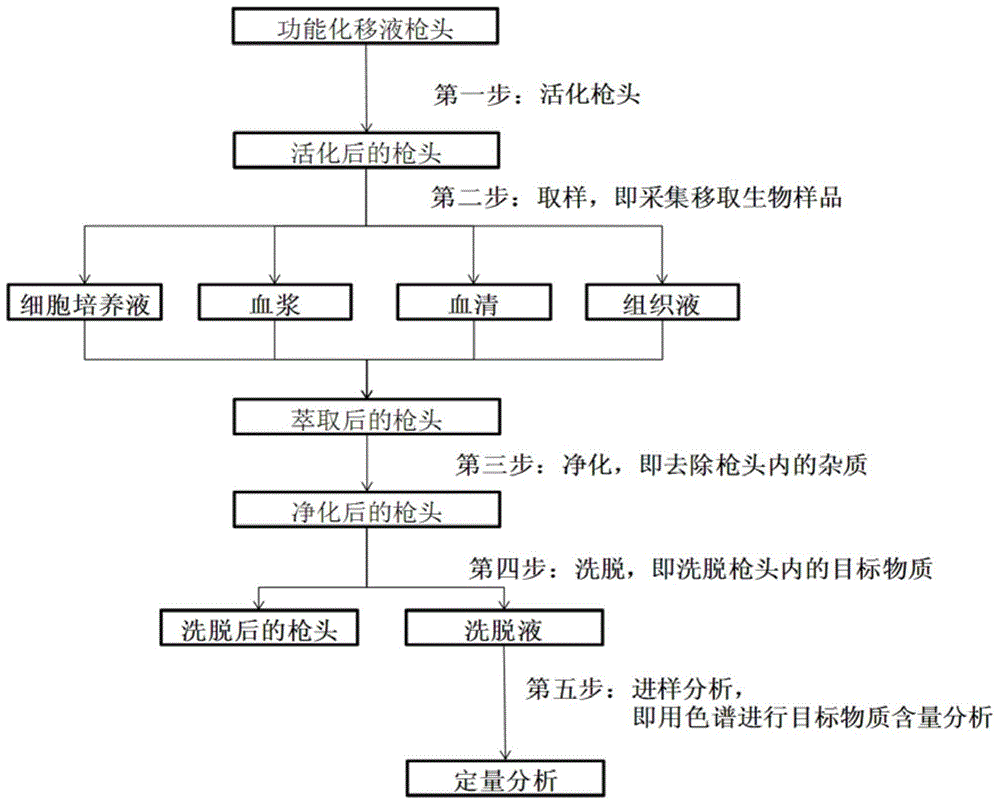
Functional pipetting head with double purification functions of ultra-filtration and solid extraction and application of functional pipetting head
ActiveCN104549593AThe production process is simple and controllableSimple structurePreparing sample for investigationUltrafiltrationAdhesive glueSolid phase extraction
The invention relates to an experiment device for pretreatment on samples, and in particular to a functional pipetting head with double purification functions of ultra-filtration and solid extraction, and application of the functional pipetting head in pretreatment and quantitative analysis on a biological sample in the medicine analysis process. The functional pipetting head comprises a pipetting head body, a solid-phase extraction material and a hollow fiber membrane, wherein the hollow fiber membrane is arranged at the sharp end of the pipetting head body; an adhesive is uniformly applied at the cross part of the sharp end of the pipetting head and the hollow fiber membrane; an upper end opening and a lower end opening are formed in the hollow fiber membrane; the upper end opening is sealed; the lower end opening is kept in an opened state; the solid-phase extraction material is arranged inside the pipetting head body. The functional pipetting head is simple, convenient and controllable in manufacturing process, flexible in extraction condition adjustment, scientific and reasonable in method, convenient in obtaining materials, and good in application prospect.
Owner:ZHEJIANG ACAD OF TRADITIONAL CHINESE MEDICINE
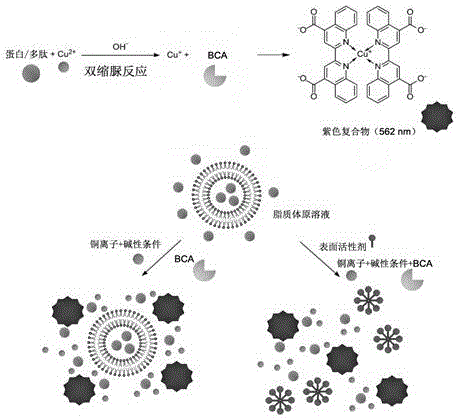
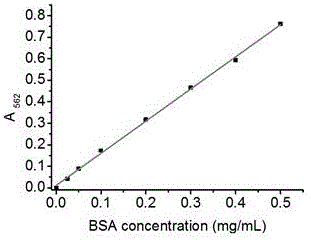
Method for detecting entrapment rate of protein or polypeptide drugs in lipid vesicles
The invention relates to a for detecting the entrapment rate of protein or polypeptide drugs in lipid vesicles, and belongs to the technical field of drug analysis. The method comprises the following steps: diluting a prepared protein or polypeptide wrapped lipid vesicle solution, taking the diluted lipid vesicle solution, adding a nonionic surfactant to carry out demulsification, taking the lipid vesicle solution before and after the demulsification, respectively adding a BCA reaction solution, and carrying out color development at a certain temperature and time; and reading the absorbance at 562 nm, and calculating the protein concentration and the entrapment rate according to a standard curve. The method uses a difference between the reaction rate of free proteins outside the lipid vesicles with a color developer and the reaction rate of the wrapped proteins in the lipid vesicles and the color developer to measure the entrapment rate of the lipid vesicle protein drugs without separating the free proteins, so the method has the advantages of convenience in operation, and good repeatability, solves the problems of tediousness and deviation in the measurement process of the entrapment rate of the lipid vesicle protein drugs, and provides an effective method and basis for relevant production application and research fields of the lipid vesicle protein drugs.
Owner:JIANGSU UNIV
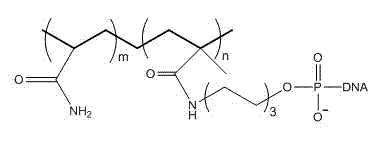
DNA hydrogel and application thereof in detection of peroxide
InactiveCN103804590AHigh sensitivityEasy to detectMaterial analysis by observing effect on chemical indicatorMicrobiological testing/measurementCross linkerPolyacrylamide
The invention belongs to the technical field of pharmaceutical analysis, relates to DNA hydrogel and an application of the DNA hydrogel in detection of a peroxide, and in particular relates to an application of high polymer material DNA hydrogel in peroxide detection by naked eyes. The hydrogel uses DNAzyme with peroxidase activity as a cross-linking agent and adopts polyacrylamide as a skeleton. The DNA hydrogel is formed by DNA-containing polyacrylamide macromolecules, T-DNAzyme-T and heme through intermolecular force. The hydrogel is in a gel state and can be used for peroxide detection by naked eyes. The DNA hydrogel has the advantages of having simplicity in operation and capability of semi-quantitative detection by naked eyes and reusability of the material and the like.
Owner:SHENYANG PHARMA UNIVERSITY +1
Signal amplification immunodetection method
The invention discloses a signal amplification immunodetection method. According to the method, signal amplification immunity quantitative detection is conducted through a filter, and the method comprises the step of adopting the filter for sequentially filtering a to-be-detected sample solution A containing objects to be detected, a detected object solution B labeled through avidin and an indicator solution C labeled through biotin, wherein a filter element of a filter layer of the filter is a solid phase material coupled to a specific binder of the objects to be detected, detected objects labeled through the avidin can be specifically bound to the objects to be detected, and thereby the quantity of the objects to be detected in the solution A is calculated according to the color or light quantity variation of an indicator. It is proved through experiments that compared with a conventional biotin-avidin amplification reaction sequence, the avidin-biotin amplification reaction sequence has a greater biological signal amplification effect. The method can be used for detecting samples which are from human bodies and animal bodies and can be subjected to disease diagnosis and health detection and applied to development of products for sample quantitative immunodetection in the fields of environment, pharmaceutical analysis, food and industrial analysis.
Owner:CHANGZHOU BIOWIN BIOPHARM
HPLC (High Performance Liquid Chromatography) analysis method for 3-aminopiperidine
The invention belongs to the technical field of medicine analysis and particularly relates to a quantitative method for 3-aminopiperidine and a detection method for the chiral purity of 3-aminopiperidine. According to the method, benzoyl chloride which is used as a derivatization reagent and 3-aminopiperidine are rapidly derived at a low temperature in a large quantity of organic solvents to generate benzoyl-3-aminopiperidine; then a detection analysis of benzoyl-3-aminopiperidine is carried out by using HPLC-UV (High Performance Liquid Chromatography-Ultraviolet). The method carries out qualitative analysis and quantitative analysis by adopting a common C18 column, and carries out enantiomeric excess rate determination by adopting a chiral chromatographic column. The method is simple to operate, high in sensitivity and high in accuracy, is easy for standardized operation.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
Fermented cordyceps taishanensis powder, preparation sterols HPLC fingerprint spectrum and building method thereof
ActiveCN108535372AImprove the quality evaluation systemEnsure quality stabilityComponent separationMethanol waterHplc fingerprint
The invention belongs to the field of drug analysis, relates a quality control method of fermented cordyceps taishanensis powder, and particularly relates to fermented cordyceps taishanensis powder, preparation sterols HPLC fingerprint spectrum and a building method thereof. The building method includes the steps of firstly, preparing a control solution; secondly, preparing a test solution; thirdly, using HPLC to determine the fingerprint spectrum, and performing similarity evaluation, to be more specific, separately precisely sucking the control solution and the test solution, performing HPLCseparation detection with the flow phase being a methanol-water elution system to obtain the sterol fingerprint spectrum of the fermented cordyceps taishanensis powder, and importing the fingerprintspectrum into similarity software to perform similarity evaluation.
