HPLC (high performance liquid chromatography) analyzing method for 3-aminopiperidine
An aminopiperidine and analytical method technology, applied in the field of pharmaceutical analysis, can solve problems such as powerlessness and large sample volume requirements, and achieve the effects of simple operation, good reproducibility, and convenient standardized operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 : Analysis of 3-Aminopiperidine by Reversed-Phase High Performance Liquid Chromatography
[0038] (1) Reverse-phase high-performance liquid chromatography analysis of (RS)-3-aminopiperidine
[0039] Weigh 2.9 g (0.029 mol) of (RS)-3-aminopiperidine, dissolve it in 40 mL of petroleum ether, stir at 40°C, and slowly add 16.4 g (0.116 mol) of benzoyl chloride dropwise. The reaction was monitored by TLC, and the solvent was evaporated to dryness after the reaction to obtain (RS)-dibenzoyl-3-aminopiperidine.
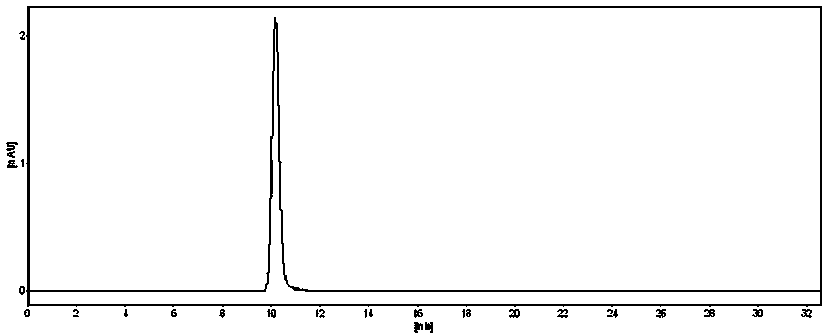
[0040] (RS)-dibenzoyl-3-aminopiperidine was dissolved in mobile phase and then detected and analyzed by high performance liquid chromatography. Liquid chromatography conditions: Dima C18 column, the mobile phase is water-methanol (40:60), the UV detection wavelength is 254nm, the flow rate is 0.8 mL / min, the column temperature is 30°C, and the injection volume is 20 μL. The retention time is shown in the following table 1, and its spectrum is shown in f...
Embodiment 2
[0048] Example 2 : Chiral purity analysis of (RS)-3-aminopiperidine
[0049] Weigh 2.9 g (0.029 mol) of (RS)-3-aminopiperidine, dissolve it in 40 mL of hexane, stir at 40°C, and slowly add 16.4 g (0.116 mol) of benzoyl chloride dropwise. The reaction was monitored by TLC, and the solvent was evaporated to dryness after the reaction to obtain (RS)-dibenzoyl-3-aminopiperidine.
[0050] (RS)-dibenzoyl-3-aminopiperidine was dissolved in mobile phase and then detected and analyzed by high performance liquid chromatography.
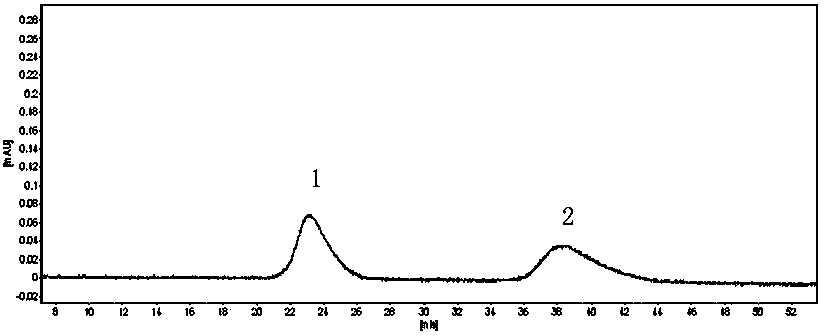
[0051] (1) Liquid chromatography condition A: ChromTech CHIRAL-AGP column, mobile phase is 0.015 mol / L phosphate aqueous solution-acetonitrile (92:8), UV detection wavelength is 254nm, flow rate is 0.8 mL / min, column temperature is 30°C, the injection volume is 20 μL. Retention time, resolution and chiral purity are shown in Table 2 below, and the spectrum is shown in figure 2 .
[0052] (2) Liquid chromatography condition B: ChromTech CHIRAL-AGP column,...
Embodiment 3
[0055] Example 3 : The chiral purity analysis of (R)-3-aminopiperidine test sample
[0056] Weigh 2.9 g (0.029 mol) of the (R)-3-aminopiperidine test sample, dissolve it in 40 mL of dichloromethane, stir at 38°C, and slowly add 16.4 g (0.116 mol) of benzoyl chloride dropwise. The reaction was monitored by TLC, and the solvent was evaporated to dryness after the reaction to obtain (R)-dibenzoyl-3-aminopiperidine.
[0057] (R)-dibenzoyl-3-aminopiperidine is dissolved in mobile phase and then detected and analyzed by high performance liquid chromatography.
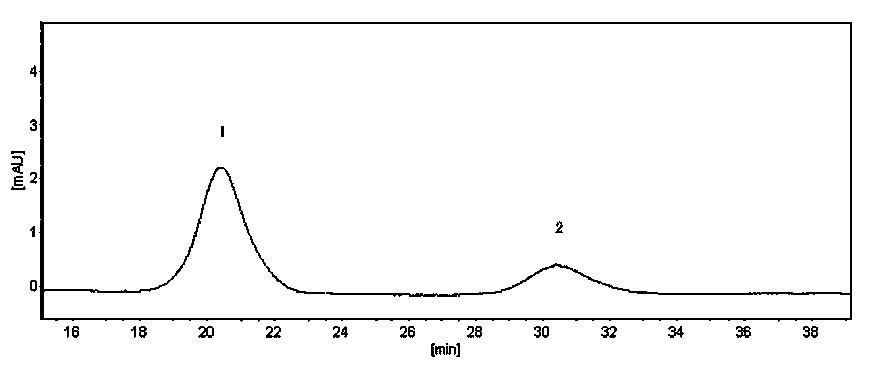
[0058] (1) Liquid chromatography condition A: ChromTech CHIRAL-AGP chromatographic column, the mobile phase is 0.02 mol / L phosphate aqueous solution-acetonitrile (92:8), the ultraviolet detection wavelength is 254nm, the flow rate is 0.8 mL / min, and the column temperature is 30°C, the injection volume is 20 μL. The peak area percentage and chiral purity are shown in Table 3 below, and the enlarged view of the liquid chrom...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com