Preparation method for 5-methyltetrahydrofolate basic amino acid salt compound, 5-methyltetrahydrofolate basic amino acid salt compound and application thereof
An amino acid salt compound, methyltetrahydrofolate technology, applied in the preparation of organic compounds, cyanide reaction preparation, chemical instruments and methods, etc., can solve the problems of low solubility, inconvenient use, inconvenient, etc., and achieve bioavailability High precision, easy to use, good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add 9.2g (20mmol) L-5-methyltetrahydrofolate in batches to 6.96g (40mmol) L-arginine solution dissolved in 100mL of water, stir at room temperature for half an hour, at this time the pH is about 6.3, The solution was concentrated to dryness in vacuo to obtain 15.8 g of L-5-methyltetrahydrofolate L-arginine salt. Among them, L-5-methyltetrahydrofolate can be synthesized by using commercially available products or referring to literature (Author Zhang Yue et al., "Fine and Specialty Chemicals", Issue 22, 2005).
[0031] HPLC detection, detection conditions:
[0032] Chromatographic column: Hypersil-ODS, 5μm; 250x4mm
[0033] Mobile phase: methanol / water (2:1, pH=8.0)
[0034] Flow rate: 1.1ml / min
[0035] Detector: UV (280nm)
[0036] Column temperature: room temperature
[0037] The content of L-5-methyltetrahydrofolate in the L-arginine salt of L-5-methyltetrahydrofolate accounts for 56.0%, and the theoretical calculation value of calcium L-5-methyltetrahydrofolate...
Embodiment 2
[0039] 9.2 grams (20 mmol) of (D, L)-5-methyltetrahydrofolate were added in batches to a solution of 5.84 grams (40 mmol) of L-lysine dissolved in 100 mL of water, and stirred at room temperature for half an hour. At this time, the pH was 6.4, and the obtained solution was spray-dried to obtain 14.4 g of (D,L)-5-methyltetrahydrofolate L-lysine salt. HPLC detection condition is the same as embodiment 1. Wherein (D, L)-5-methyltetrahydrofolate is the racemate of 5-methyltetrahydrofolate, reference can be made to the literature (Author Zhang Yue et al., "Fine and Specialty Chemicals", Issue 22, 2005) etc. The method in is synthesized, and commercially available products can also be used.