Owner:JIANGXI GUOYAO PHARMA LLC +1
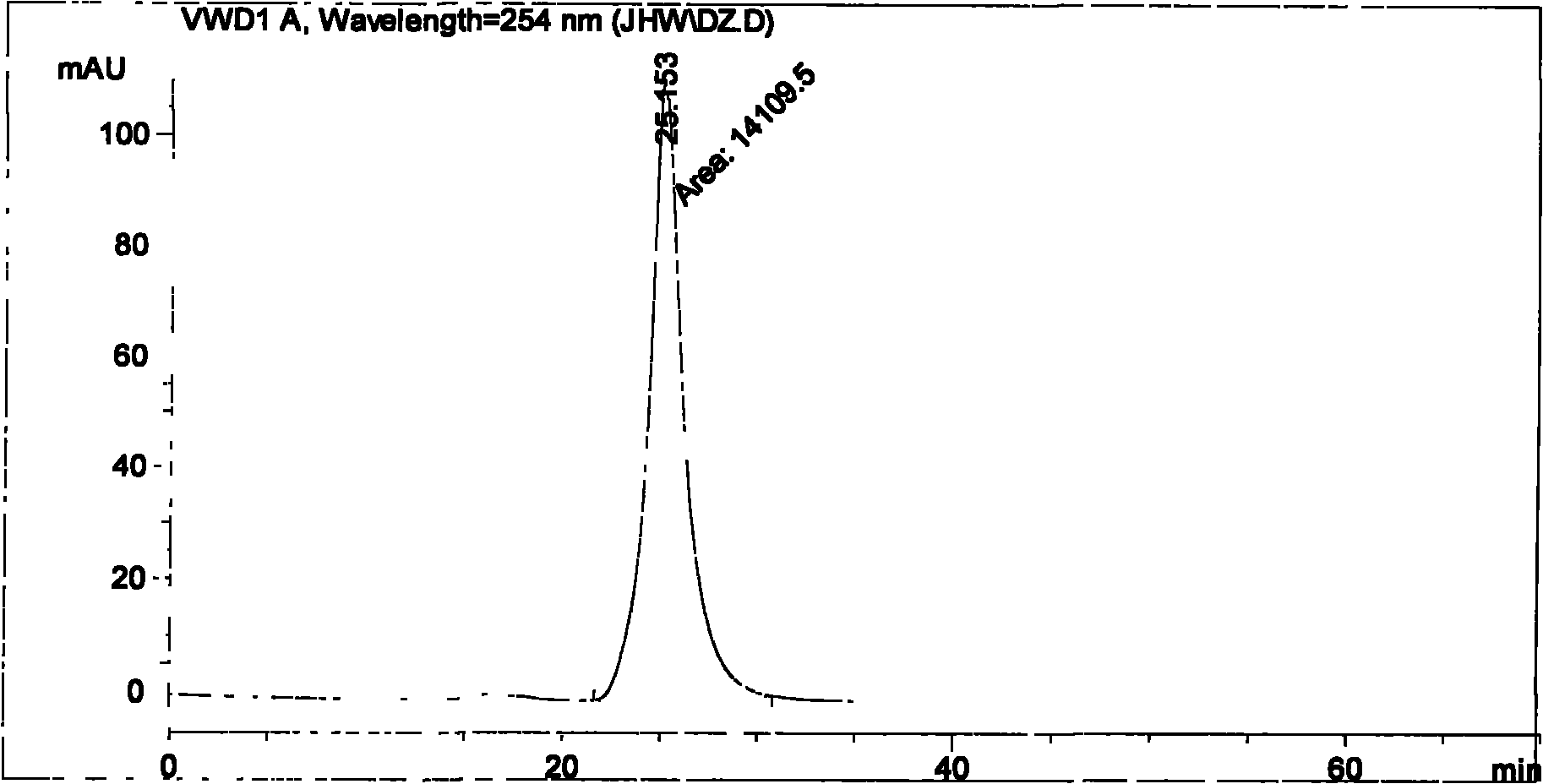
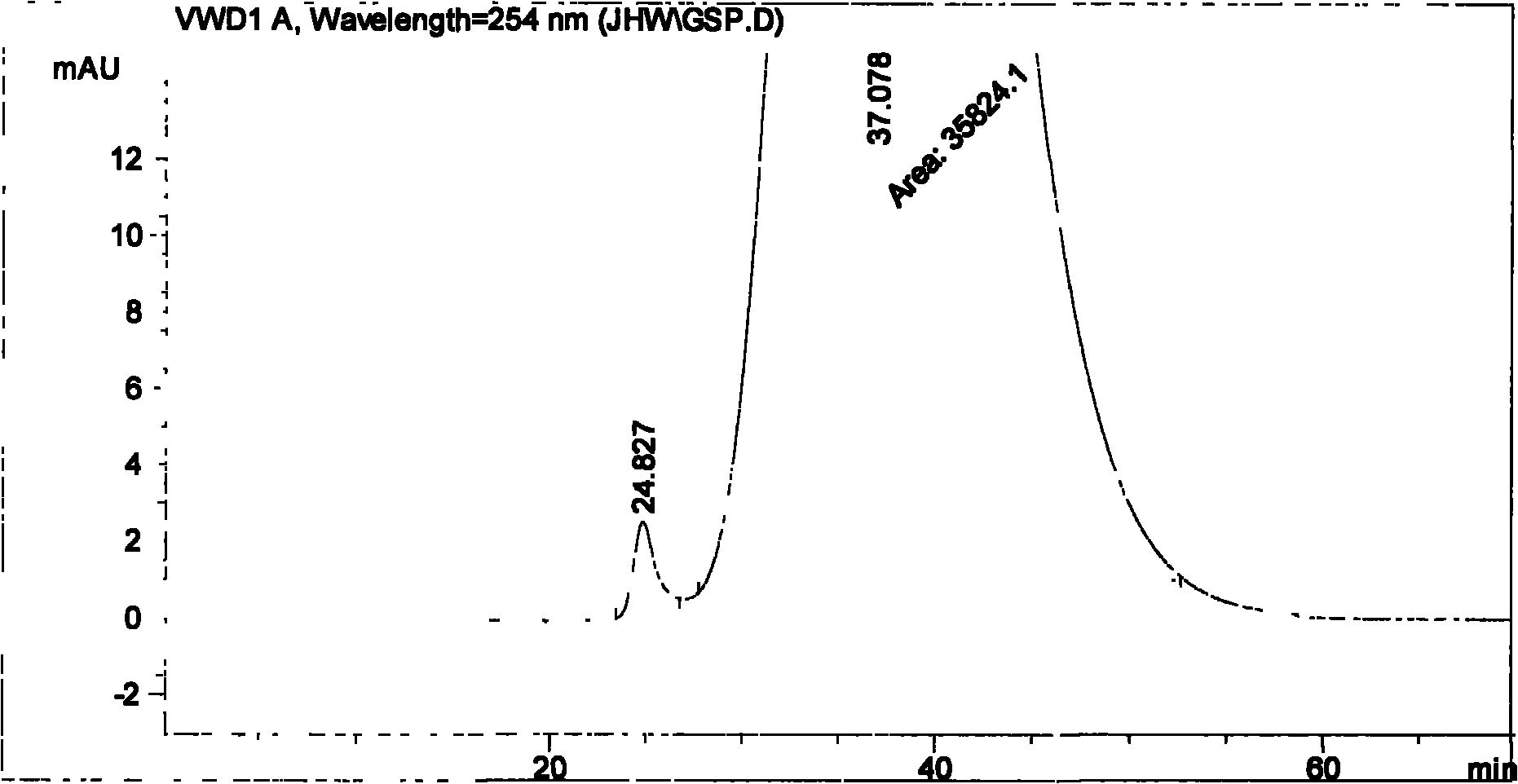
Method for determining impurity F in captopril tablets through high performance liquid chromatography
The invention discloses a method for determining an impurity F in captopril tablets through high performance liquid chromatography and belongs to the technical field of pharmaceutical analysis. Detection is performed under the conditions as follows: an amylase-tris(5-chloro-2-methyl phenyl carbamate) coated chromatographic column is used, normal hexane-absolute ethyl alcohol-trifluoroacetic acid serves as a mobile phase, a volume ratio of the normal hexane to absolute ethyl alcohol to trifluoroacetic acid is 80:20:0.1, a detection wavelength is 215nm, flow velocity is 1ml / min, a column temperature is 35 DEG C and a sample amount is 20[mu]l. A structural formula of the impurity F is as shown in the description. According to the method disclosed by the invention, the content of the impurityF in the captopril tablets can be quantitatively determined, so that the quality of the captopril tablets is effectively controlled. According to the method provided by the invention, the captopril and the impurity F can be proved to be effectively separated in a system suitability solution, and the method has high precision and high separation degree. A signal to noise ratio of a self-contrast solution is more than 10, and if the sample contains the impurity F, the impurity F can be detected.
Owner:ZHENGZHOU TAIFENG PHARMA CO LTD
HPLC (high-performance liquid chromatography) detection method for escitalopram oxalate related substances
ActiveCN106324141AGood linear relationshipStrong specificityComponent separationAcetonitrileGradient elution
The invention belongs to the field of pharmaceutical analysis and particularly relates to a HPLC (high-performance liquid chromatography) detection method for escitalopram oxalate related substances. The escitalopram oxalate related substances are determined with HPLC, a C18 column is taken as a chromatographic column, a phosphate buffer solution