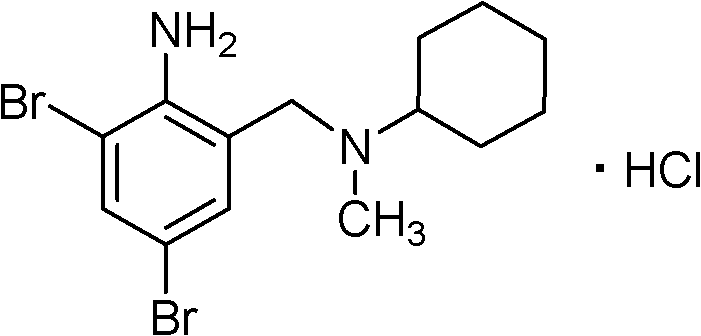
Method for preparing bromhexine hydrochloride
A technology of bromhexine hydrochloride and salt-forming reaction, which is applied in the field of preparation of bromhexine hydrochloride, can solve problems such as inability to realize large-scale production, high requirements for production equipment conditions, and difficulty in achieving reaction temperature, etc., and achieve safe, reliable and comparatively safe production process. The effect of less pollution and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
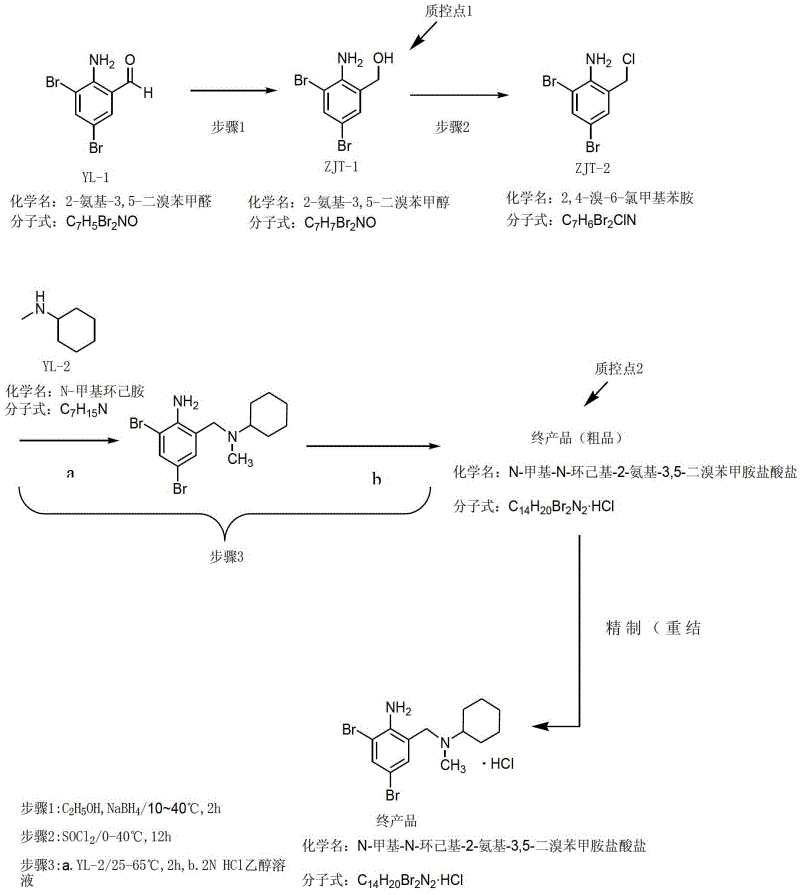
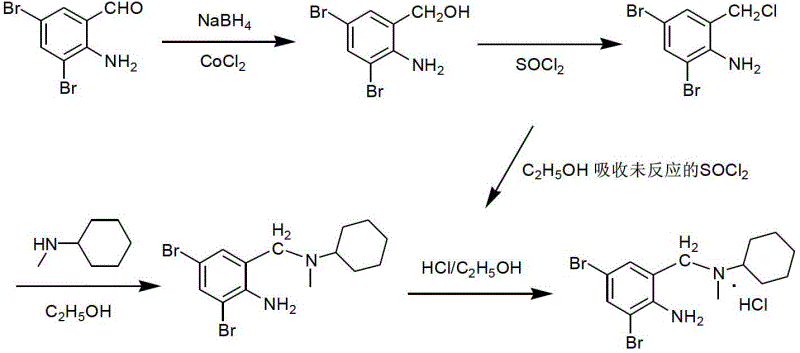
[0056] (1) Synthesis of 2-amino-3,5-dibromobenzyl alcohol
[0057]
[0058] The reaction type is a reduction reaction. The starting material 2-amino-3,5-dibromobenzaldehyde (YL-1) reacts with the reducing agent sodium borohydride at room temperature, and the product is 2-amino-3,5-dibromo Benzyl alcohol (ZJT-1).
