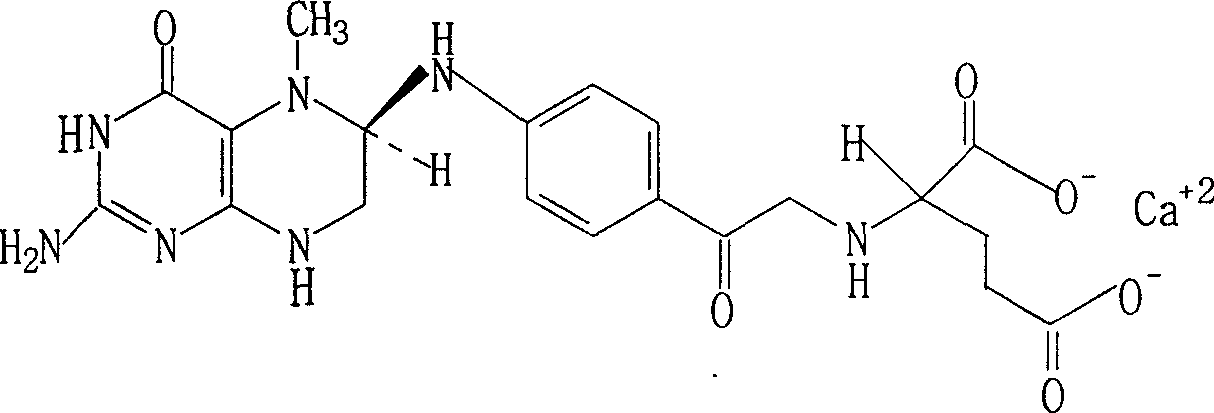
Resolution for 5-methyltetrahydrofolic acid and salifying method thereof
A technology of methyltetrahydrofolate and calcium methyltetrahydrofolate, which is applied in the directions of organic chemistry methods, chemical instruments and methods, separation of optically active compounds, etc. And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Add 56.0g (0.4mol) (±)-α-phenylethylamine and 300ml water in a 1000ml three-necked flask, then add 300ml aqueous solution containing 107.1g (0.2mol) (6R, S)-5-MTHF, stir, Heat the reaction to 80°C, cool to 60°C, and filter to remove slightly soluble 6R-acid acid. Add activated carbon to the filtrate for decolorization, filter, cool to 20°C, crystallization occurs, vacuum filter to obtain 42.8g of 6S-salt acid crystals, yield 36.9%, [α] 20 D : +22.1° (C=1, ethanol).
[0017] Suspend the 6S-salt acid salt obtained above in 200ml of water, add 5.9g (0.08mol) of calcium hydroxide powder in portions, and heat to 40°C while stirring until it is completely dissolved; add 95% ethanol while cooling, gradually A white or light yellow precipitate is produced, filtered and dried to obtain (6S)-5-MTHF·Ca·5H 2 O39.1g, primary crystallization yield 95.1%, [α] 20 D : +34.5° (C=1, H 2 O) Optical purity ≥ 98.5%.
Embodiment 2
[0019] In a 1000ml three-necked flask, add 28.0g (0.2mol) (-)-α-phenylethylamine and 150ml of water, then add 300ml of aqueous solution containing 107.1g (0.2mol) of (6R, S)-5-MTHF, Heated to 75°C, cooled to 55°C, filtered to remove undissolved 6R-acid acid salt, added activated carbon to the mother liquor, filtered, cooled to 18-20°C, crystallization occurred, vacuum filtered to obtain 47.4g of crystalline 6S-acid acid salt, Yield 40.8%, [α] 20 D : +22.5° (C=1, ethanol).
[0020] Suspend the 6S-acid salt obtained above in water, add 10% sodium hydroxide solution, make the pH value 8-9, make the temperature not exceed 30°C, then add 7.4g (0.1mol) calcium hydroxide powder in portions, Warm while stirring. The temperature should not exceed 40°C. It should be completely dissolved. Add 95% ethanol while stirring, and a white precipitate or light yellow precipitate will form. Filter and dry to obtain 46.4g (6S)-5-MTHF·Ca·5H 2 O, primary crystallization yield 96.2%, [α] 20 D : ...
Embodiment 3
[0022] In a 1000ml three-necked flask, add 28.0g (0.2mol) (+)-α-phenylethylamine and 150ml of water, then add 300ml of aqueous solution containing 107.1g (0.2mol) of (6R, S)-5-MTHF, The operation is the same as in Example 1 to obtain 44.496S-acid acid salt with a yield of 38.2%, [α] 20 D : +21.8° (C=1, ethanol).
[0023] Suspend the 6S-acid salt obtained above in 200ml of water, add it to the aqueous sodium carbonate solution, make the pH value of the solution 7.5-8.5, then add 0.1mol calcium hydroxide powder in portions, and warm while stirring, the temperature should not exceed 40°C, it should be completely dissolved, under stirring, add 95% ethanol, a white precipitate or light yellow precipitate is formed, filter and dry to get 45.2g (6S)-5-MTHF·Ca·5H 2 O, primary crystallization yield 96.1%, [α] 20 D : +34.5° (C=1, H 2 O) Optical purity ≥ 99.0%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com