is taken as a mobile phase A, the mobile phase A and an acetonitrile solution are mixed in a certain ratio to form a mobile phase B, the mobile phase A and the acetonitrile solution are mixed in a certain ratio to form a mobile phase C, and the escitalopram oxalate related substances are detected with a gradient elution method. The method can separate impurities, is high in specificity and sensitivity and good in repeatability and durability, can well control known impurities and unknown impurities of escitalopram oxalate and guarantees safety of escitalopram oxalate.
Owner:山东锐顺药业有限公司
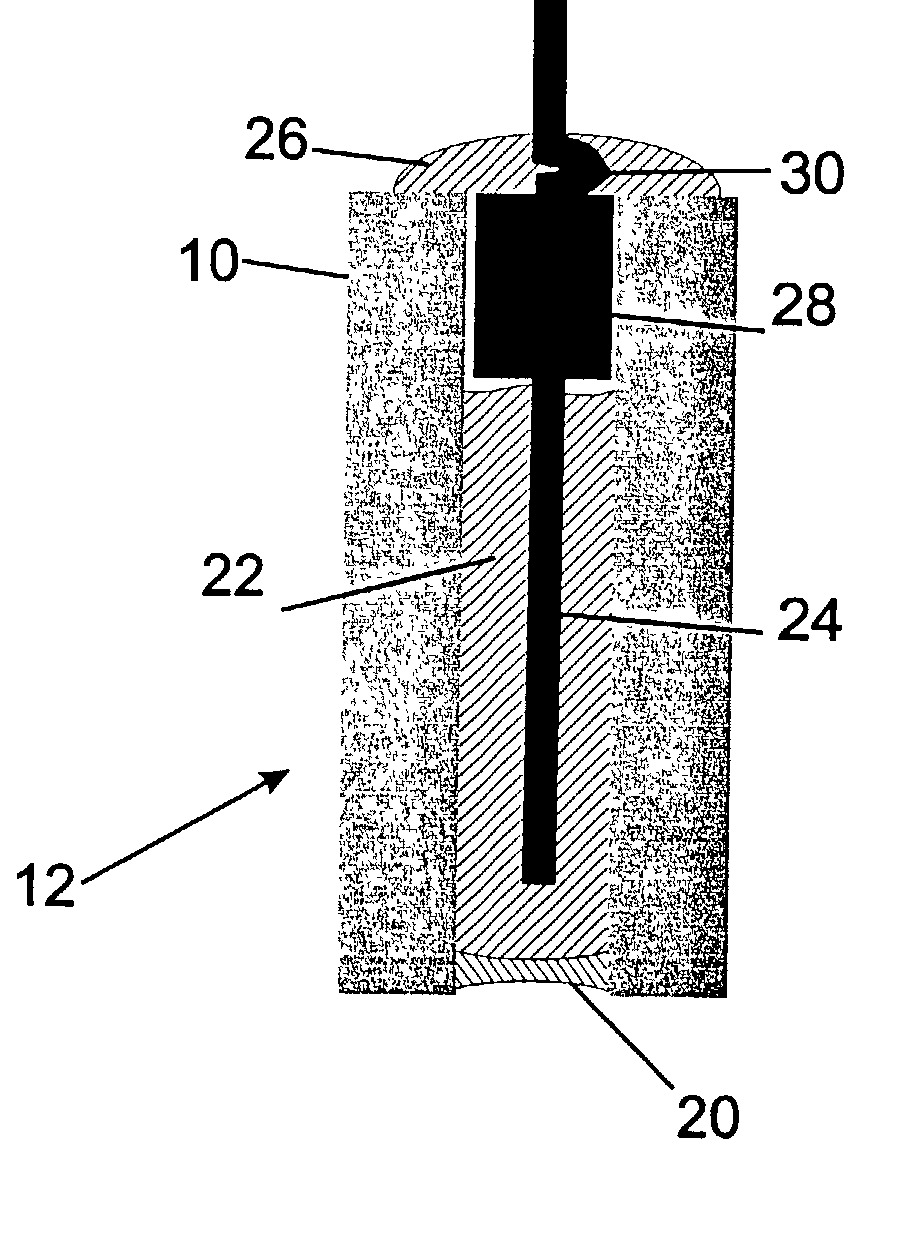
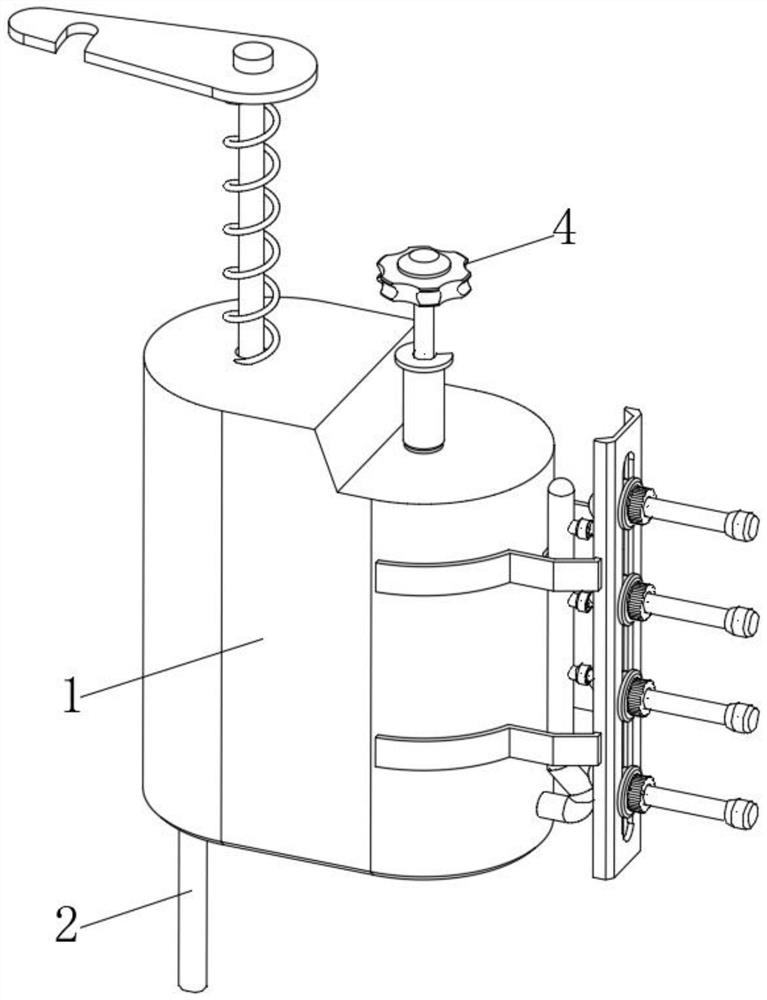
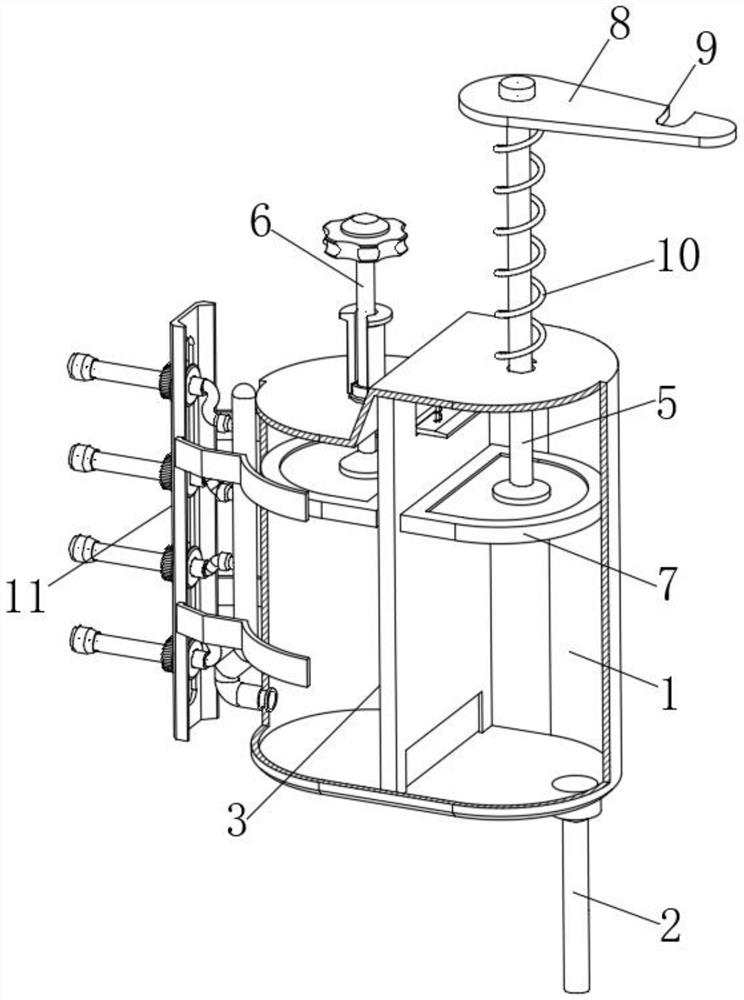
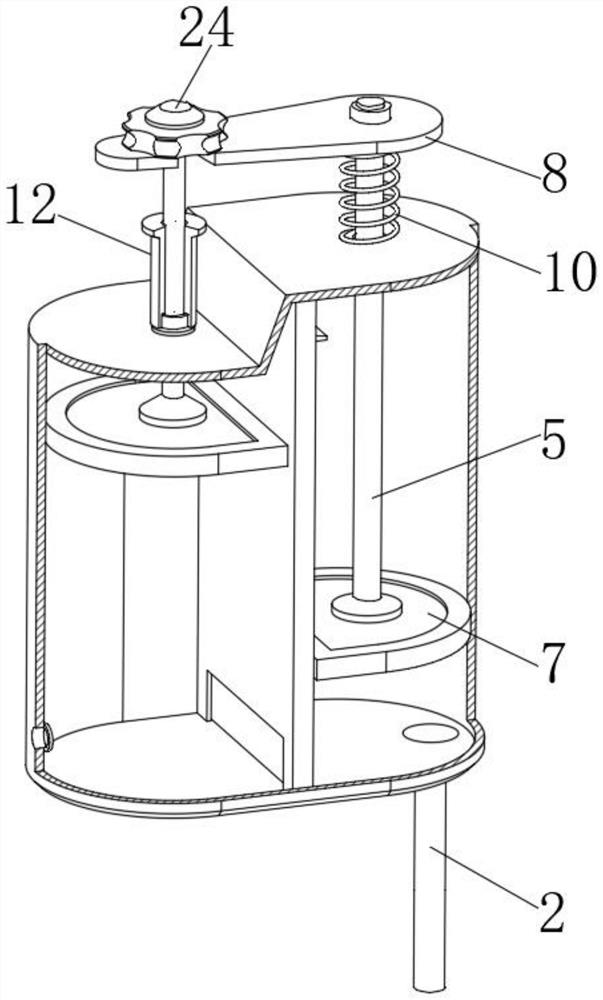
Fluid sampling device for drug analysis
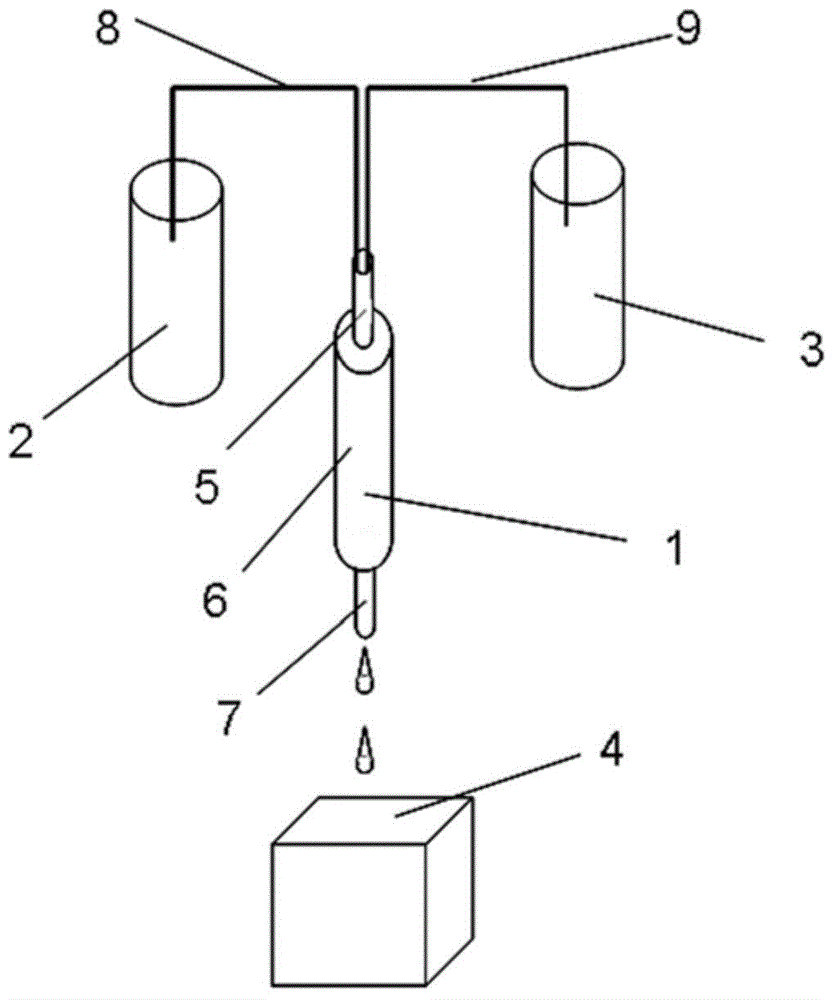
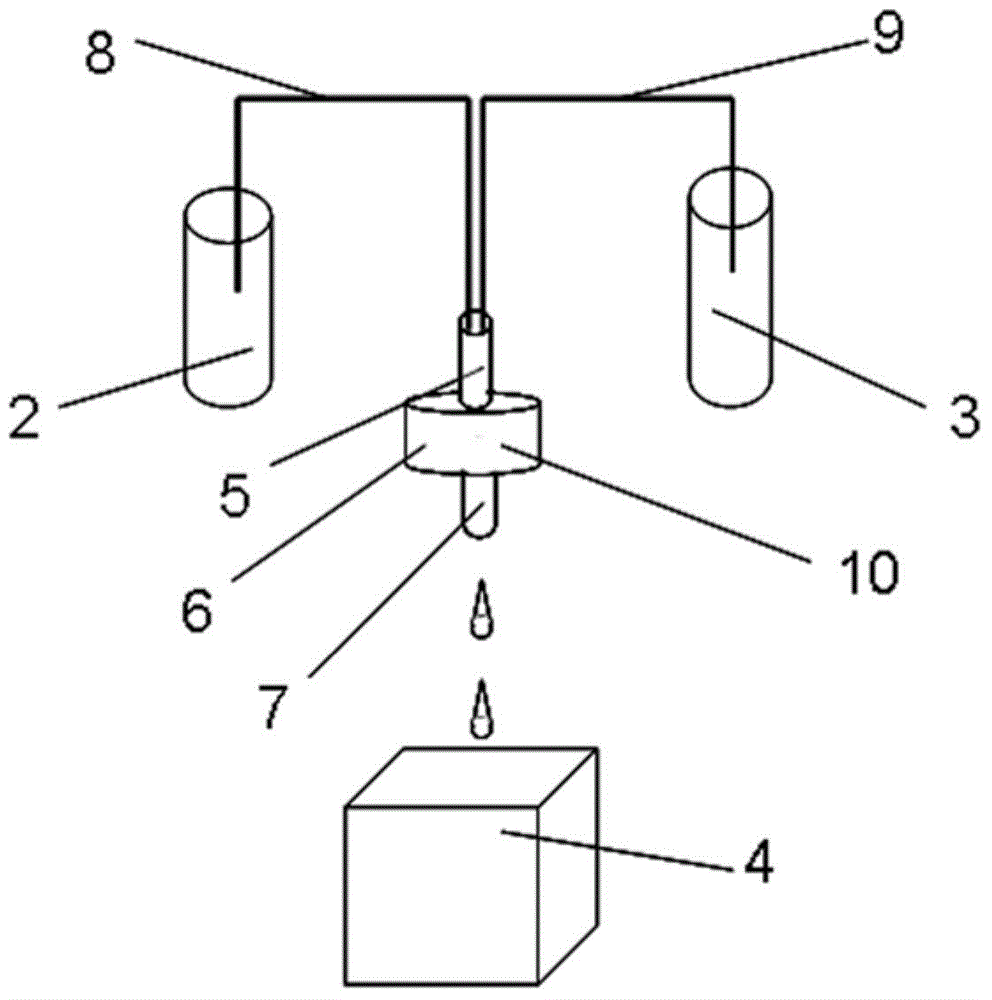
ActiveCN111693336ASampling implementationFast titrationChemical analysis using titrationWithdrawing sample devicesPharmaceutical drugSurgery
The invention discloses a fluid sampling device for drug analysis. The fluid sampling device comprises a sampling barrel, the bottom of the sampling barrel is communicated with a sampling needle tube;two air outlets for adjusting air pressure are formed in the top of the sampling barrel; an isolation sleeve plate is fixed in the sampling barrel; the sampling barrel is divided into two cavities through the isolation sleeve plate; a multi-bottle sampling device for multi-bottle quantitative sampling for drug analysis is mounted on the sampling barrel; the first piston rod in the multi-bottle sampling device stretches out and draws back to realize sampling of drugs, the quantitative adjusting piece and the flow dividing assembly are matched with each other so that equivalent medicine samplesare titrated in a plurality of test tubes at a time, compared with an existing mode that personnel titrate the medicine samples in the test tubes one by one, the titration speed is high, the titration amount of a solution in each test tube does not need to be measured, and a large amount of time is saved.