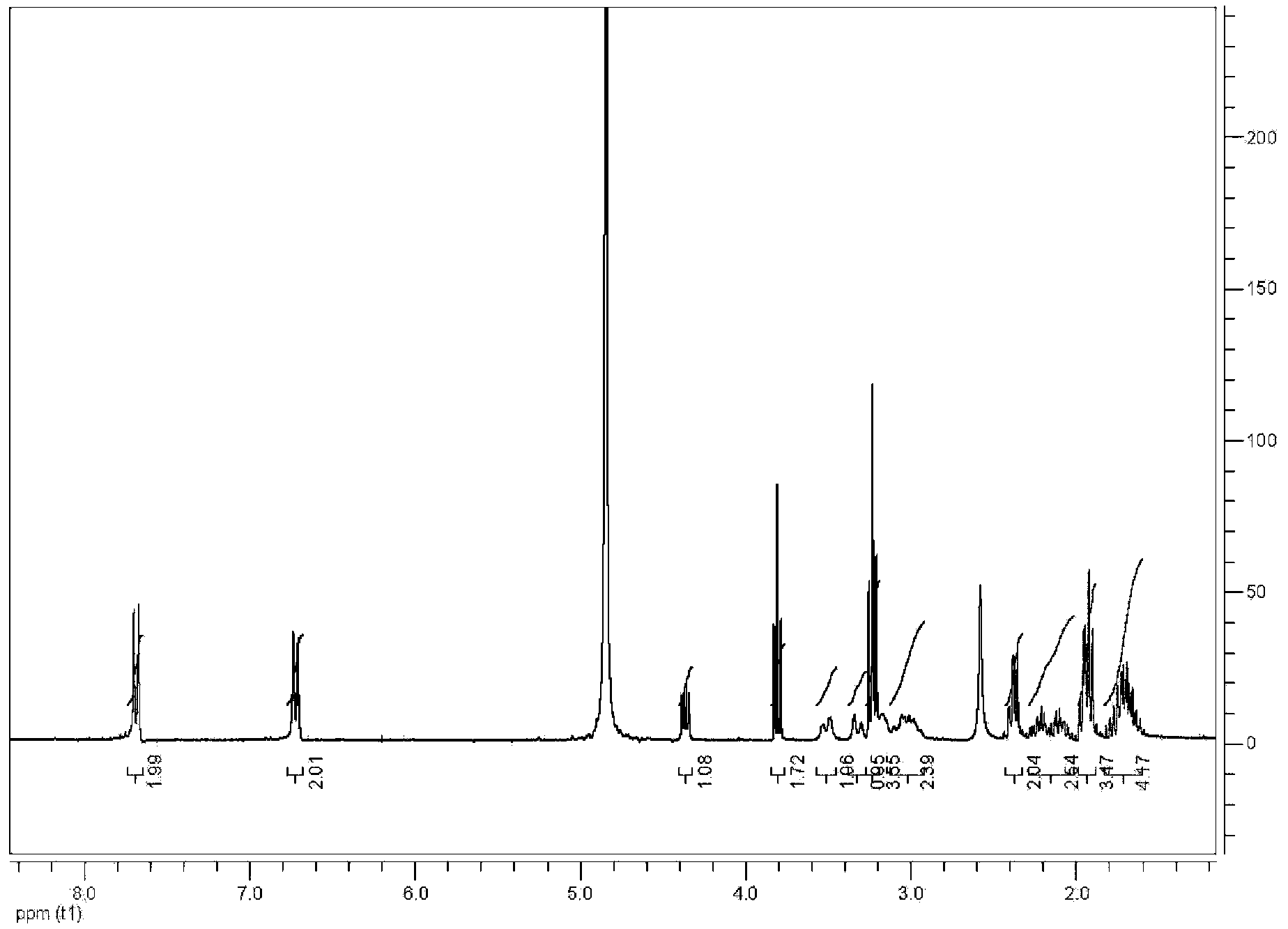
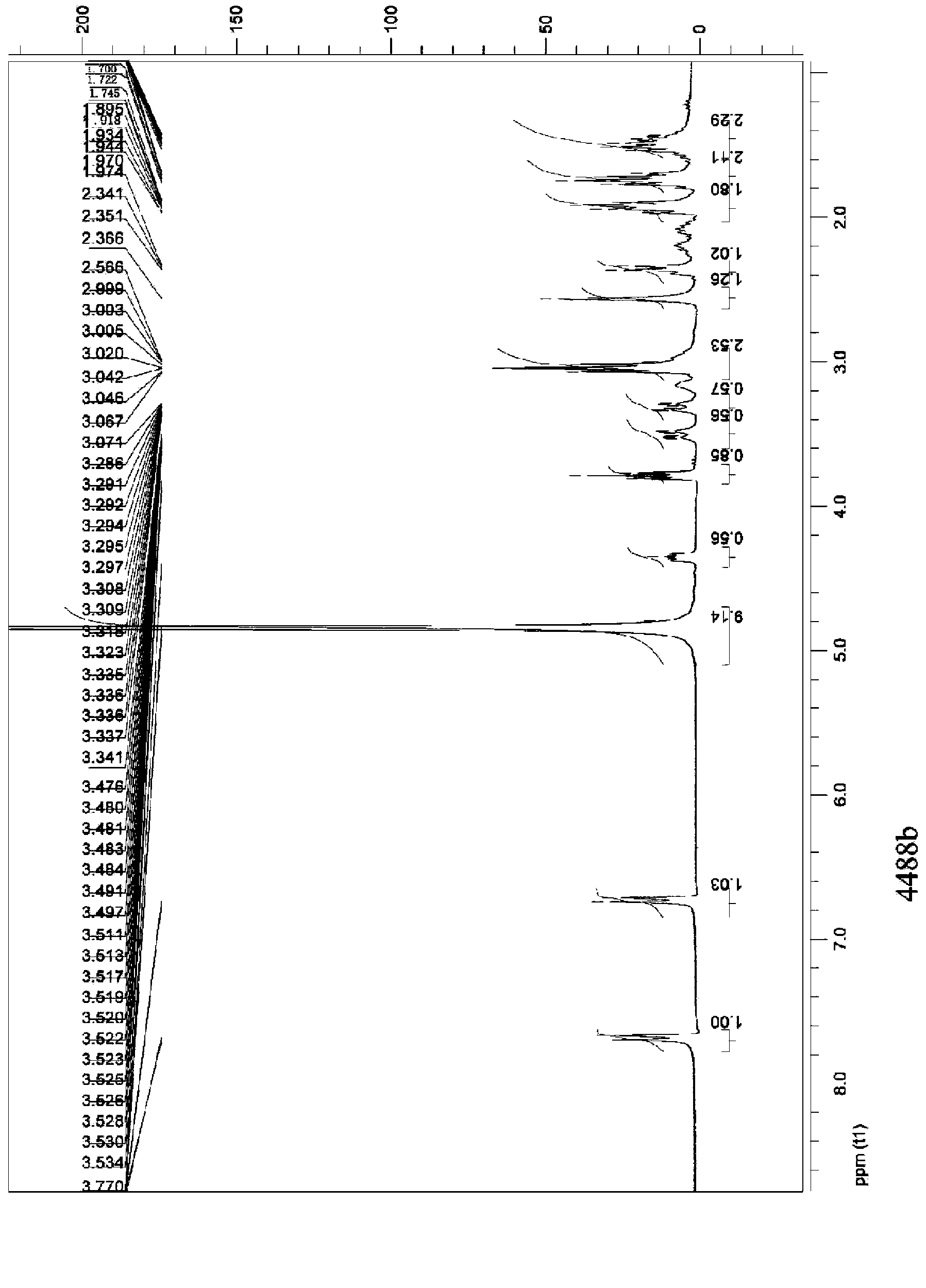
[0040] The content of (D,L)-5-methyltetrahydrofolate in (D,L)-5-methyltetrahydrofolate L-lysine salt accounts for 59.1%, and the theoretical calculation value is 60.0%. Its HPLC spectrum is shown in image 3 , the compound's 1 See HNMR Figure 4 .
Embodiment 3
[0042]Add 9.2 g (20 mmol) (D, L)-5-methyltetrahydrofolate in batches to a solution of 6.2 g (40 mmol) L-histidine dissolved in 100 mL of water, and stir at room temperature for half an hour. At this time, the pH was 6.3, and the resulting solution was spray-dried to obtain 15.1 g of (D, L)-5-methyltetrahydrofolate L-histidine salt. The HPLC detection conditions were the same as in Example 1. Wherein (D, L)-5-methyltetrahydrofolate is the racemate of 5-methyltetrahydrofolate, reference can be made to the literature (Author Zhang Yue et al., "Fine and Specialty Chemicals", Issue 22, 2005) etc. The method in is synthesized, and commercially available products can also be used.
