Patents
Literature
78 results about "Oxidative cyclization" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
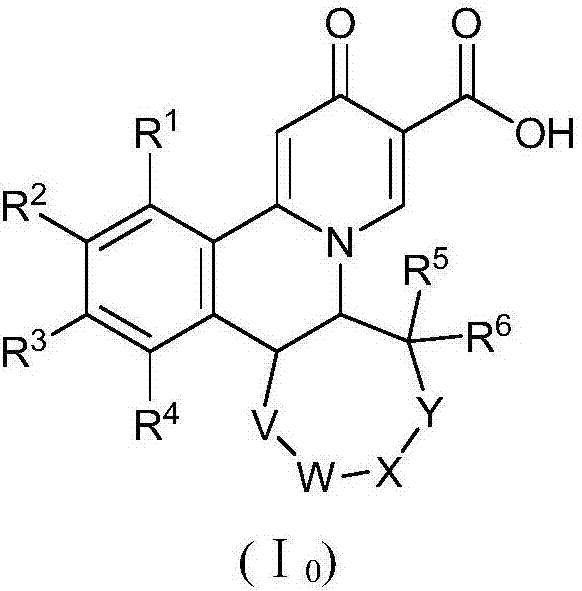
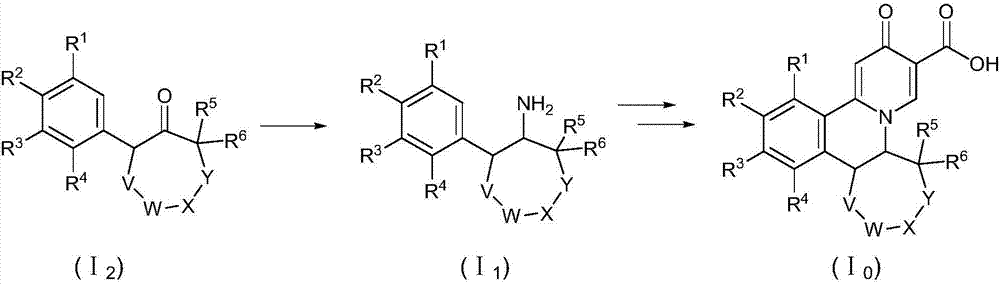
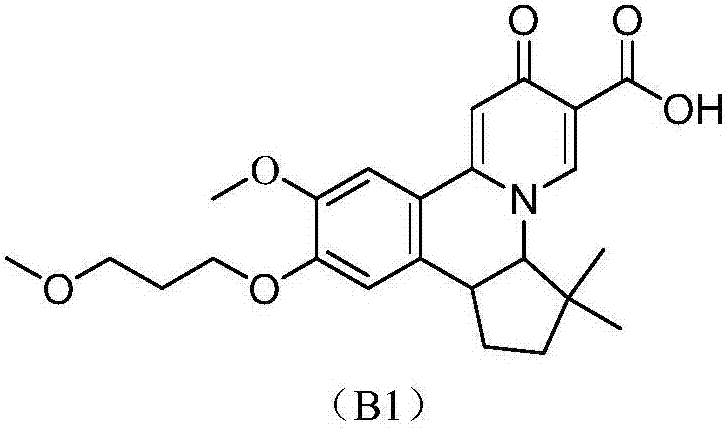
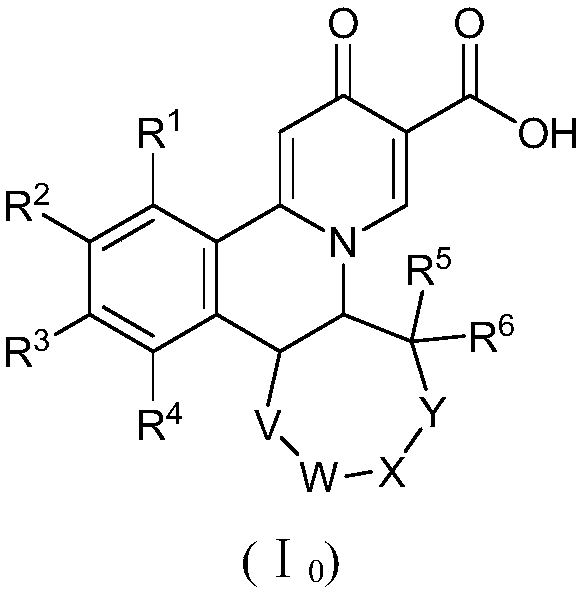
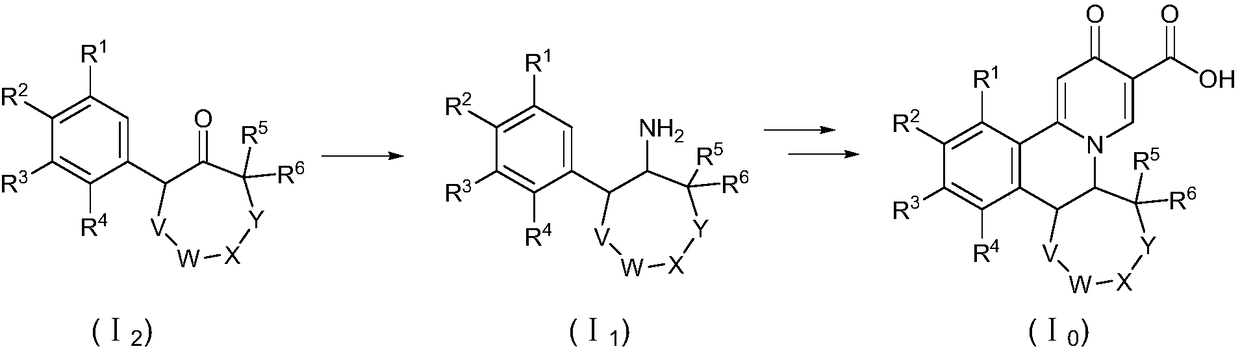
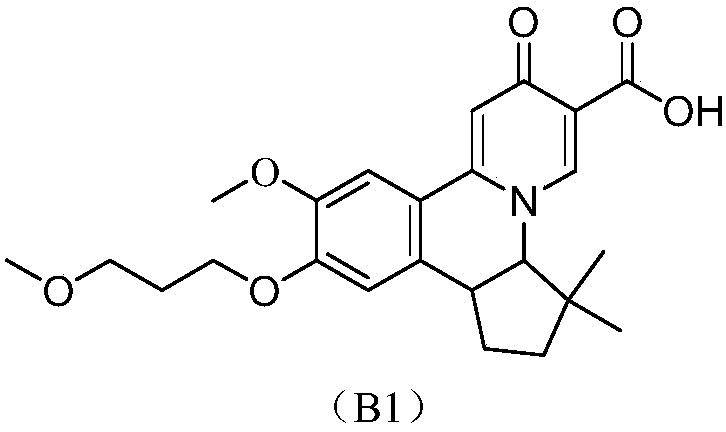
Preparation method of quinolizinones compound
ActiveCN106928215AHigh yieldGood effectOrganic chemistry methodsAntiviralsOxidative cyclizationSolvent
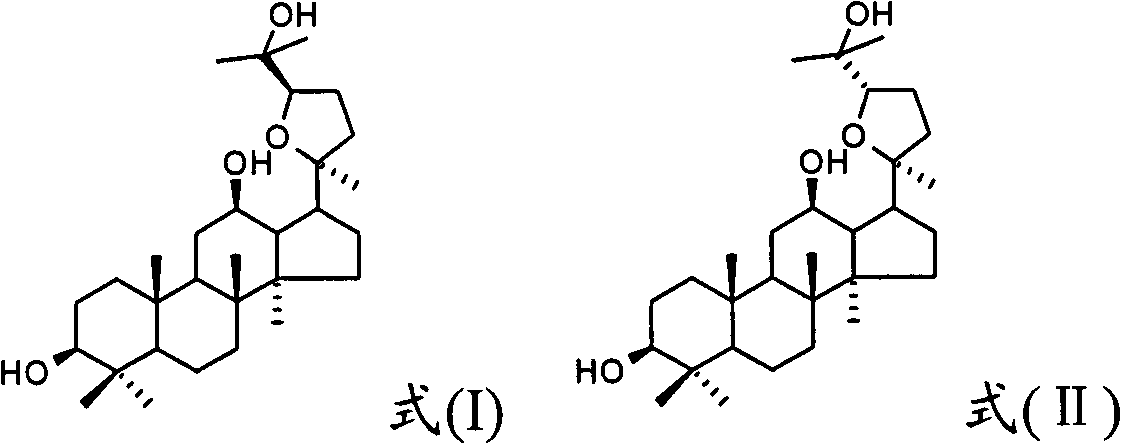
The invention discloses a method for preparing a quinolizinones compound in a formula I, which comprises: S (A), oxidizing a compound in a formula III in a solvent under