Owner:山东四君子集团汉邦生物医药有限公司
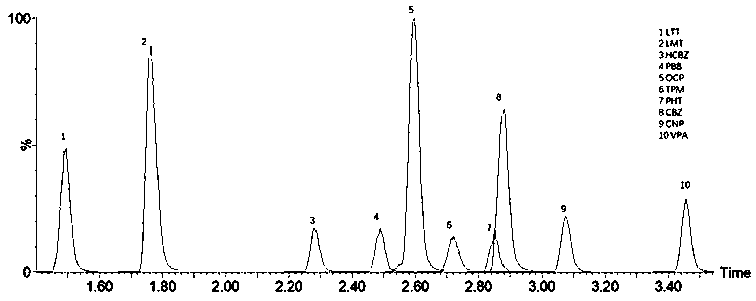
Detection kit for antiepileptic drugs in serum and application thereof
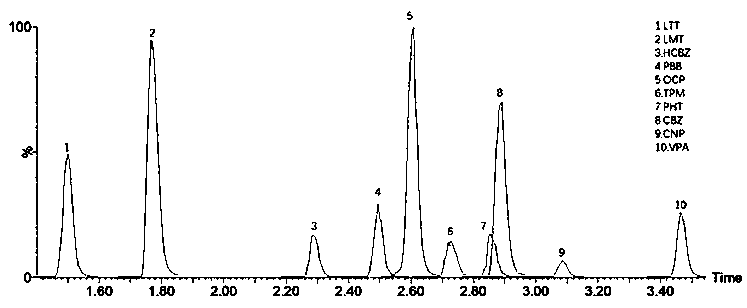
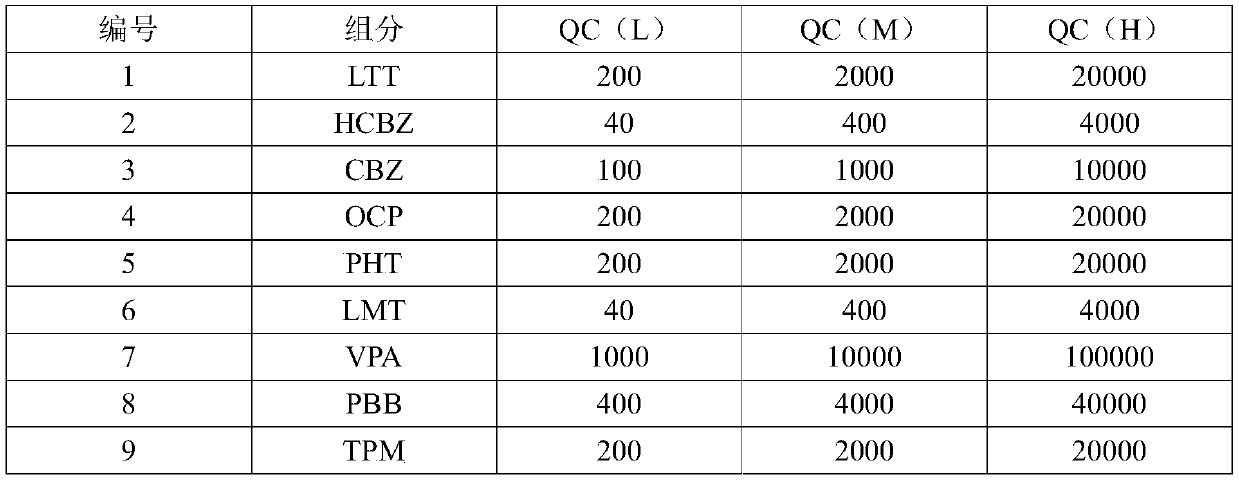
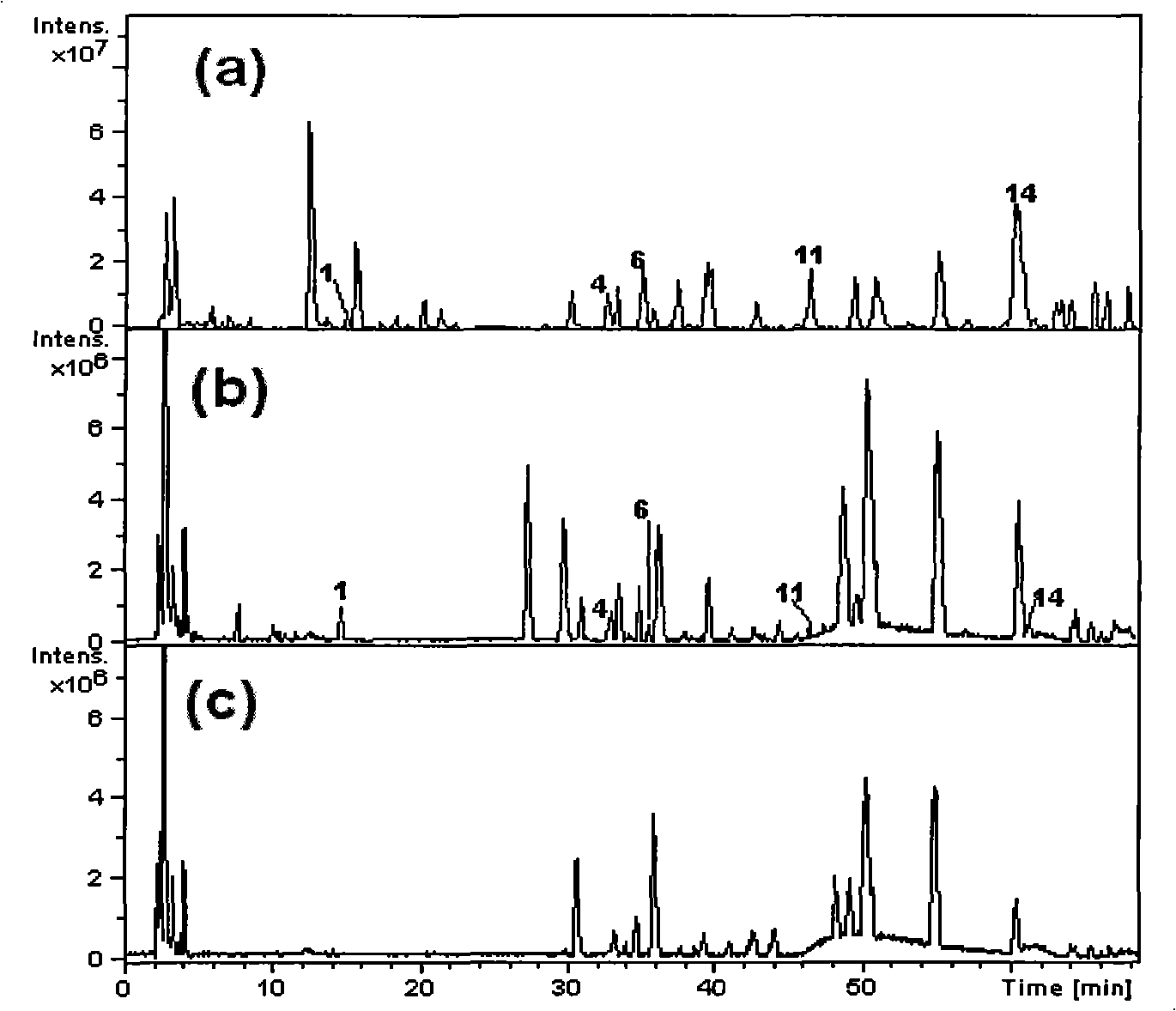
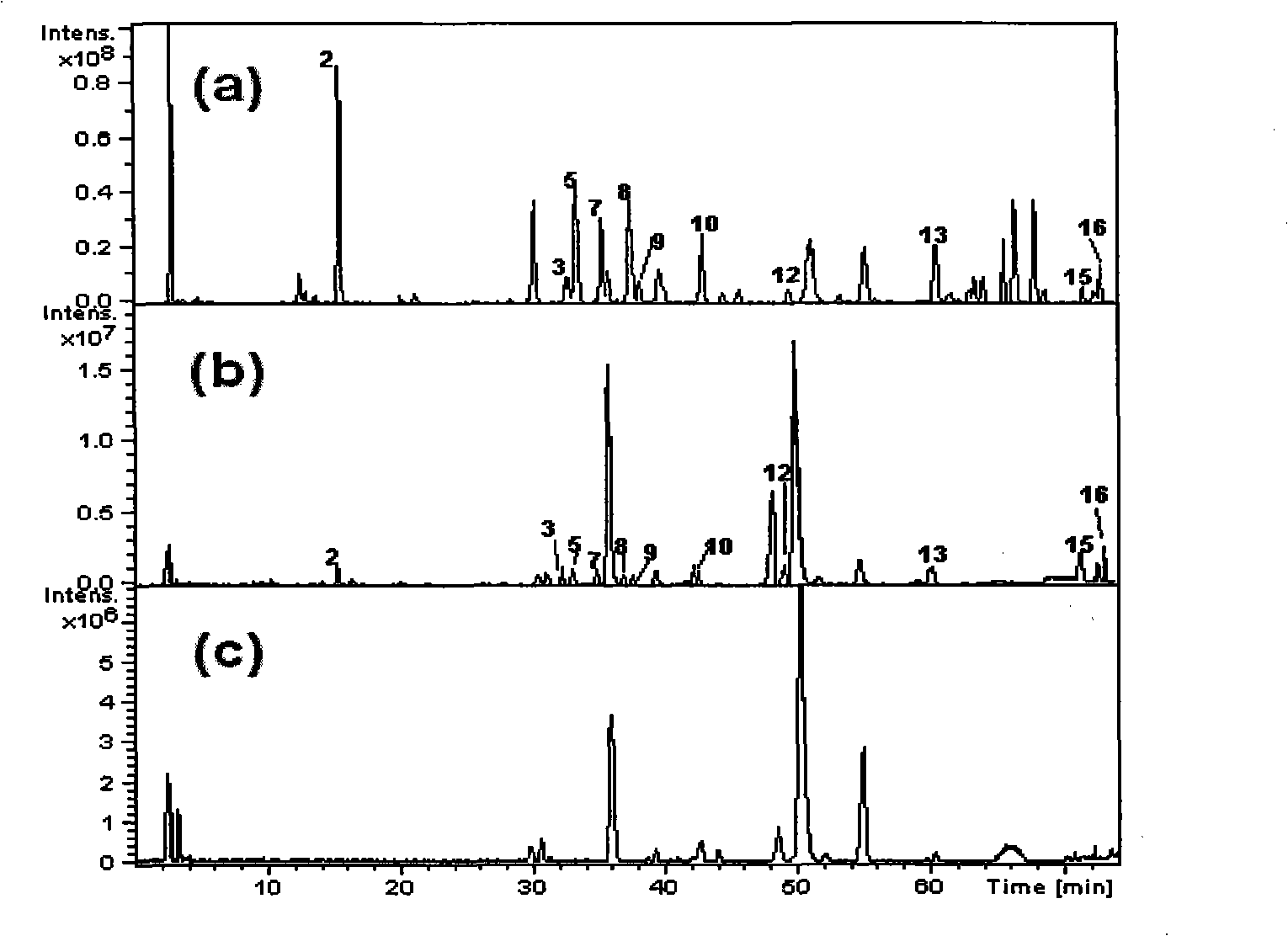
The invention discloses a detection kit for antiepileptic drugs in serum and application thereof, and belongs to the technical field of drug analysis. The kit disclosed by the invention adopts an LC-MS / MS method; ten common antiepileptic drugs can be detected at one time; the kit is simple in pretreatment process, low in cost, high in sensitivity and high in specificity; separation and detection of the antiepileptic drugs are completed within 6 min, the accuracy and precision basically meet the requirements, the kit can be used for quantitative analysis of the clinical antiepileptic drugs, anda reliable detection method is provided for monitoring the treatment concentration of the clinical antiepileptic drugs.
Owner:NANJING PINSHENG MEDICAL TECH CO LTD
Method for determining medicine active component in serum
The invention belongs to the pharmaceutical analysis field, relates to a method for determining a medicine active component in serum, especially relates to a method for determining active components of a compound traditional Chinese medicine in serum. The invention employs a high performance liquid chromatography-mass spectrometry method, and extracts of rat serum, blank rat serum and medicament after medication can be analyzed, active component which is capable of entering to blood and performing drug effect in the medicine and is material foundation of the medicine can be determined, and the content of the material foundation in the medicine can be determined on the base. According to the invention, the compound traditional Chinese medicine can be taken as an integral body for researching, the contained compound component group from the medicine in serum can be analyzed, the material foundation of the medicine can be revealed, and the content of medicine active component can be determined, the method for determining the medicine active component in serum has the advantages of good stability and reappearance, strong operationality and the like.
Owner:HEHUANG PHARMA SHANGHAI
HPLC analytic method for Nicorandil-related substances
The invention belongs to the technical field of pharmaceutical analysis, and particularly relates to an HPLC analytic method for Nicorandil-related substances. The HPLC analytic method is characterized in that a stationary phase is a reversed-phase column of carbon octadecyl silane, and a moving phase is a mixed solution of a buffer solution, methyl alcohol and tetrahydrofuran. The HPLC analytic method is quick, simple and accurate, is good in repeatability and suitable for control and stability study of the related substances, lays a foundation for formulating quality standards of the related substances, and makes drug quality control and medication safety possible. Through scaling down organic solvents and volatile salt, which have high toxicity, the acquisition time is shortened, the economic cost is lowered, the HPLC analytic method is suitable for large-scale production, and meanwhile, harms to experimenters and environments are reduced.
