New application of pharmaceutical composition of metformin and 5-methyltetrahydrofolate
A technology of methyltetrahydrofolate and metformin, applied in the field of medicine, can solve the problems of folic acid absorption disorder, neglect of early prevention and treatment of diabetic vascular disease, and reduction of folic acid level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~3
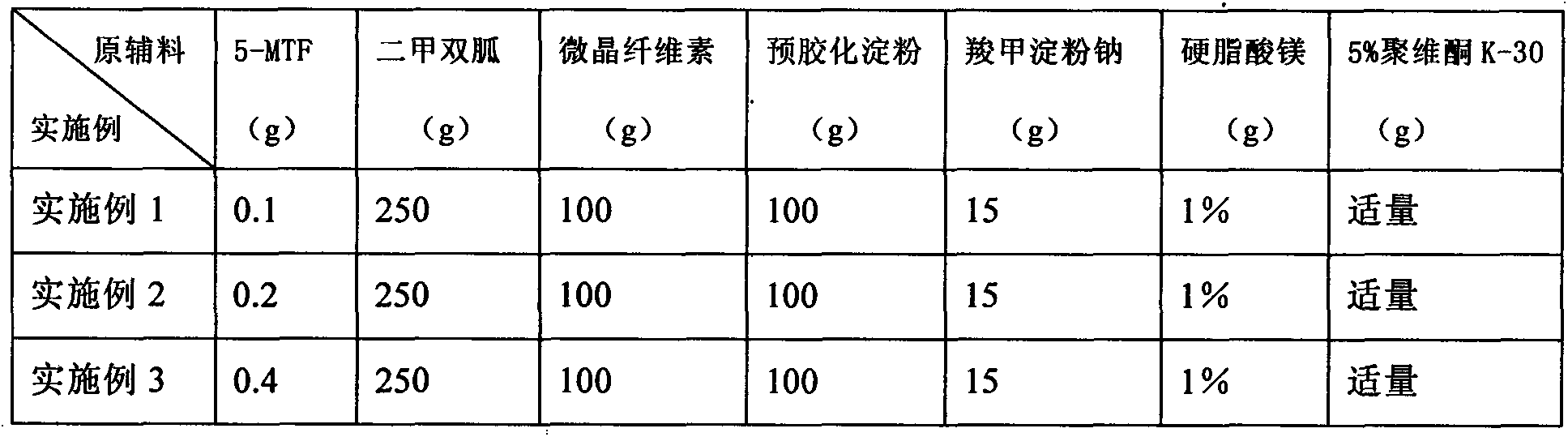
[0022] Table 1 Example 1~3 Tablet Prescription Composition
[0023]
[0024] Preparation Process:
[0025] (1) The raw and auxiliary materials are respectively passed through a 100-mesh sieve for subsequent use;
[0026] (2) According to Table 1, the raw and auxiliary materials of the prescription amount are weighed and mixed for subsequent use;
[0027] (3) Add an appropriate amount of binder to make soft materials, granulate with a 24-mesh sieve, and dry at 40-45°C;
[0028] (4) 20 mesh sieve granulation;
[0029] (5) Add an appropriate amount of magnesium stearate to the dry granules, mix them evenly, and compress them into tablets after content determination.
Embodiment 4
[0030] Example 4 Preparation of Compound Metformin 5-MTF Capsules (1000 Capsules)
[0031] formula:
[0032]
[0033]
[0034] Preparation Process:
[0035](1) Pass the raw and auxiliary materials through a 100-mesh sieve for later use; (2) Mix the raw and auxiliary materials according to the above prescription amount and mix them for later use; (3) Add an appropriate amount of binder to make soft materials, granulate with a 24-mesh sieve, Dry at 45°C; (4) sieve through a 20-mesh sieve; (5) add an appropriate amount of magnesium stearate to the dry granules and mix well, and fill the capsules after content determination. Each capsule contains 250mg metformin and 0.2mg 5-MTF.
Embodiment 5
[0036] Example 5 Preparation of compound metformin 5-MTF enteric-coated tablets (quantity of 1000 tablets)
[0037] formula:
[0038]
[0039] Preparation Process:
[0040] (1) Pass the raw and auxiliary materials through a 100-mesh sieve for later use; (2) Weigh metformin, povidone K30, Tween-80 and sodium hydroxide in absolute ethanol as the adhesive solution according to the above; ( 3) Weigh microcrystalline cellulose (model pH101) and croscarmellose sodium according to the above, mix them evenly, add binder, and dry at 40-45°C; (4) granulate with 24-mesh sieve; (5) Dry granules Add an appropriate amount of magnesium stearate and talcum powder and mix evenly. After the content is determined, the tablet is pressed, and then the isolation layer coating and the enteric coating are performed sequentially. Each tablet contains 250mg of metformin and 0.2mg of 5-MTF.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com