the action of an oxidizing agent and under the electro-oxidation and reduction condition or under the photo-oxidation and reduction condition, and then processing the compound by acid to obtain a ringed syn-oxime compound in a formula II; and S (B), carrying out hydrolysis on the compound in the formula II to obtain the compound in the formula I. The invention further discloses another method for preparing the quinolizinones compound in the formula I on the basis of the same conception. The two methods have key points that before oxidative cyclization, a pyrazine ring is closed in a raw material compound, and due to the influence of a steric hindrance, when oxidative cyclization is carried out to form a five-membered ring, ring closing can only be carried out selectively at the same side of the pyrazine ring so as to obtain a syn-oxime structure, i.e., a syn-oxime isomer can be highly selectively generated.
Owner:河南春风医药科技有限公司
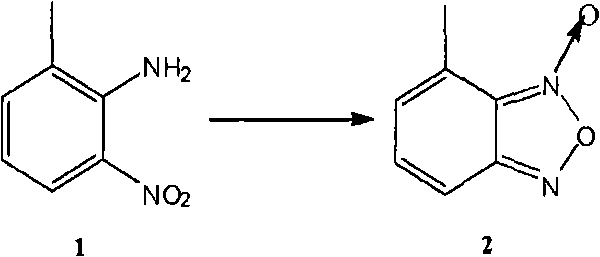
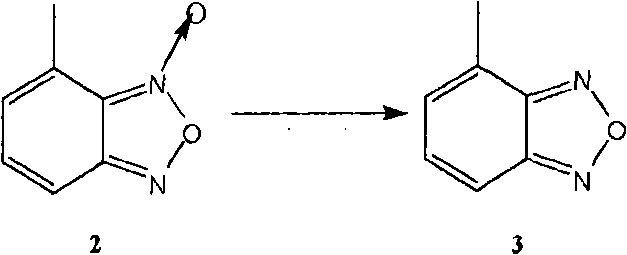
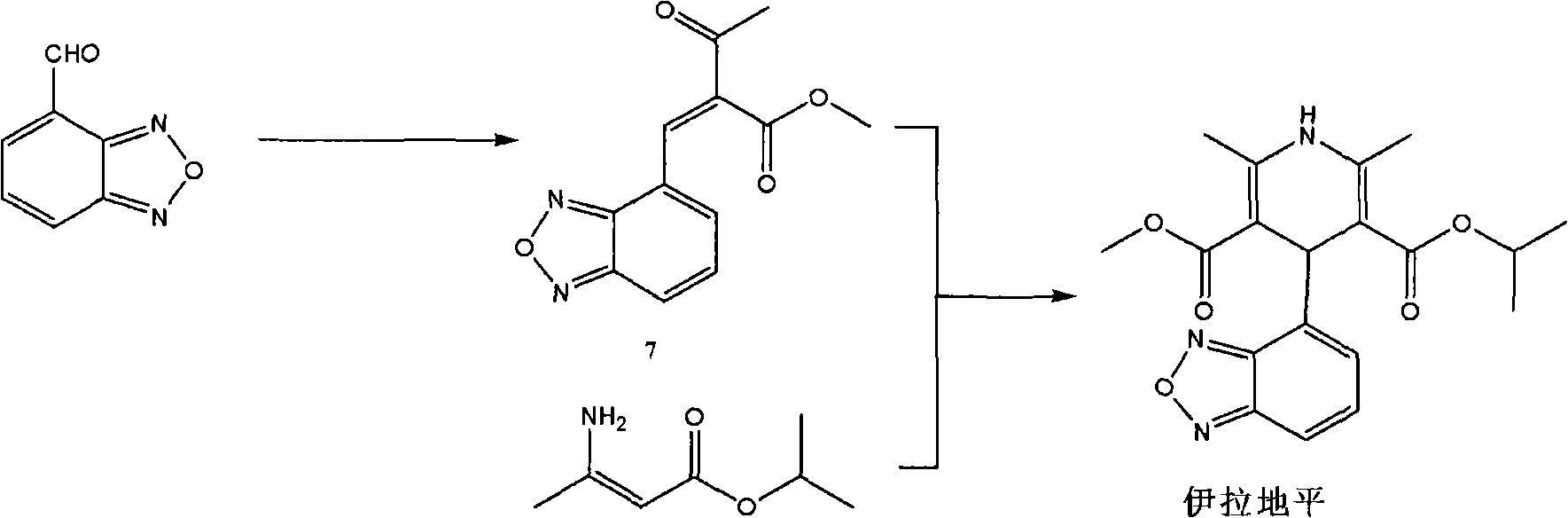
Method for preparing israbipine medicament for treating hypertension
InactiveCN101768153AProcess stabilityImprove product qualityOrganic active ingredientsOrganic chemistryMedicineNitrobenzene
The invention relates to a method for preparing an israbipine medicament for treating hypertension. Particularly the method of the invention comprises the following steps: (a) carrying