Owner:XIAN KAIBEI NAITE INTELLIGENT ENG
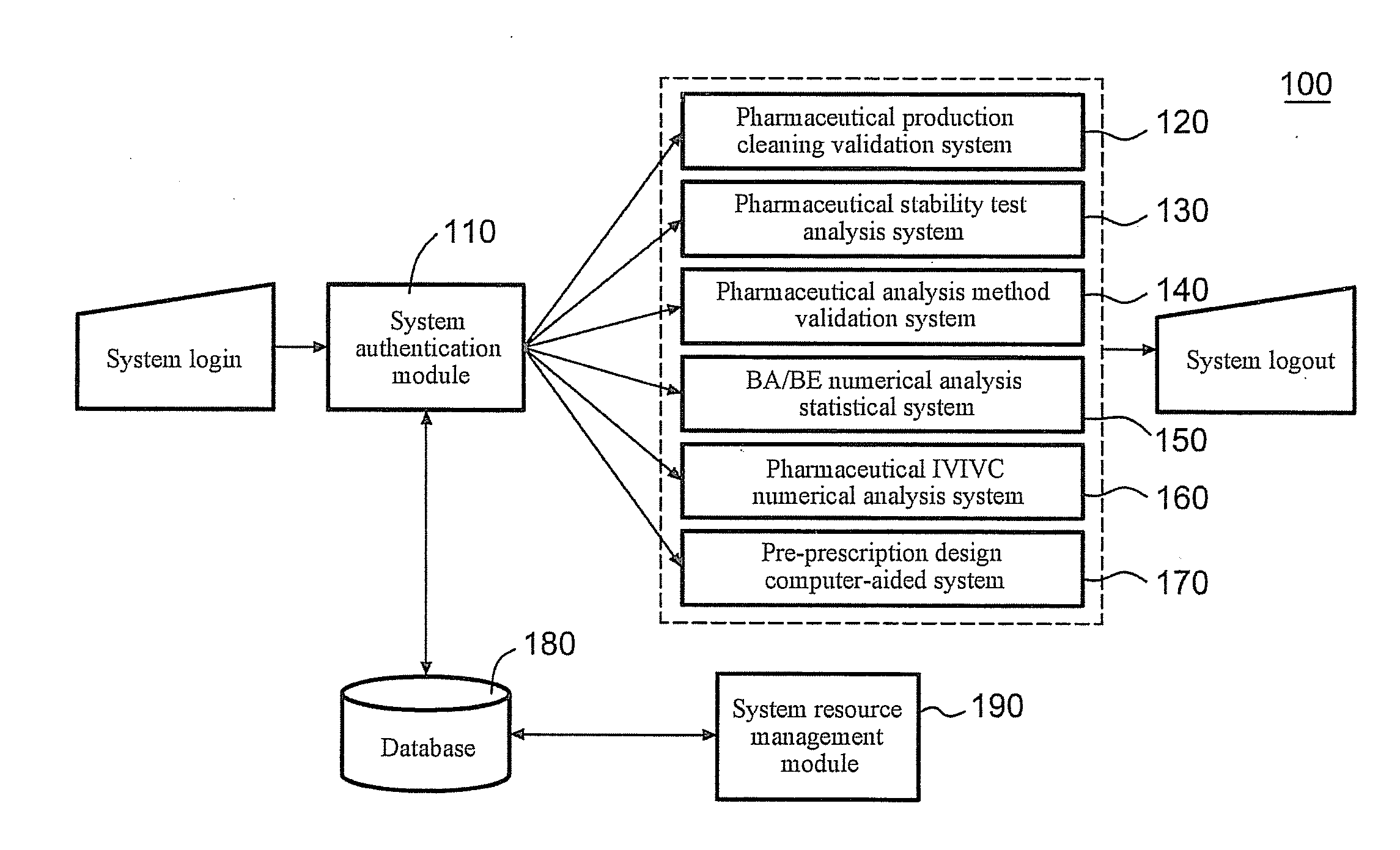
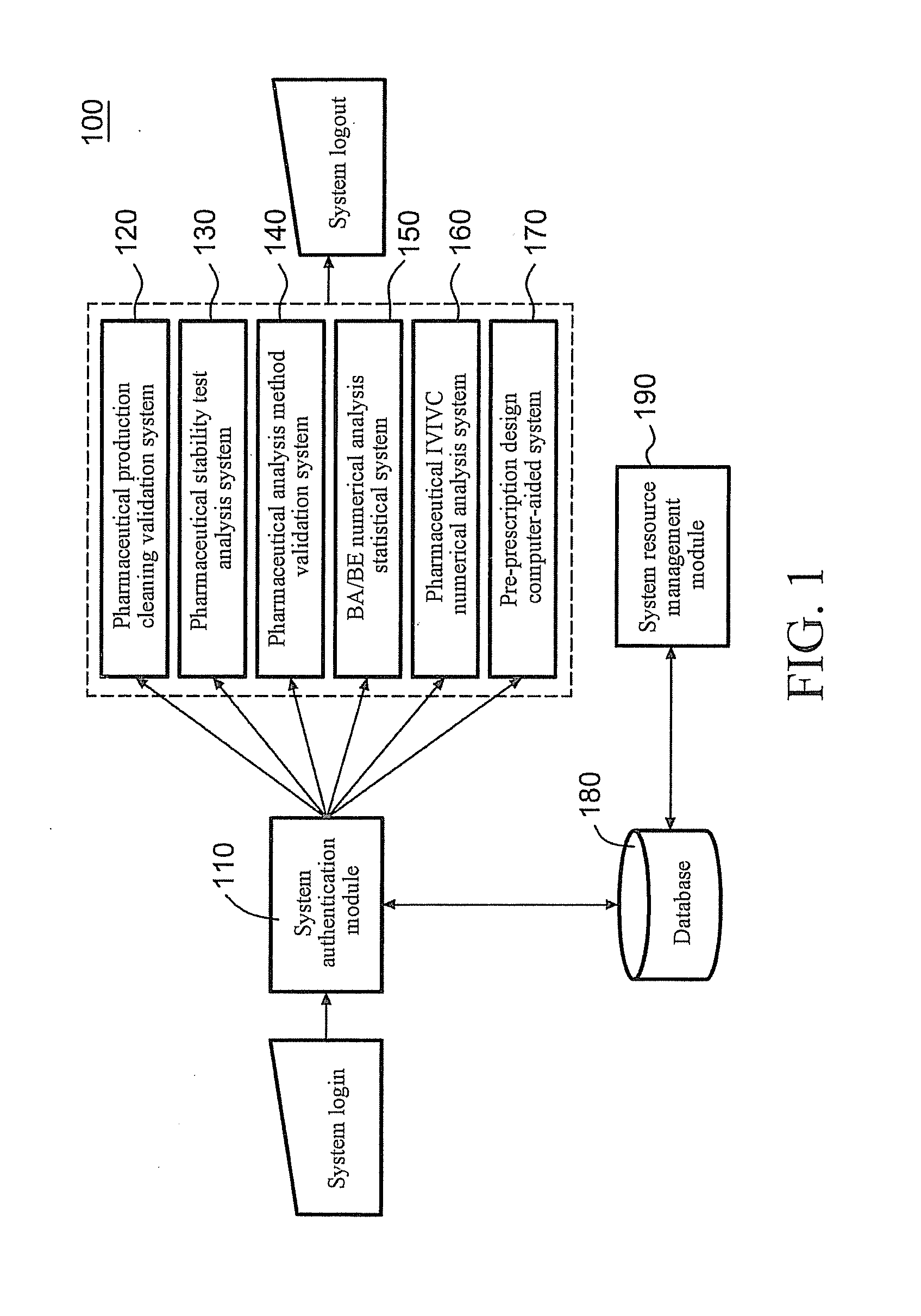
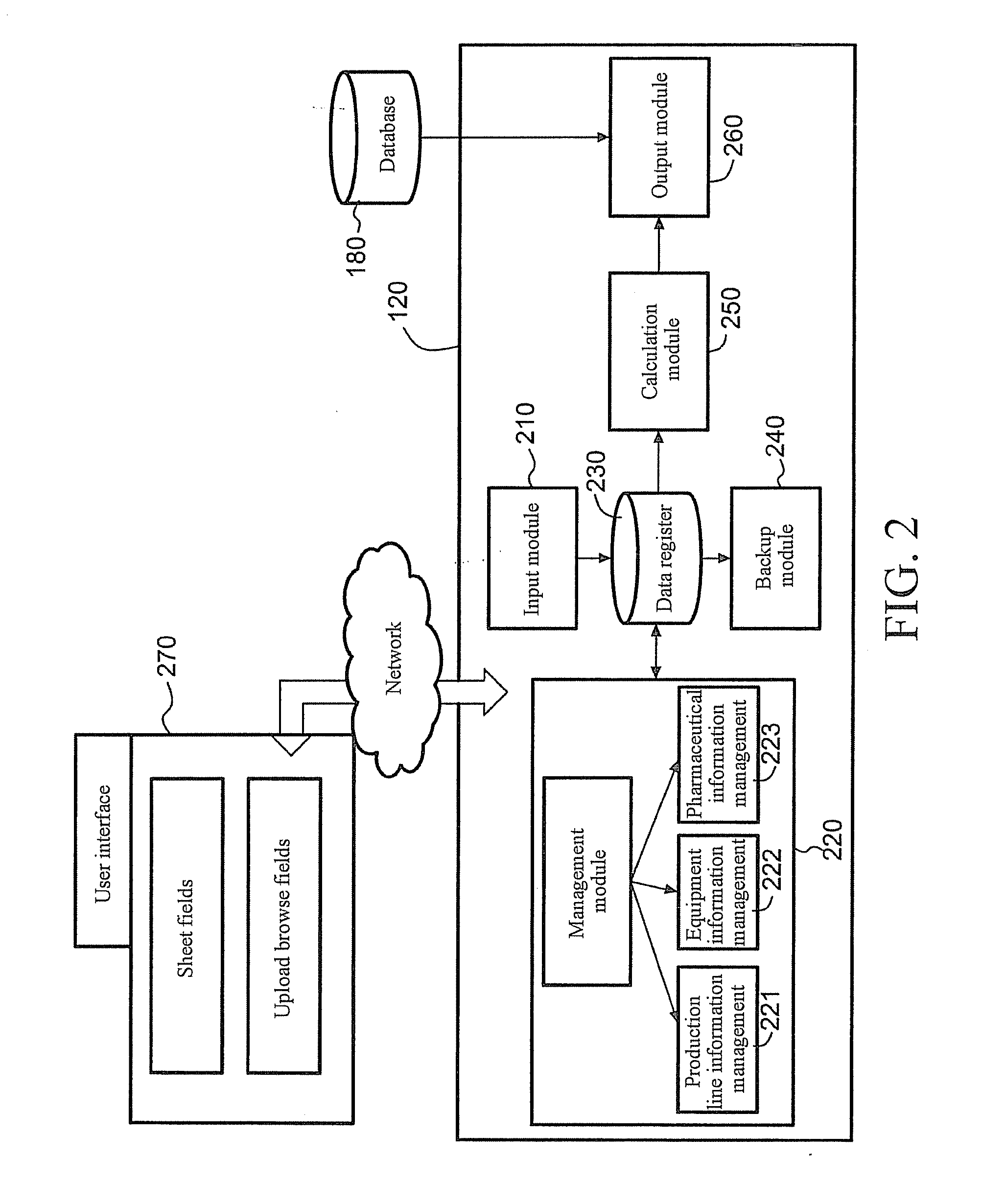
Server for Integrated Pharmaceutical Analysis and Report Generation Service, Method of Integrated Pharmaceutical Manufacturing and Research and Development Numerical Analysis, and Computer Readable Recording Medium
ActiveUS20110276161A1Easy to operateImprove accuracyDrug and medicationsOffice automationPharmaceutical manufacturingComputer science
A web-based tool (as a server) for integrated pharmaceutical analysis and report generation service is provided in the present invention. The server can be used for numerical analysis and report generation for pharmaceutical manufacturing, research and development, and has advantages such as simple operation, complicated but fast calculation and professional report generation, and high accuracy. The server includes at least one pharmaceutical manufacturing and research and development numerical analysis system configured to perform different pharmaceutical manufacturing and research and development numerical analyses and generate different reports. Each of the at least one pharmaceutical manufacturing and research and development numerical analysis system includes an input module configured to receive, via a user interface, at least one of a template file and a backup file previously output by the server as at least one input file, wherein the at least one input file includes a plurality of data fields to provide corresponding data; at least one calculation module, each configured to execute a built-in pharmaceutical manufacturing and research and development numerical analysis calculation program, thereby automatically performing a pharmaceutical manufacturing and research and development numerical analysis calculation on at least one of the data of the at least one input file and on-line filled data; and an output module configured to generate at least one of a backup file and a report file as at least one output file based on the result of the pharmaceutical manufacturing and research and development numerical analysis calculation performed by the at least one calculation module and provide the at least one file via the user interface.