[0059] In the study, the reaction solvent was first screened. According to experience, it is easier to reduce the carbonyl group with sodium borohydride. Commonly used solvents include: water, lower alcohols (methanol, ethanol, isopropanol), ethers (tetrahydrofuran, ether, methyl alcohol, etc.) base tert-butyl ether). We have carried out experimental screening with above-mentioned solvent respectively, the result finds that this step reaction is carried out comparatively slowly in ether solvent, and thin-layer chromatography (TLC) detection shows in the test: in tetrahydrofuran, diethyl ether, methyl tert-butyl ether, initial The raw material YL-1 still has a lo...
Embodiment 2
[0092] (1) Synthesis of 2-amino-3,5-dibromobenzyl alcohol
[0093] Suspend 11.2g (0.04mol) of 2-amino-3,5-dibromobenzaldehyde in 20ml of ethanol, below 25 degrees (cooled by ice-water bath), add 0.95g (0.025mol) of sodium borohydride solid in batches within 15min After the addition, stir at room temperature for 1.5 h, dilute with 50 ml of distilled water, adjust the pH value to 6 with 6% hydrochloric acid at room temperature, and stir vigorously for 0.5 h. Filter, wash with distilled water (20ml×3), suck dry, and dry (70°C for 2h) to obtain off-white solid 2-amino-3,5-dibromobenzyl alcohol.
[0094] (2) Synthesis of 2,4-dibromo-6-chloromethylaniline
[0095] Under cooling in an ice-water bath (5-25 degrees), add 30ml of SOCl within 10 minutes 2 10 g (0.0357 mol) of 2-amino-3,5-dibromobenzyl alcohol was added in batches, and after the addition was complete, it was stirred overnight at room temperature. Evaporate excess SOCl under reduced pressure below 30 degrees 2 , a larg...
Embodiment 3
[0120] Suspend 25g of 2-amino-3,5-dibromobenzaldehyde (YL-1) in 40ml of ethanol, add 1.5g of sodium borohydride solid in batches within 15min at 15-40°C (under ice-water bath cooling), After the addition, stir at room temperature for 1.5 h, add 120 ml of purified water to dilute, adjust the pH value to 6.0 with 36% hydrochloric acid at room temperature, and stir vigorously for 0.5 h. Filter, wash with purified water (20ml×3), suck dry, and dry at 80°C for 2h to obtain off-white solid 2-amino-3,5-dibromobenzyl alcohol (ZJT-1). The operation was repeated three times, and the reaction yields were 98.6%, 98.0%, and 98.0%, respectively. After testing, the melting points were 152.7-153.3°C, 152.8-153.2°C, and 152.7-153.2°C, respectively.
[0121] (2) Synthesis of 2,4-dibromo-6-chloromethylaniline
[0122] At 10~35°C (cooled by ice-water bath), add 75ml SOCl within 10min 2 Add 24 g of 2-amino-3,5-dibromobenzyl alcohol in batches, and stir at room temperature for 1 h after the addi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com