Venlafaxine hydrochloride sustained-release tablet preparation and preparation method thereof
A technology for venlafaxine hydrochloride and sustained-release tablets, which is applied in the direction of pharmaceutical formulas, medical preparations containing no active ingredients, medical preparations containing active ingredients, etc., and can solve the problem of large fluctuations in blood drug concentration, large side effects, and rapid metabolism And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
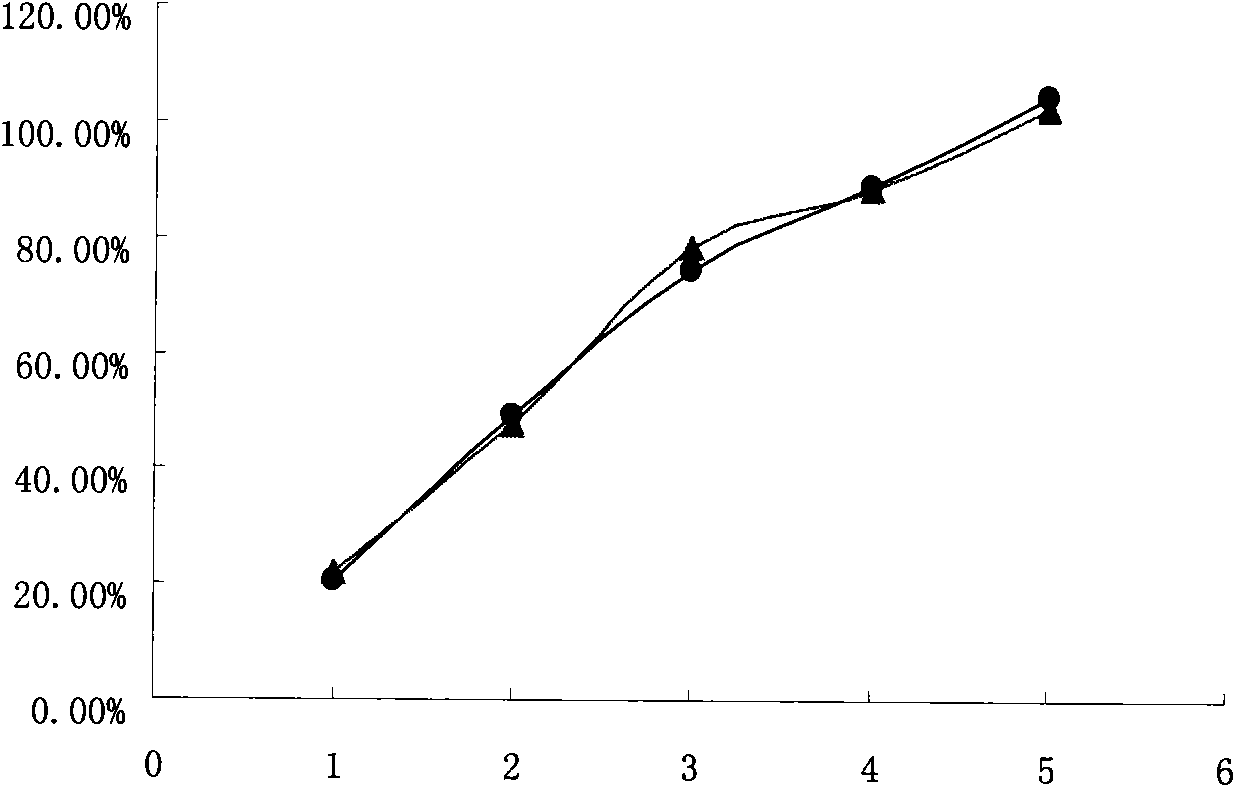