Owner:TAIWAN BIOTECH
Separation method of effective components of sharpleaf galangal fruit aqueous extract
ActiveCN104557505AClear structureCarbonyl compound separation/purificationCarboxylic compound separation/purificationCoumaric acidEthyl acetate
The invention discloses a separation method of effective components of a sharpleaf galangal fruit aqueous extract, belonging to the field of pharmaceutical analysis. The separation method comprises the following steps: (A) fetching dried sharpleaf galangal fruit, grinding, carrying out reflux extraction with water, combining filtrate, carrying out reduced pressure distillation to obtain extractum, dissolving the extractum into distilled water, respectively extracting by virtue of petroleum ether, ethyl acetate and normal butanol, and recycling solvents, so as to obtain ethyl acetate extract dry powder; (B) dissolving the dry powder obtain in the step (A), filtering, and processing by virtue of a sephadex LH20 gel column, so as to obtain six components from F1 to F6; and (C) carrying out HPLC separation and purification on the components from F2 to F6, so as to obtain compounds including protocatechualdehyde, protocatechuic acid, p-coumaric acid, (-)-epicatechin and (+)-catechinic acid. According to the separation method, the effective active components are separated and extracted from the sharpleaf galangal fruit, the structures of the effective components are further defined, the basis is provided for later medication, and required components can be extracted according to different requirements.
Owner:HEBEI YILING MEDICINE INST
Fingerprint spectrum of immature bitter orange medicinal material and construction method and application of fingerprint spectrum
ActiveCN103837621AComprehensive characteristic peak informationImprove stabilityComponent separationHplc fingerprintComputer science
The invention belongs to the technical field of pharmaceutical analysis and relates to a fingerprint spectrum of an immature bitter orange medicinal material and a construction method and application of the fingerprint spectrum. Specifically, the invention relates to a high performance liquid chromatography (HPLC) fingerprint spectrum of the immature bitter orange medicinal material and a construction method of the fingerprint spectrum, as well as application of the HPLC fingerprint spectrum and / or a standard fingerprint spectrum of the immature bitter orange medicinal material in identification of the immature bitter orange medicinal material. The method for constructing the HPLC fingerprint spectrum of the immature bitter orange medicinal material specifically comprises the following steps: (1) preparing an immature bitter orange test sample; and (2) performing HPLC analysis. The invention also relates to a method for identifying the immature bitter orange medicinal material. The method is applied to identifying the authenticity and quality of the immature bitter orange medicinal material.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
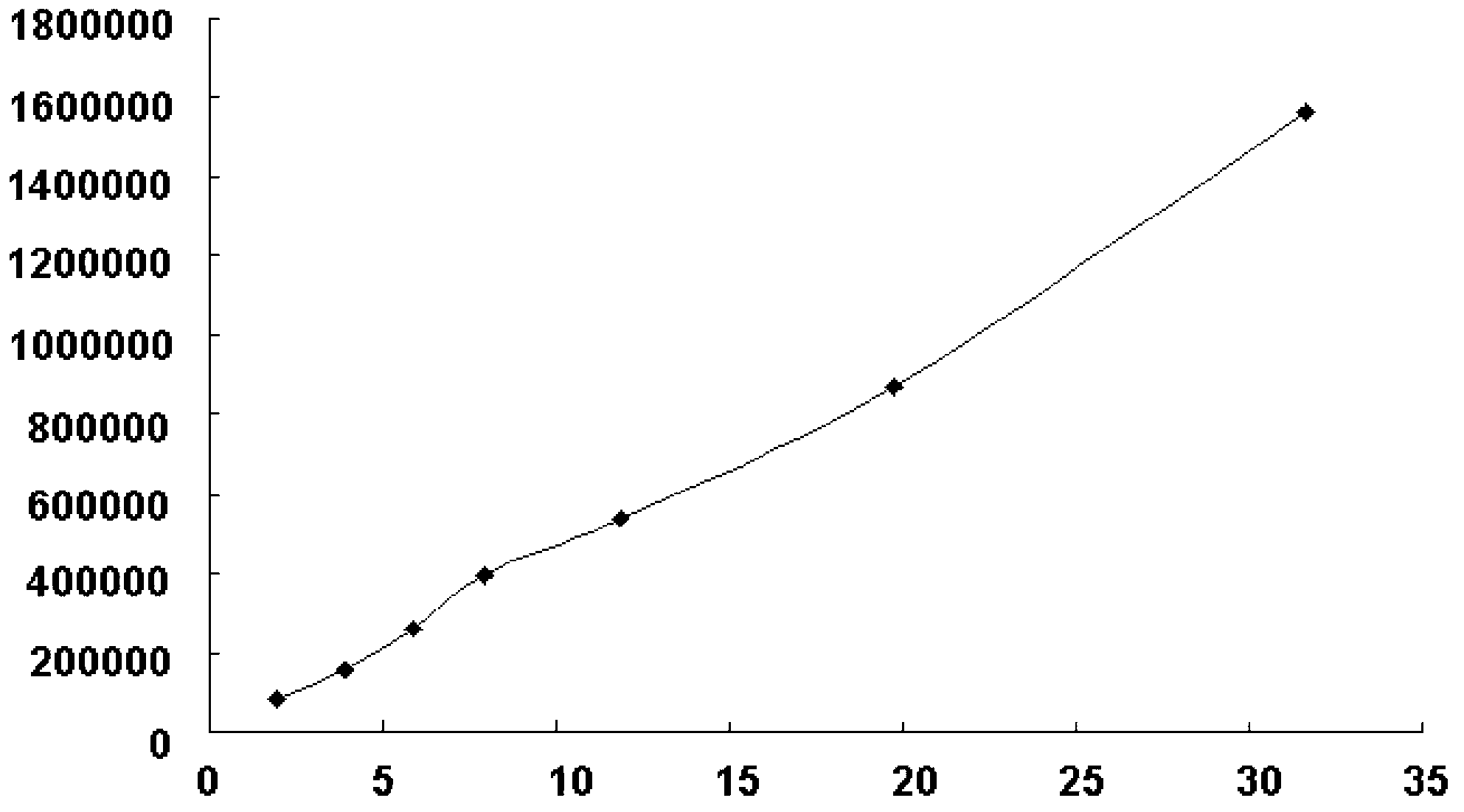