out oxidative cyclization on 2-amino-3-methyl nitrobenzene to form 4-methyl benzofuroxan oxide; (b) reducing the 4-methyl benzofuroxan oxide to form 4-methyl benzofuroxan; (c) carrying out substitution bromination on the 4-methyl benzofuroxan to form 4-bromomethyl benzofuroxan; (d) carrying out hydrolyzation on the 4-bromomethyl benzofuroxan to form 4-hydroxymethyl benzofuroxan; (e) carrying out oxidation on the 4-hydroxymethyl benzofuroxan to form 4-formaldehyde benzofuroxan; and (f) performing a reaction of the 4-formaldehyde benzofuroxan amd alpha-aminocrotonic acid isopropyl ester to form israbipine. The method of the invention has high yield and simple and convenient operation and can prepare high-purity israbipine.
Owner:SHANGHAI SUN SAIL PHARMA SCI & TECH CO LTD +1
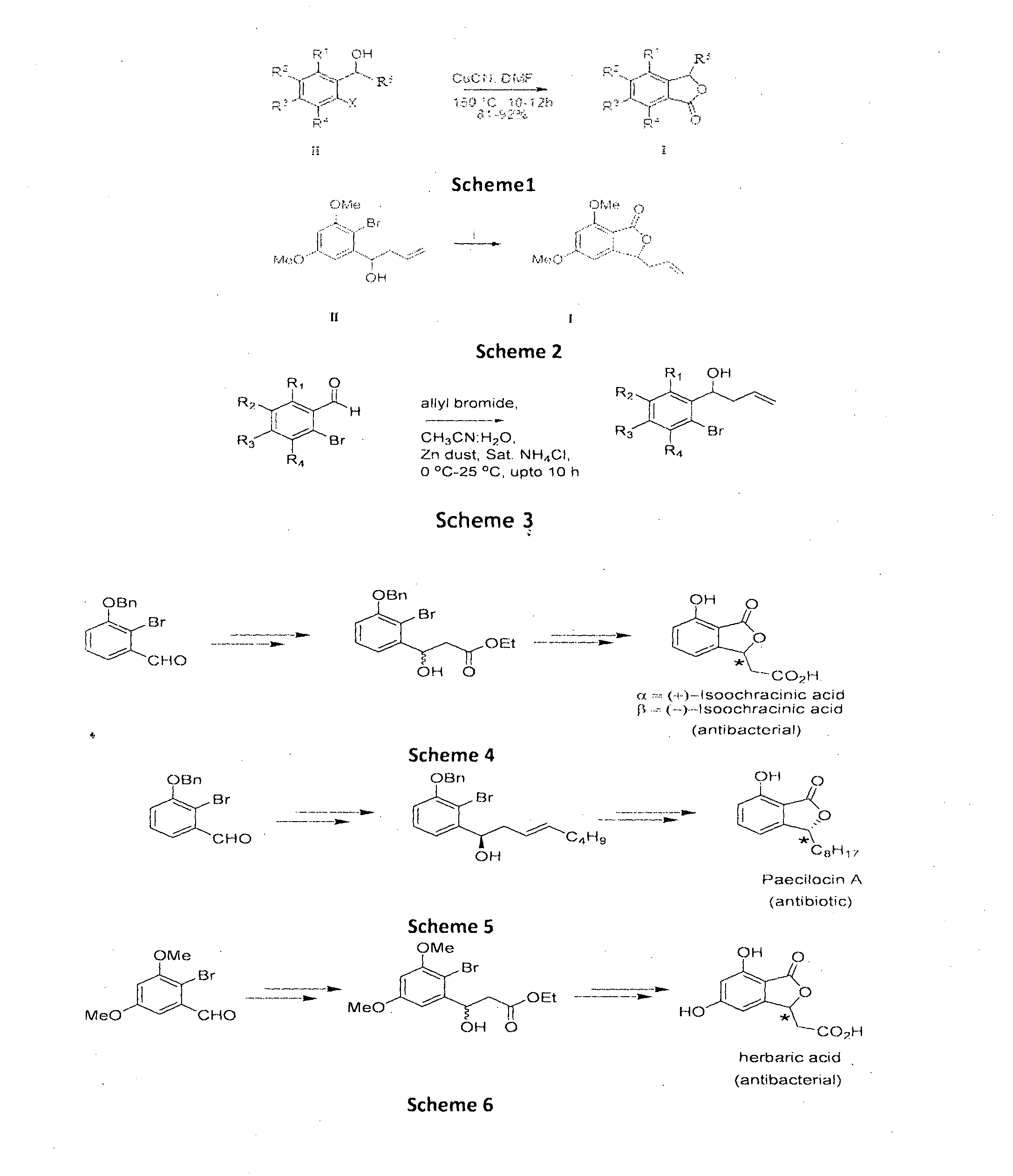
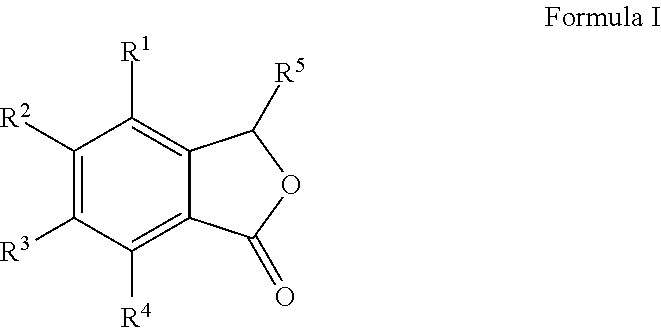
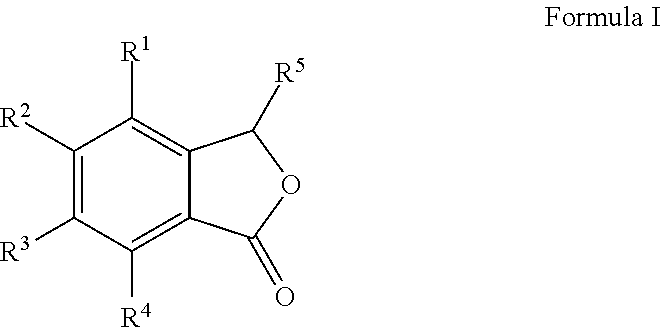
Cu-MEDIATED ANNULATION FOR THE EFFECTIVE SYNTHESIS OF 3-SUBSTITUTED PHTHALIDES
The present invention disclosed herein is a novel commercially feasible, one pot synthesis of library of 3-substituted phthalides of formula I via CuCN mediated oxidative cyclization in high yield. Formula I
Owner:COUNCIL OF SCI & IND RES
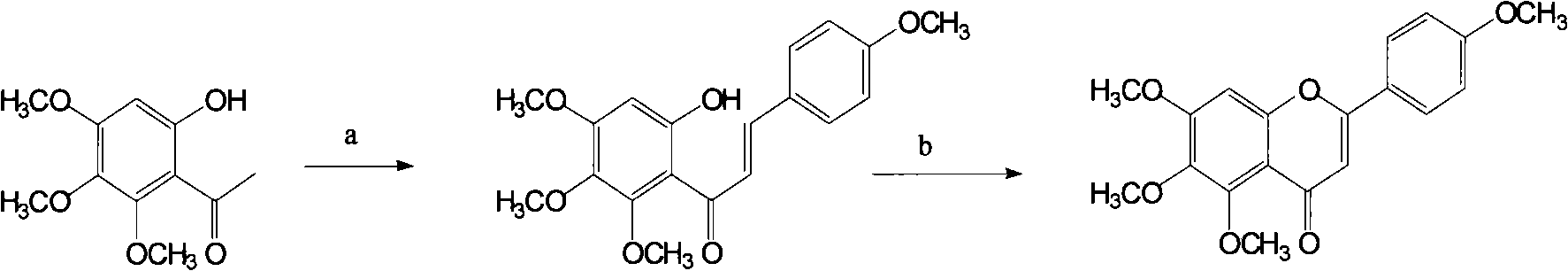
Preparation method of 5,6,7,4'-tetramethoxy flavones of scutellarin and aglucone key intermediate thereof