Embodiment 1
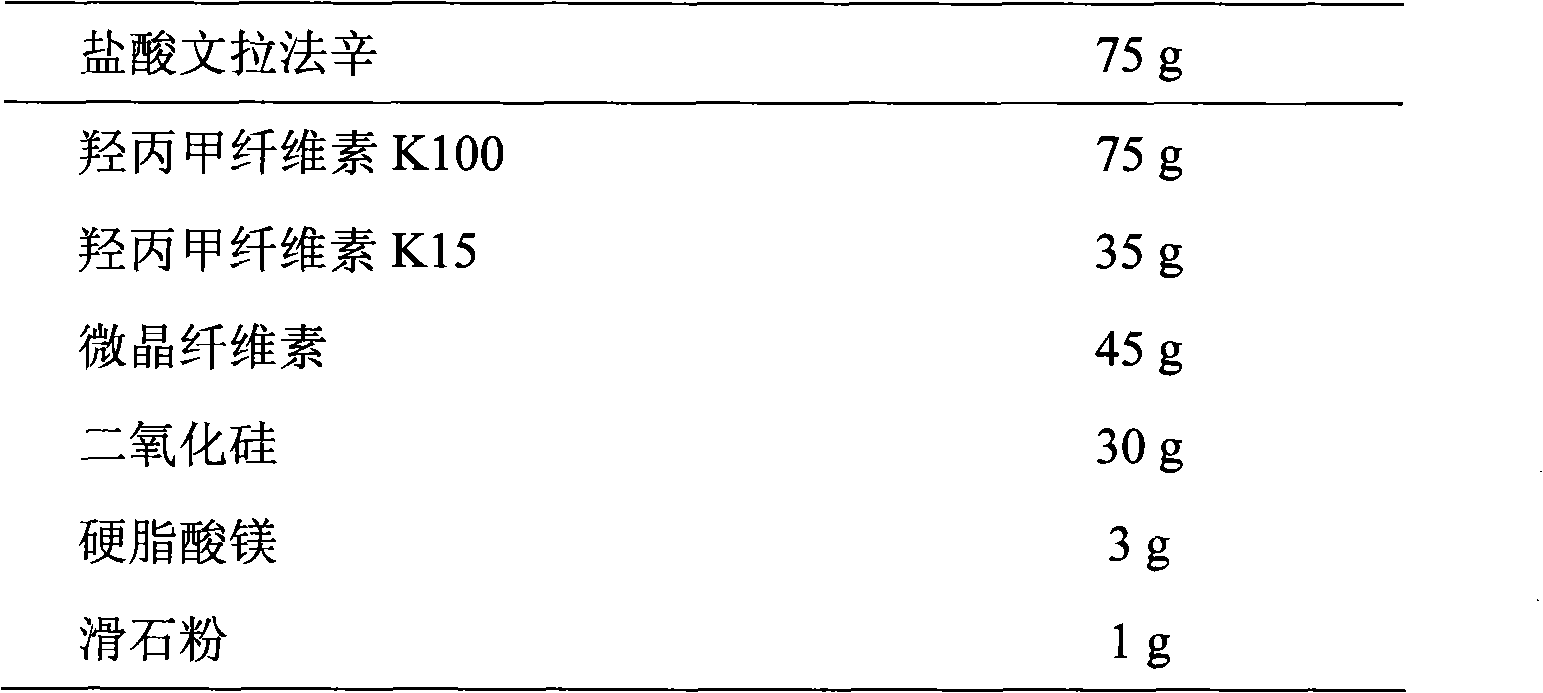
[0060] Tablet formula: (made into 1000 tablets, each containing venlafaxine hydrochloride 75mg)
[0061]
[0062] Coating solution: aqueous dispersion of ethyl acrylate-methyl methacrylate copolymer
[0063] Preparation process: pass the above raw materials and supplements through 80-mesh sieve respectively, then mix evenly, directly compress into tablets, then coat with the above coating solution, and add talcum powder. Place the wrapped finished product at 40°C and keep it warm for 24 hours.