Method for measuring impurity content of faropenem polymers in faropenem sodium raw materials and preparations
ActiveCN101852782ASignificantly progressiveVolume controlOrganic chemistryComponent separationColumn temperatureImpurity
The invention particularly relates to a method for measuring the content of faropenem polymers in faropenem sodium raw materials and preparations by high-efficiency liquid chromatography, which belongs to the field of medicine analysis. The measuring method of the invention is gel chromatography. The high-efficiency liquid chromatogram is used for detection, and the chromatogram conditions are as follows: (1) the gel permeation chromatography has the exclusion molecular weight between 600 and 800 Daltons; (2) the mobile phases as two mobile phases, wherein the mobile phase A is a water phase solution with the pH value between 5.0 and 8.5 and the concentration between 0.01 and 0.2 mol / L, and the mobile phase B is water or a sodium dodecyl sulfate water solution or a glycin solution between 0.005 and 0.02 percent; (3) the column temperature is the room temperature; (4) the flowing rate is between 0.5 and 1.0 ml / min; and (5) the detection wavelength is between 210 and 300 nm. The method of the invention has the advantages of strong specificity, good repetitiveness and high automation degree, and the polymers in the faropenem sodium raw materials and the preparations can be accurately qualified.
Owner:LUNAN PHARMA GROUP CORPORATION
Methods for validating medication
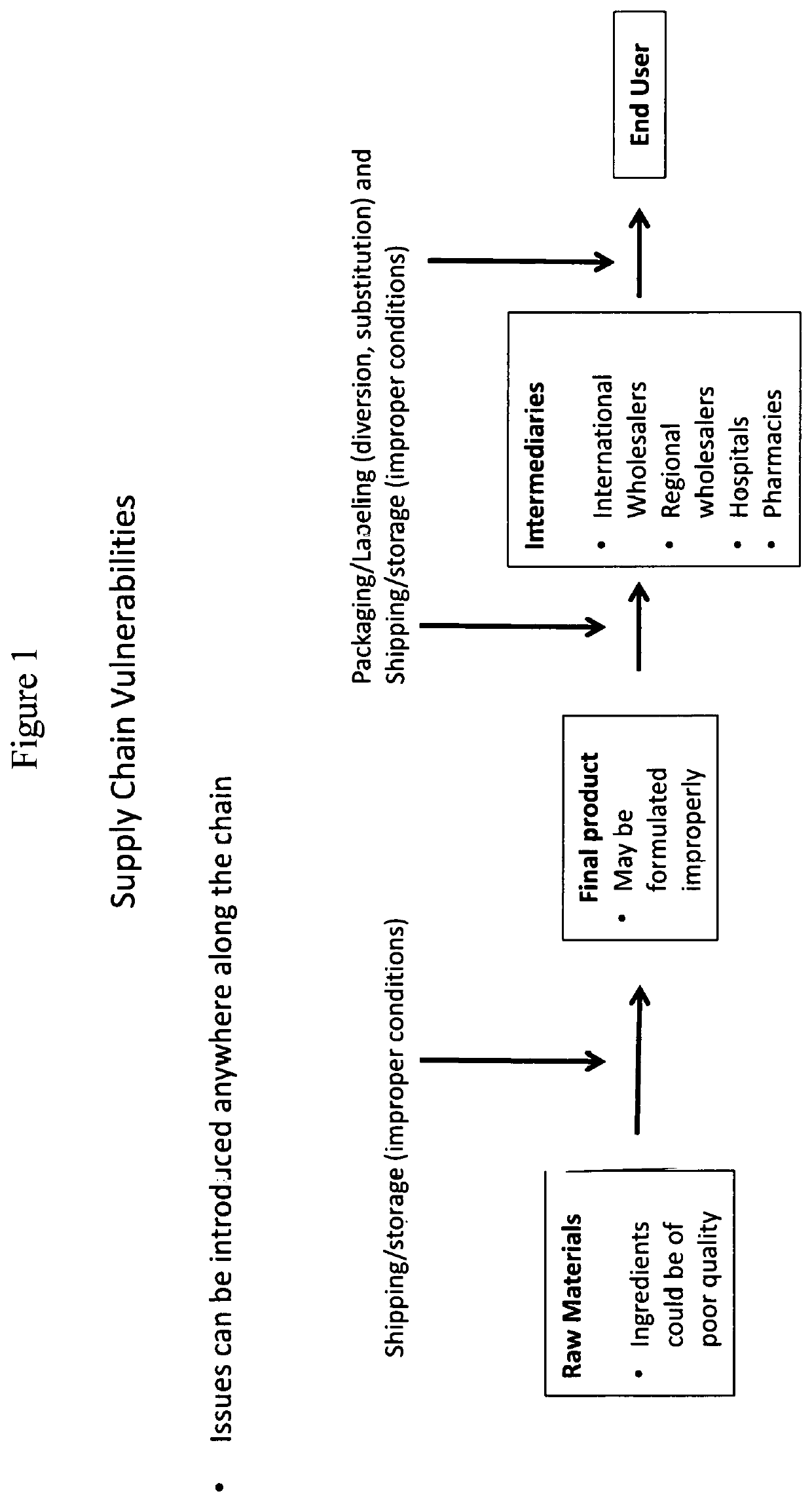
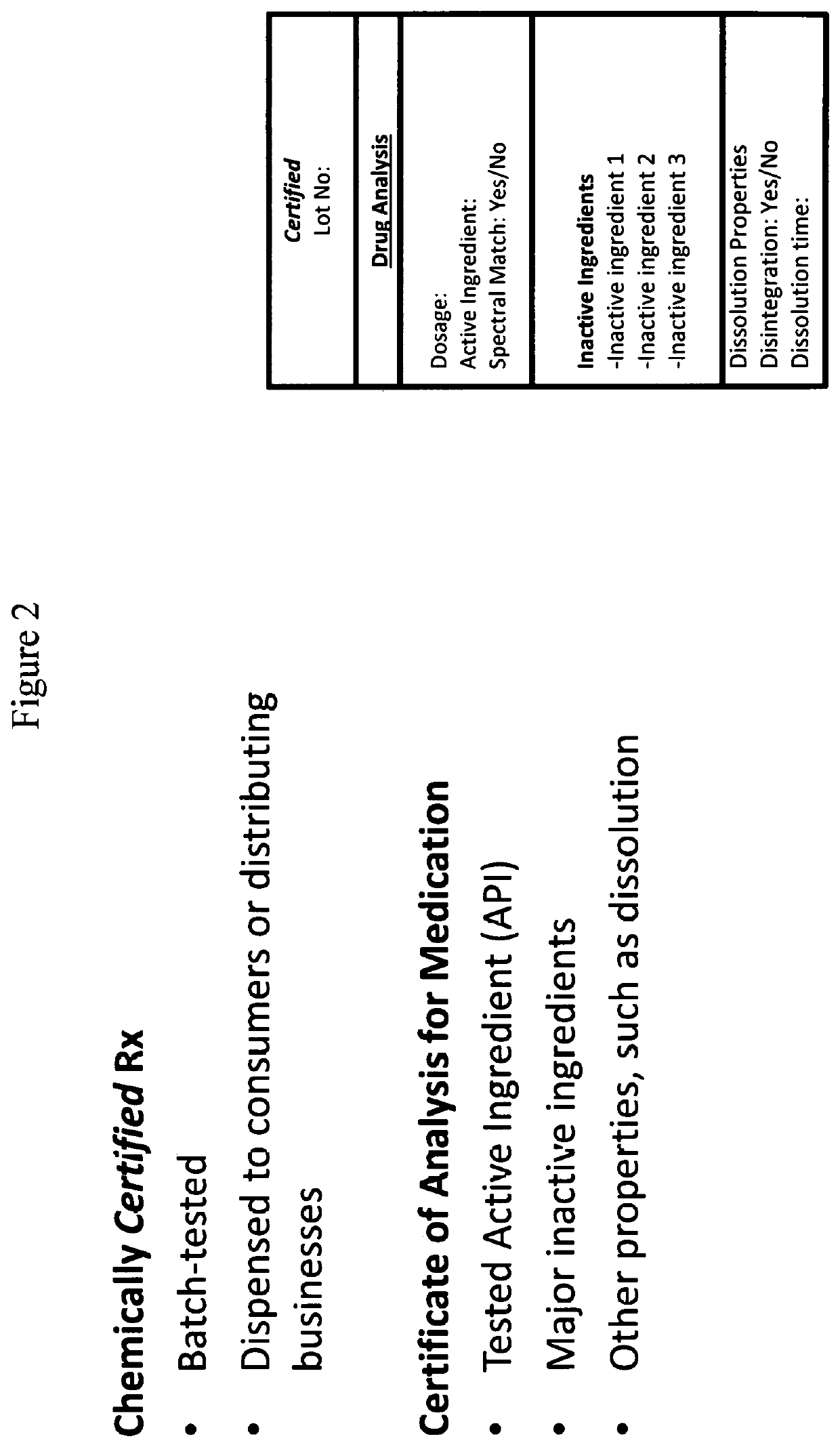
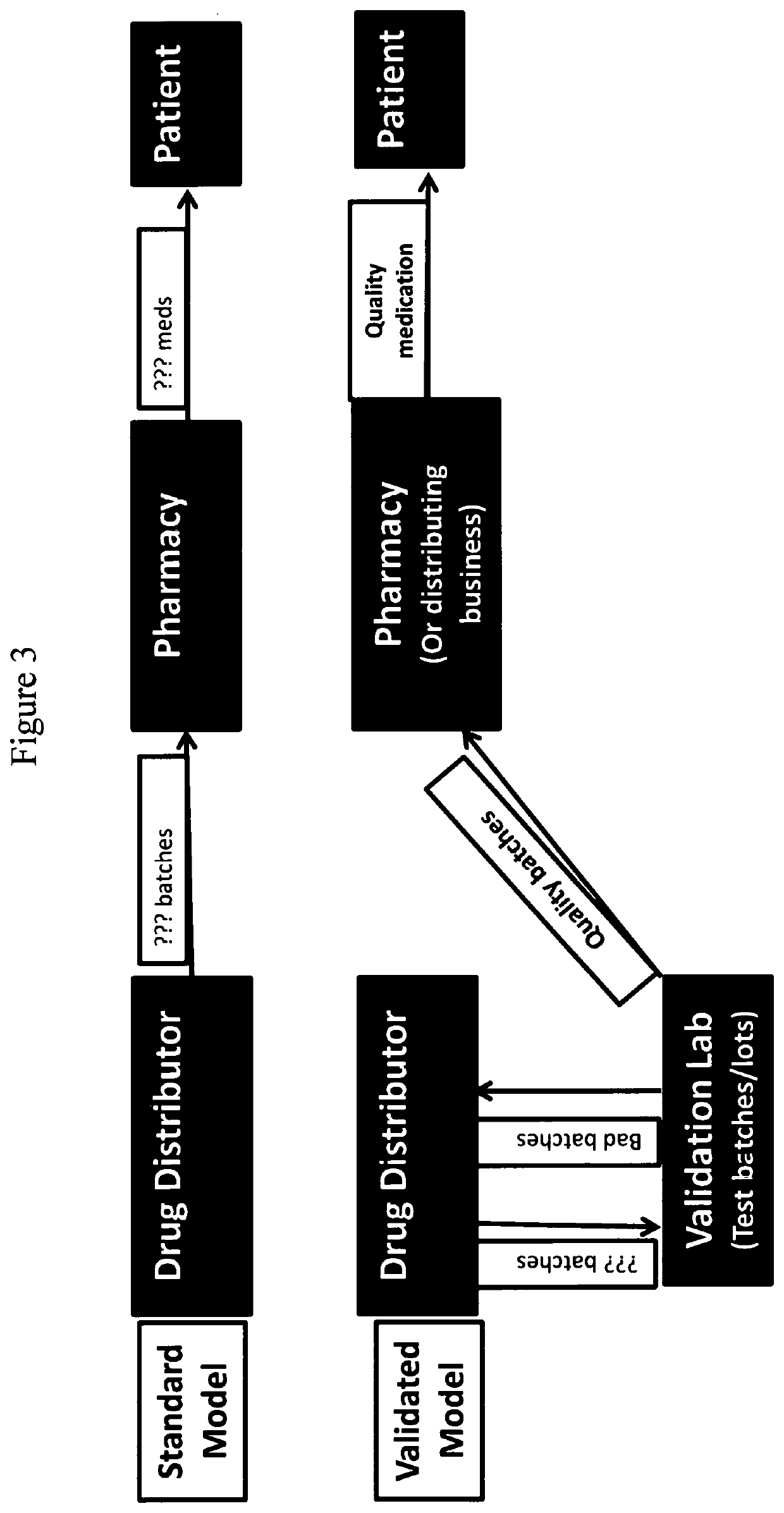
ActiveUS20200080980A1Accurate identificationAccurate verificationDrug and medicationsRaman scatteringMedicinePharmaceutical drug
The present disclosure provides methods and systems for providing a validated drug to an end-user or distributing business. Also provided herein are methods and systems for providing a certificate of analysis for a drug to provide more information to a user.
Owner:VALISURE LLC
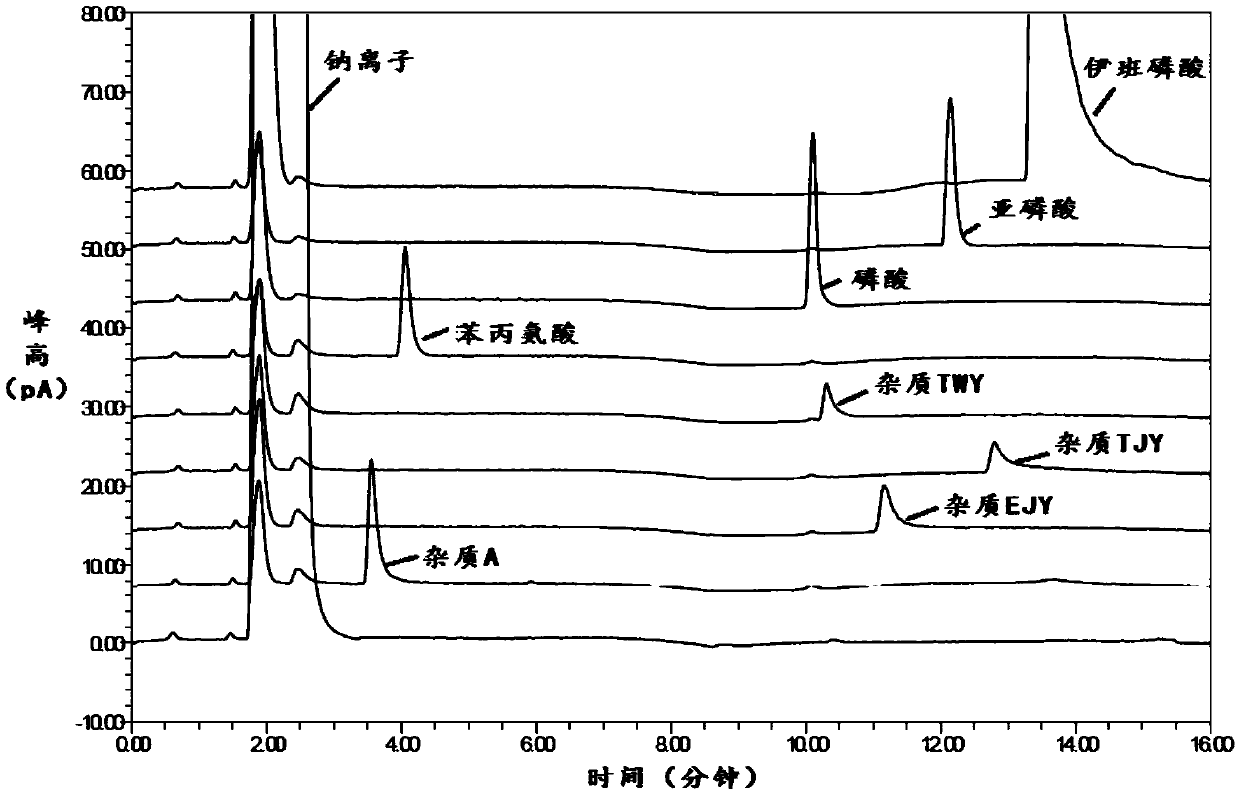
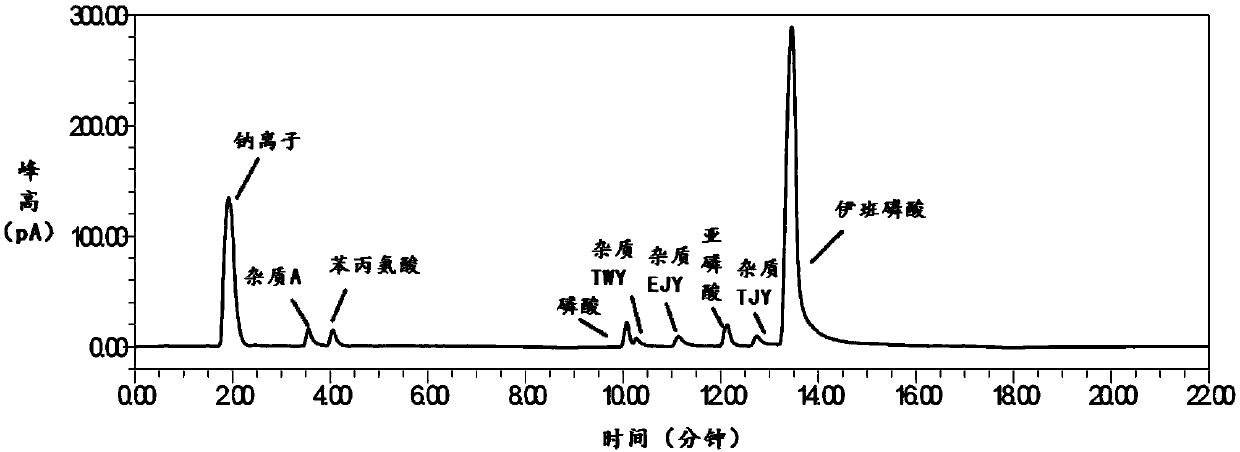
Method for detecting related substances in sodium ibandronate injection
InactiveCN109668975AOptimal pre-processing methodEnsure safetyComponent separationSilanesCharged aerosol detector
The invention provides a method for detecting related substances in sodium ibandronate injection, and belongs to the technical field of drug analysis. According to the method for detecting the relatedsubstances in the sodium ibandronate injection, a high performance liquid chromatograph-charged aerosol detector (CAD) is adopted, a chromatographic column with octadecyl silane chemically bonded silica embedded in a strong anion exchange group as a filler is adopted, acetonitrile-water-trifluoroacetic acid serves as a flowing phase for gradient elution, the injection is subjected to Ag / H type pretreatment small column treatment, and thus the related substances in the sodium ibandronate injection can be effectively separated and determined.
