Chiral zinc complex and copper complexes of alpha-phenylethylamine
A technology of zinc phenylethylamine acetate and chirality, which is applied to the preparation of organic compounds, organic compound/hydride/coordination complex catalysts, compounds of Group 4/14 elements of the periodic table, etc., and can solve complex operations, low efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
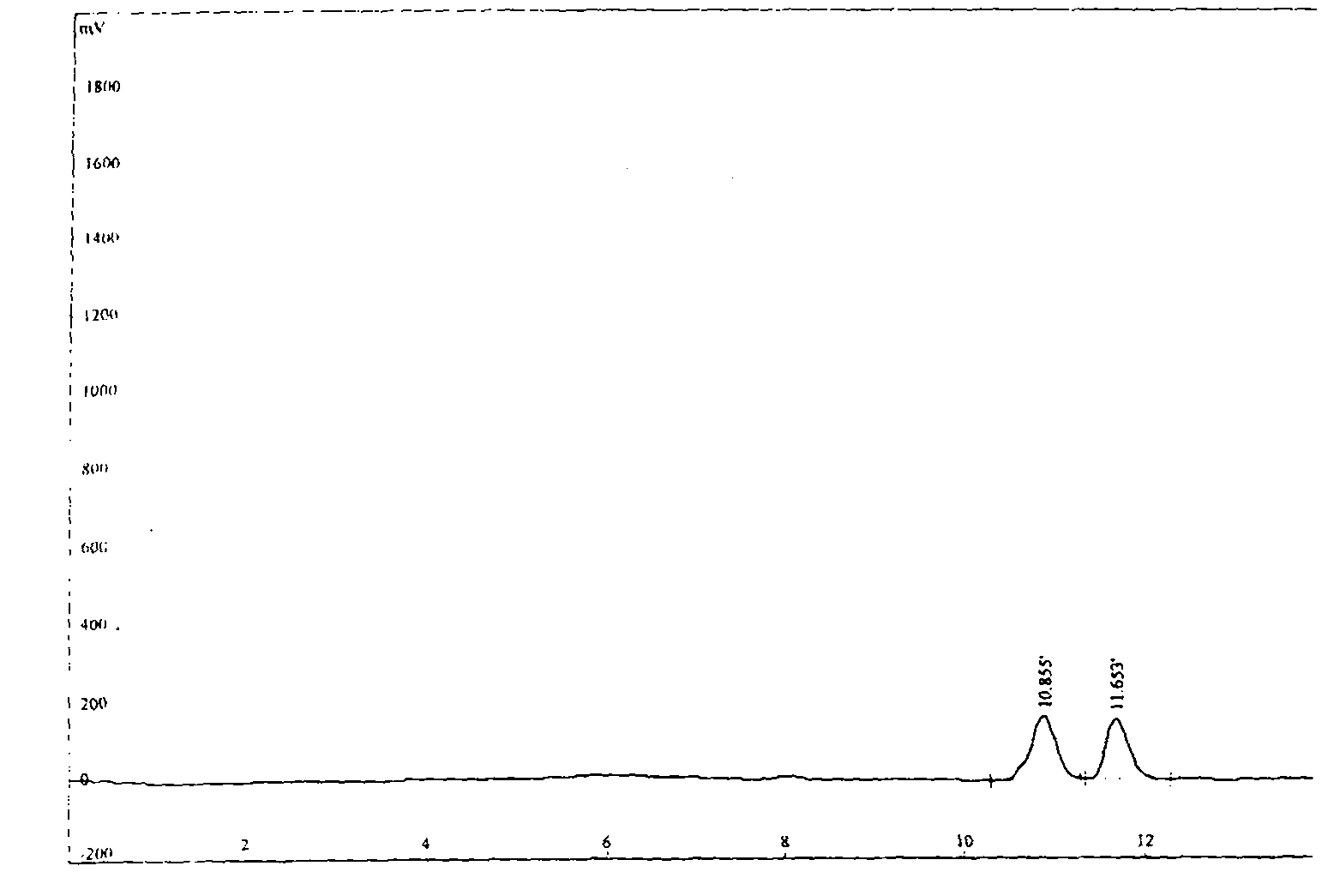
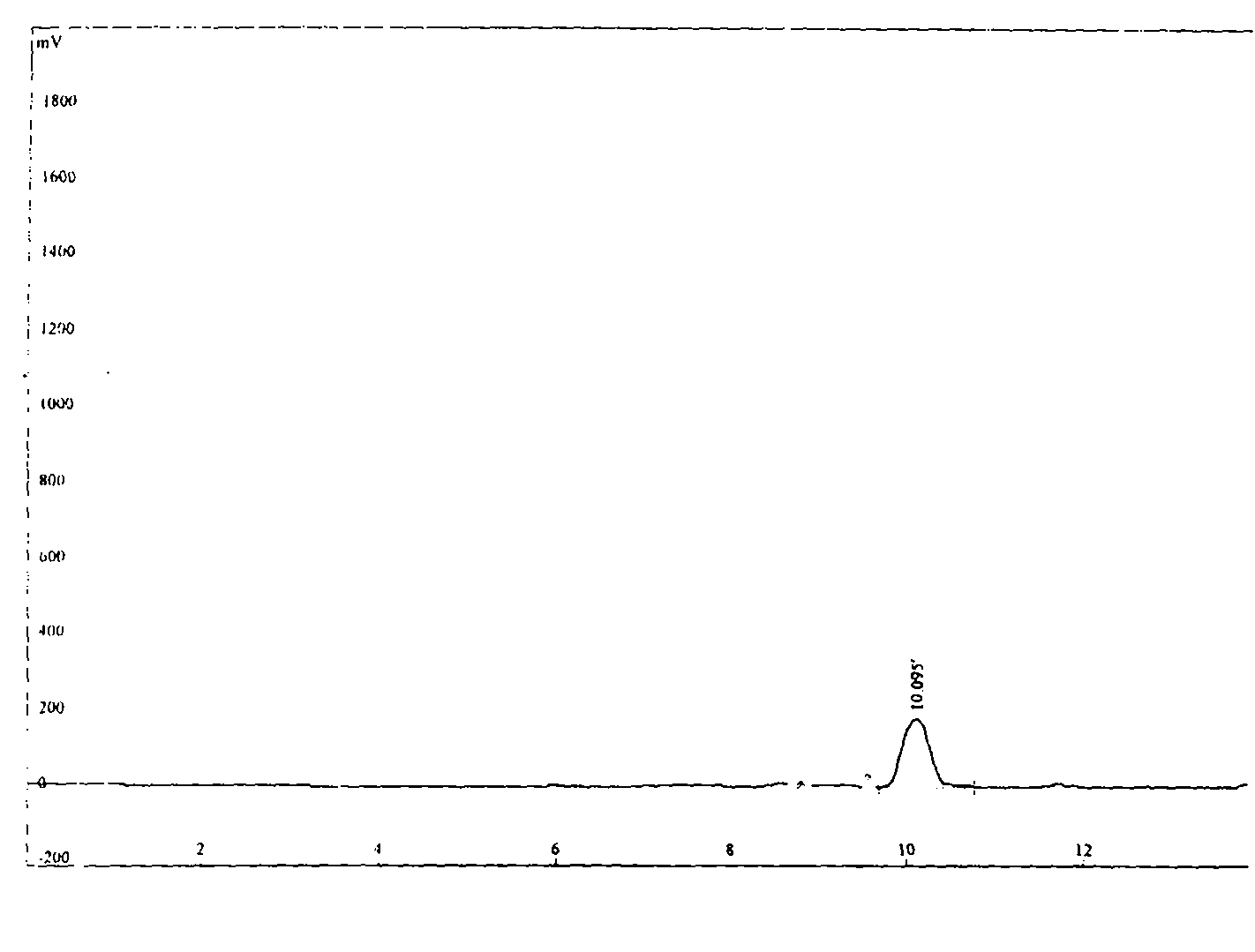
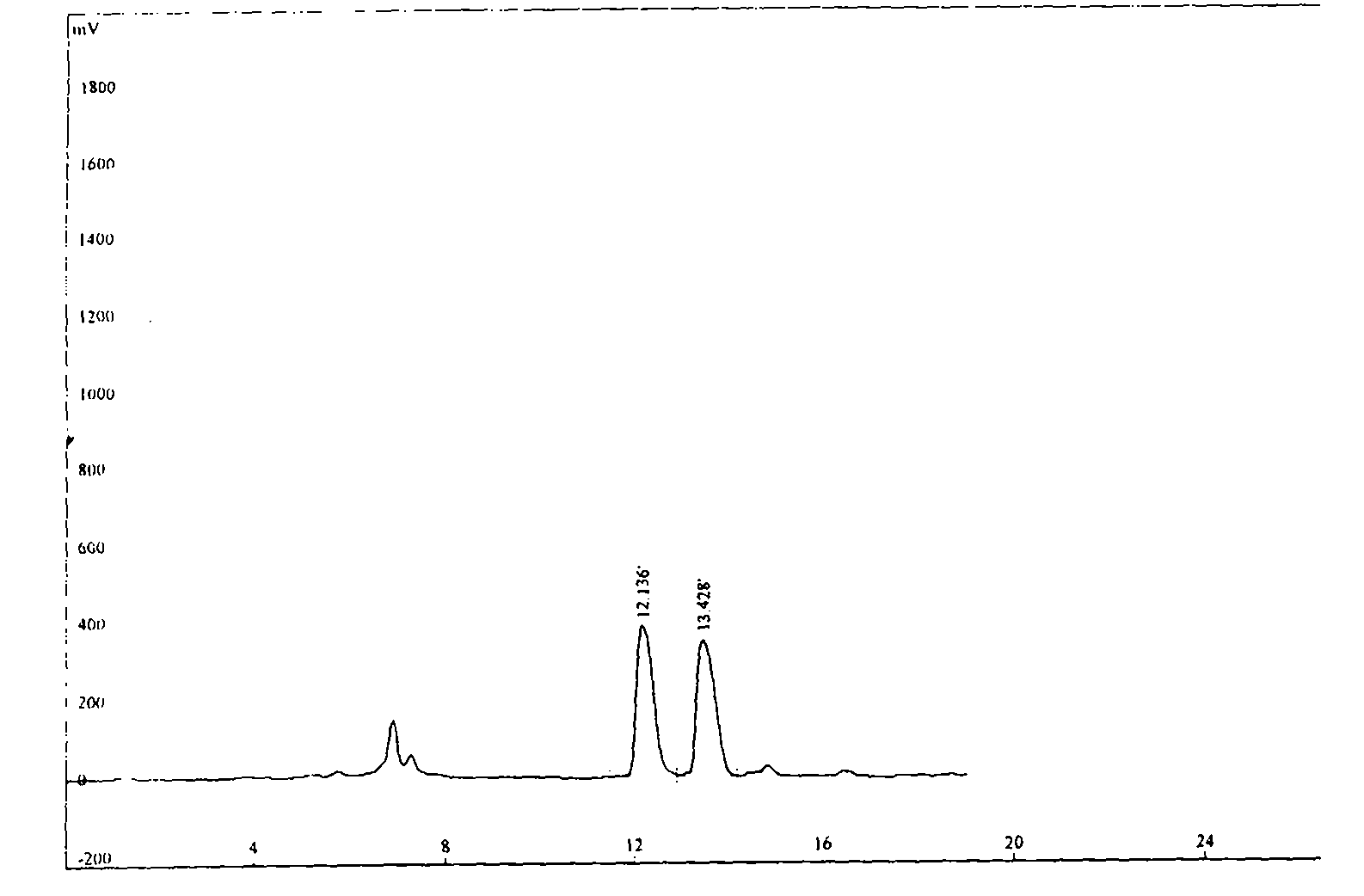
Image