Embodiment 2
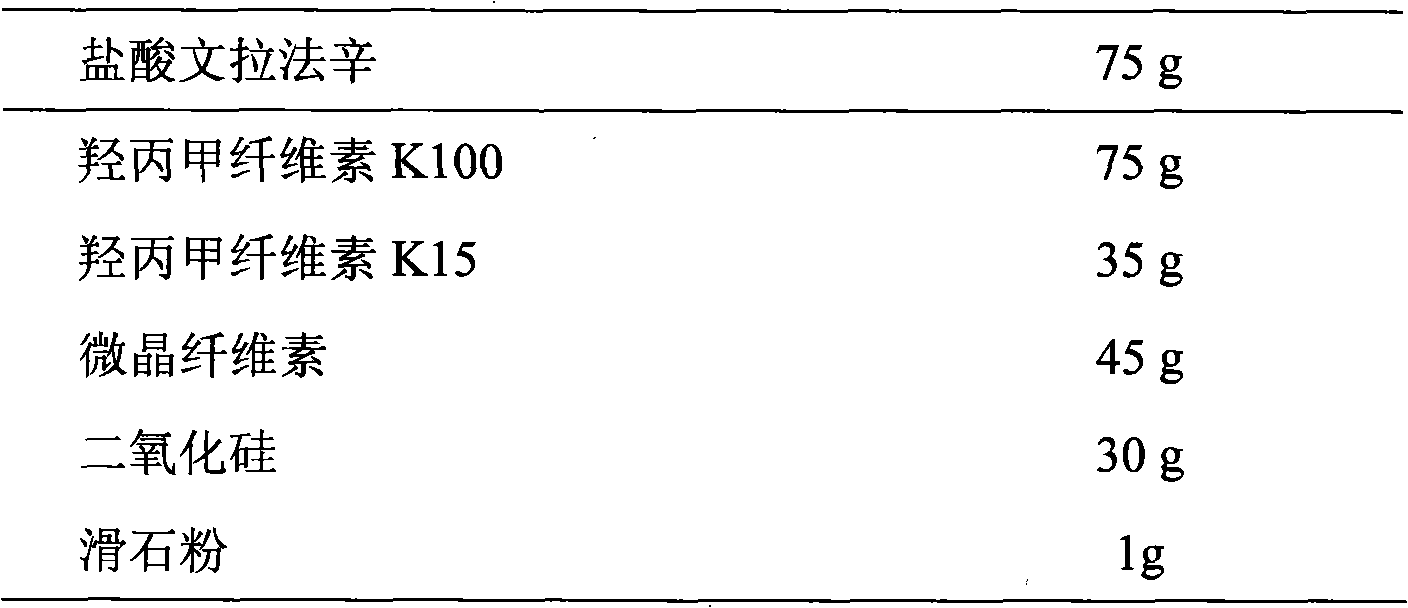
[0065] Tablet prescription: (made into 1000 tablets, each containing 75mg of venlafaxine hydrochloride)
[0066]
[0067] Coating solution: aqueous dispersion of ethyl acrylate-methyl methacrylate copolymer
[0068] Preparation process: pass the above raw materials and supplements through 80-mesh sieve respectively, then mix them evenly, make 16-mesh granules by dry method, compress into tablets, then coat with the above-mentioned coating solution, and add appropriate amount of talcum powder. Place the wrapped finished product at 40°C and keep it warm for 24 hours.
Embodiment 3
[0070] Tablet prescription: (made into 1000 tablets, each containing 75mg of venlafaxine hydrochloride)
[0071]
[0072] Coating solution: aqueous dispersion of ethyl acrylate-methyl methacrylate copolymer
[0073] Preparation process: pass the above raw materials and supplements through 80-mesh sieve respectively, then mix them evenly, make 16-mesh granules by wet method, dry them, add the prescribed amount of magnesium stearate, mix them evenly, press into tablets, then coat with the above coating solution, and Add talc. Place the wrapped finished product at 40°C and keep it warm for 24 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com