Owner:QILU PHARMA
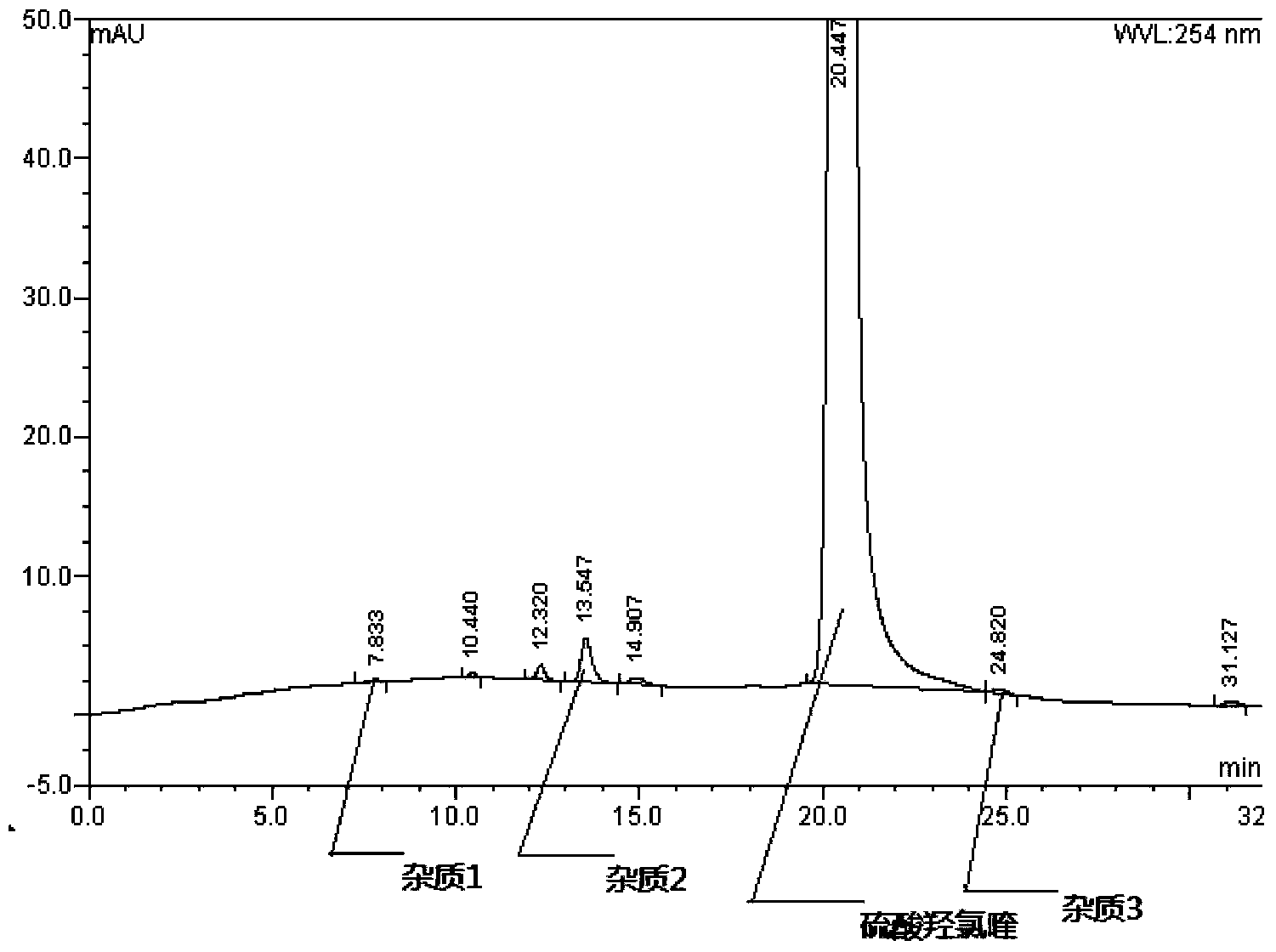
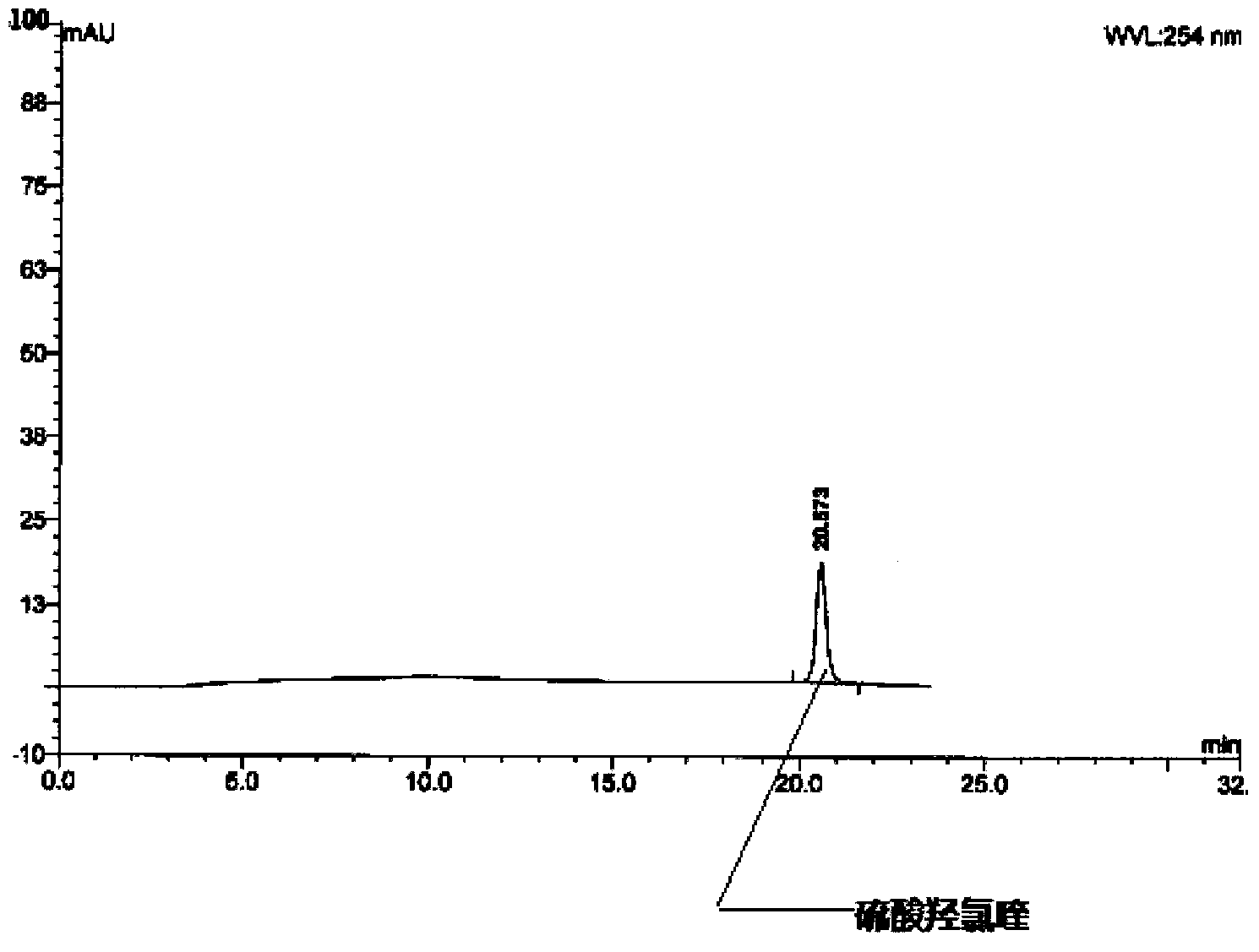
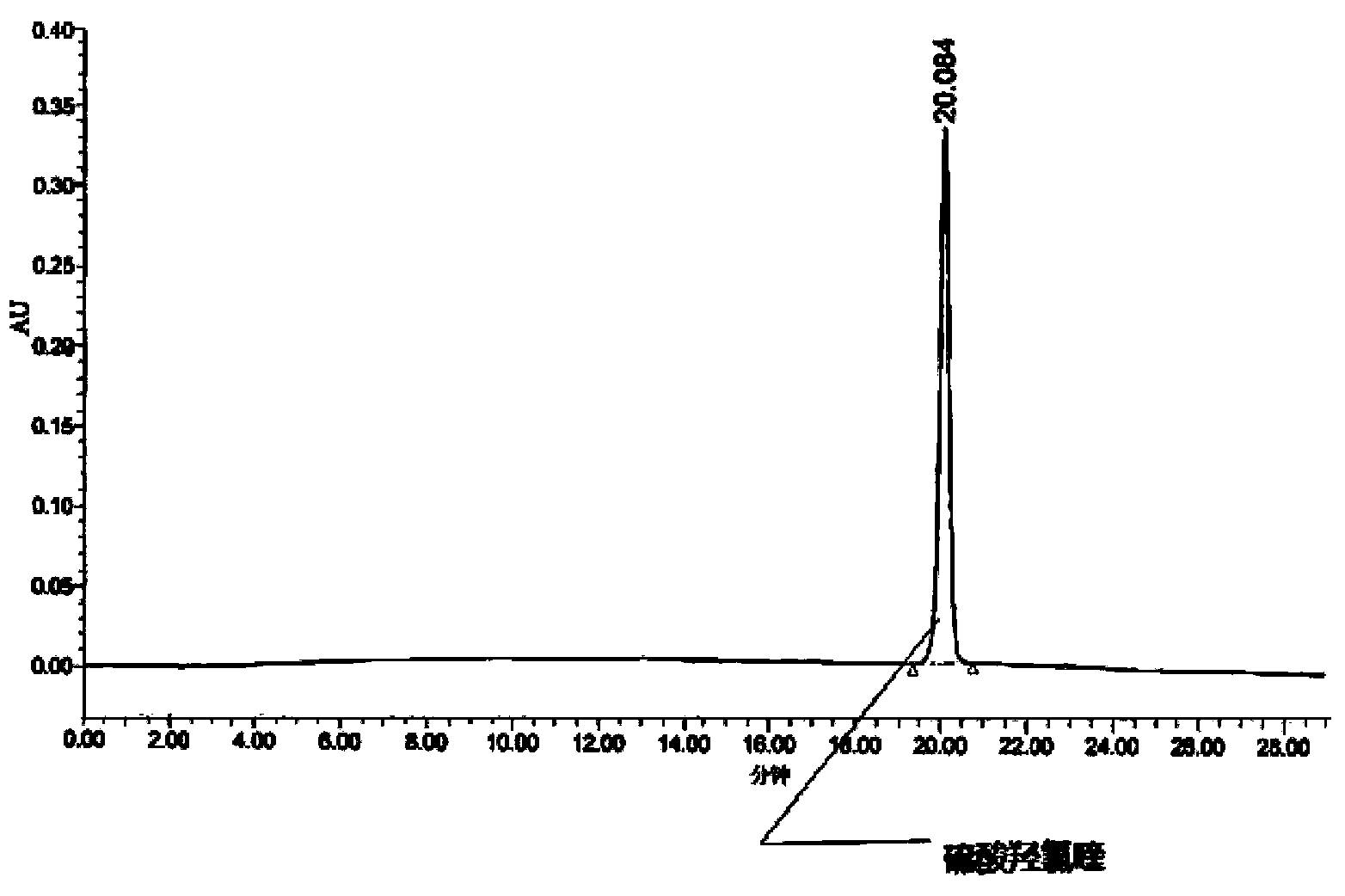
Method for analysis of hydroxychloroquine sulfate raw material and preparation by high performance liquid chromatography
InactiveCN103472154AOvercoming the lack of specificityOvercoming sensitivityComponent separationHydroxychloroquine SulfateHigh-performance liquid chromatography
The invention belongs to the field of drug analysis and discloses a method for analysis of a hydroxychloroquine sulfate raw material and preparation by high performance liquid chromatography. The method can realize effective separation of related substances in hydroxychloroquine sulfate so that hydroxychloroquine sulfate quality is controlled, hydroxychloroquine sulfate raw material and preparation content is accurately determined, and hydroxychloroquine sulfate stability is indicated. The method has the advantages of strong specificity, high accuracy and simple operation.
Owner:WUHAN WUYAO SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com