The invention relates to a preparation method of 5,6,7,4'-tetramethoxy flavone of scutellarin and aglucone key intermediate thereof. The 5,6,7,4'-tetramethoxy flavones is prepared by utilizing 2-hydroxyl-4,5,6-trimethoxy hypnone as the raw material and comprising a reaction a step and a reaction b step, wherein in the reaction a, 2-hydroxyl-4,5,6-trimethoxy hypnone and p-methoxybenzaldehyde are condensed to prepare a chalcone derivative; and in the reaction b, the chalcone derivative is processed in a oxidative cyclization mode to prepare the 5,6,7,4'-tetramethoxy flavone. The method has the advantages of simple operation steps and high product yield.
Owner:KPC PHARM INC +1
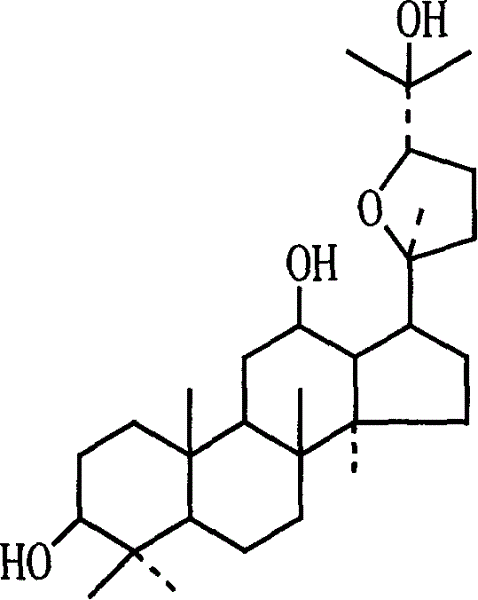
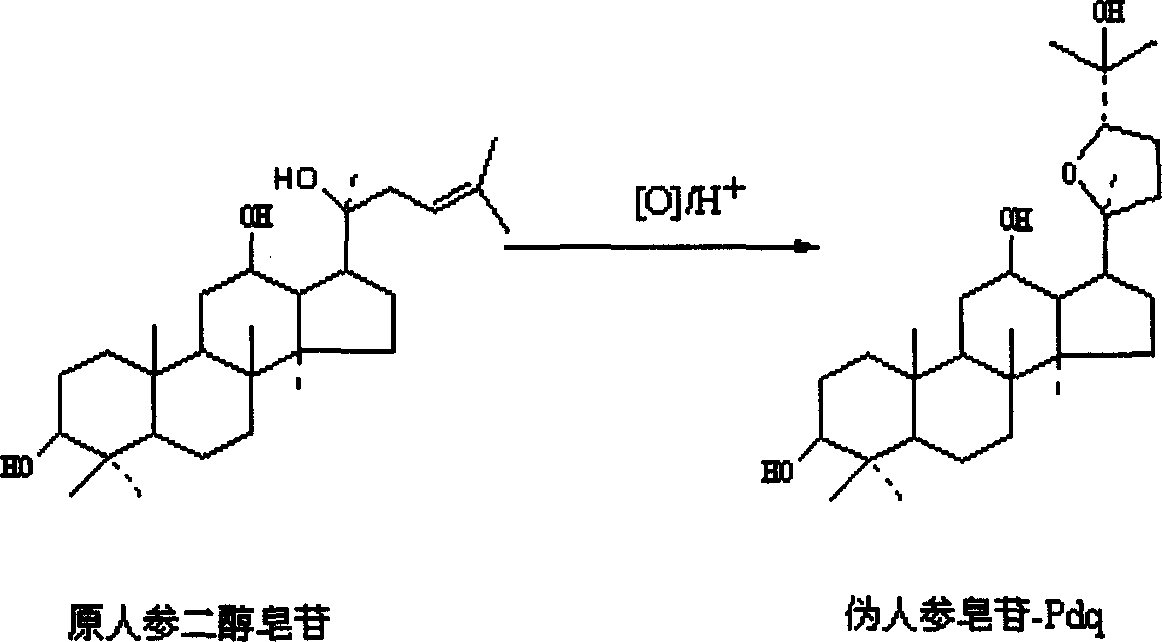