Examples
Embodiment Construction
[0075] (1) Examples of preparation of chiral complexes
[0076] 1, 1a: Synthesis of chiral (S)-α-phenylethylamine zinc acetate metal-organic complexes
[0077] Weigh 2.191g Zn(CH 3 COO) 2 2H 2 Add O (0.01mol) into a 100mL round-bottomed flask, add 25mL tetrahydrofuran as a solvent, add a stirrer, measure 2.7mL α-phenylethylamine (0.02mol) with a syringe under stirring, add it to the flask, Put on the condensing tube, connect to tap water, place on a magnetic heating stirrer and heat to reflux for 24 hours, remove the solvent with a rotary evaporator, and recrystallize with petroleum ether to obtain colorless crystals with a yield of 90%.
[0078] 1 HNMR (500MHz, CDCl 3 )7.22~7.31(m, 10H), 4.06~4.07(d, J=6.5Hz, 1H), 3.06(br, 1H), 1.94(s, 6H), 1.40~1.42(d, J=6.5Hz, 6H ). 13 C NMR (75MHz, CDCl 3 )179.70, 144.04, 128.94, 127.92, 125.92, 51.82, 23.94, 23.10. IR (KBr) cm -1 : 3130, 1620, 1390, 1170, 702.
[0079] 2. 1b: Synthesis of chiral (S)-α-phenylethylamine acetate co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com