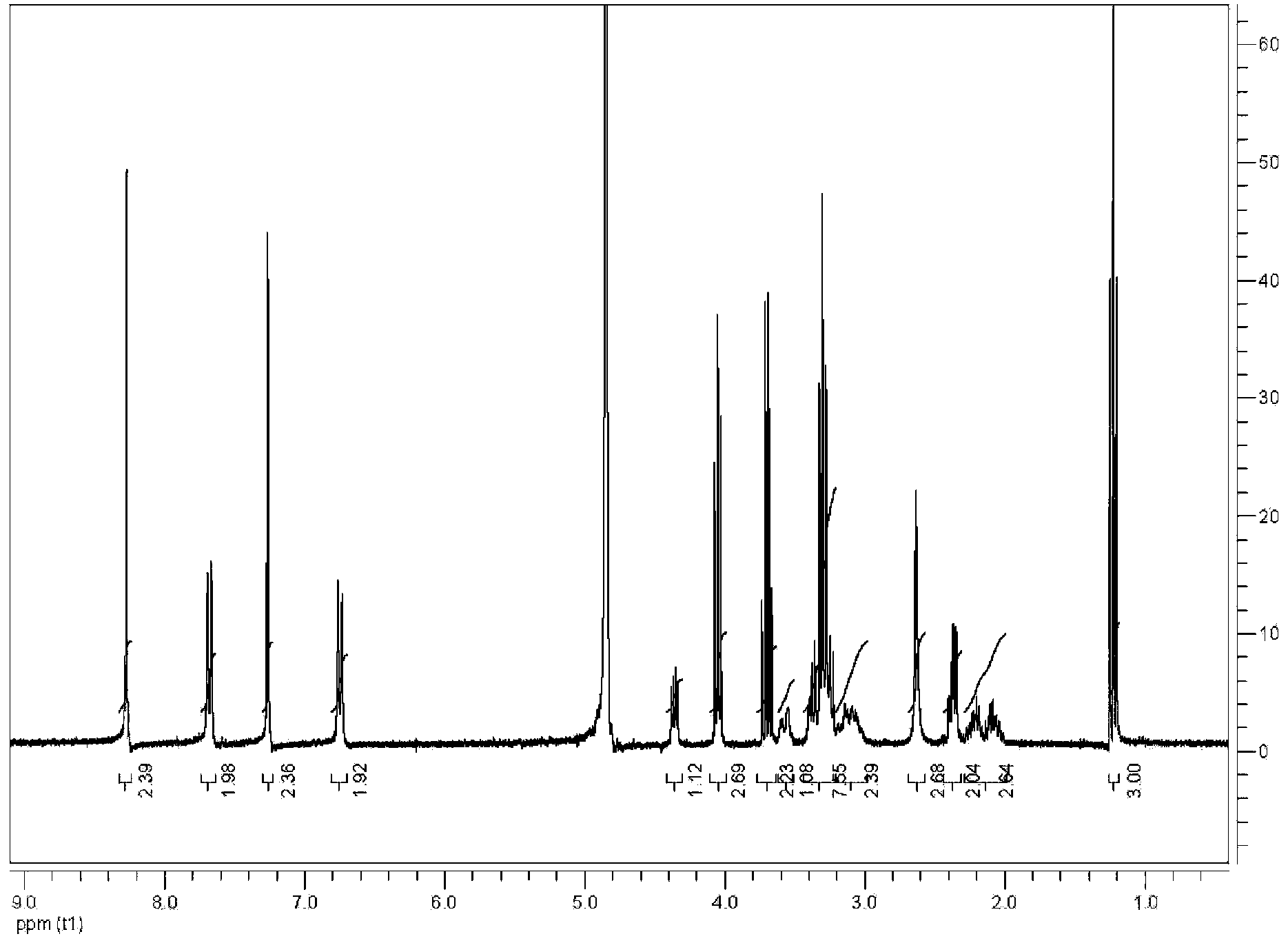
[0043] The content of L-5-methyltetrahydrofolate in (D, L)-5-methyltetrahydrofolate L-histidine salt accounts for 58.8%, and the theoretical calculation value is 59.7%. Its HPLC spectrogram is shown in Figure 5 ,. The compound's 1 See HNMR Figure 6 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com