Pseudoginsenoside Pdq and its semi-synthesis process and medicine use
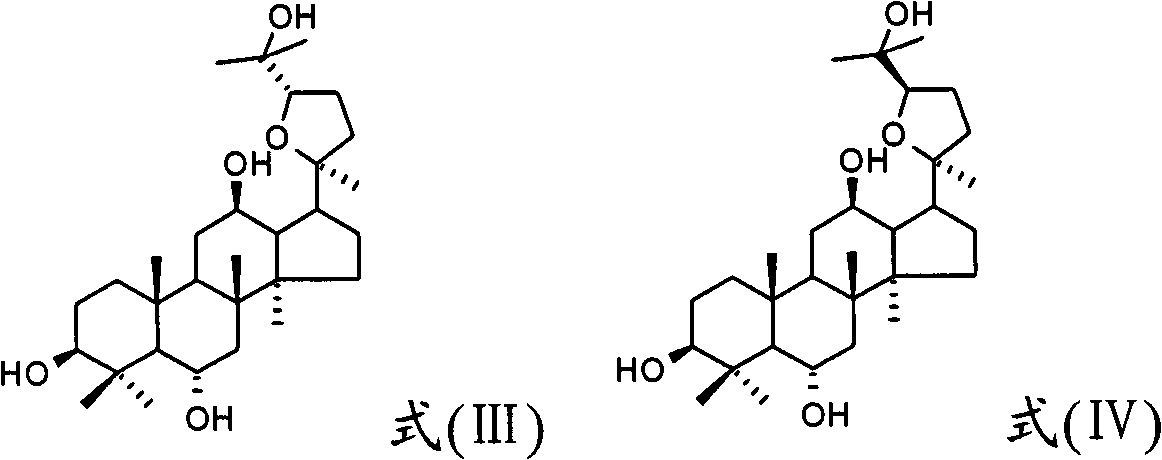
The present invention relates to pseudoginseenoside-Pdq and its semi-synthesis process and medicinal use, and belongs to the field of new compound and its synthesis and medicinal use. The material 20(S)-protopanoxadiol saponin or 20(R)-protopanoxadiol saponin is processed through oxidation, cyclization and other steps under acid condition to produce pseudoginseenoside-Pdq as one new compound, named chemically as damar-20S, 20R-epoxy-3beta, 12beta, 25-triol. Pseudoginseenoside-Pdq has wide application in preparing medicine for treating coronary heart disease, myocardial ischemia, ischemic shock, arrhythmia, etc.
Owner:李平亚
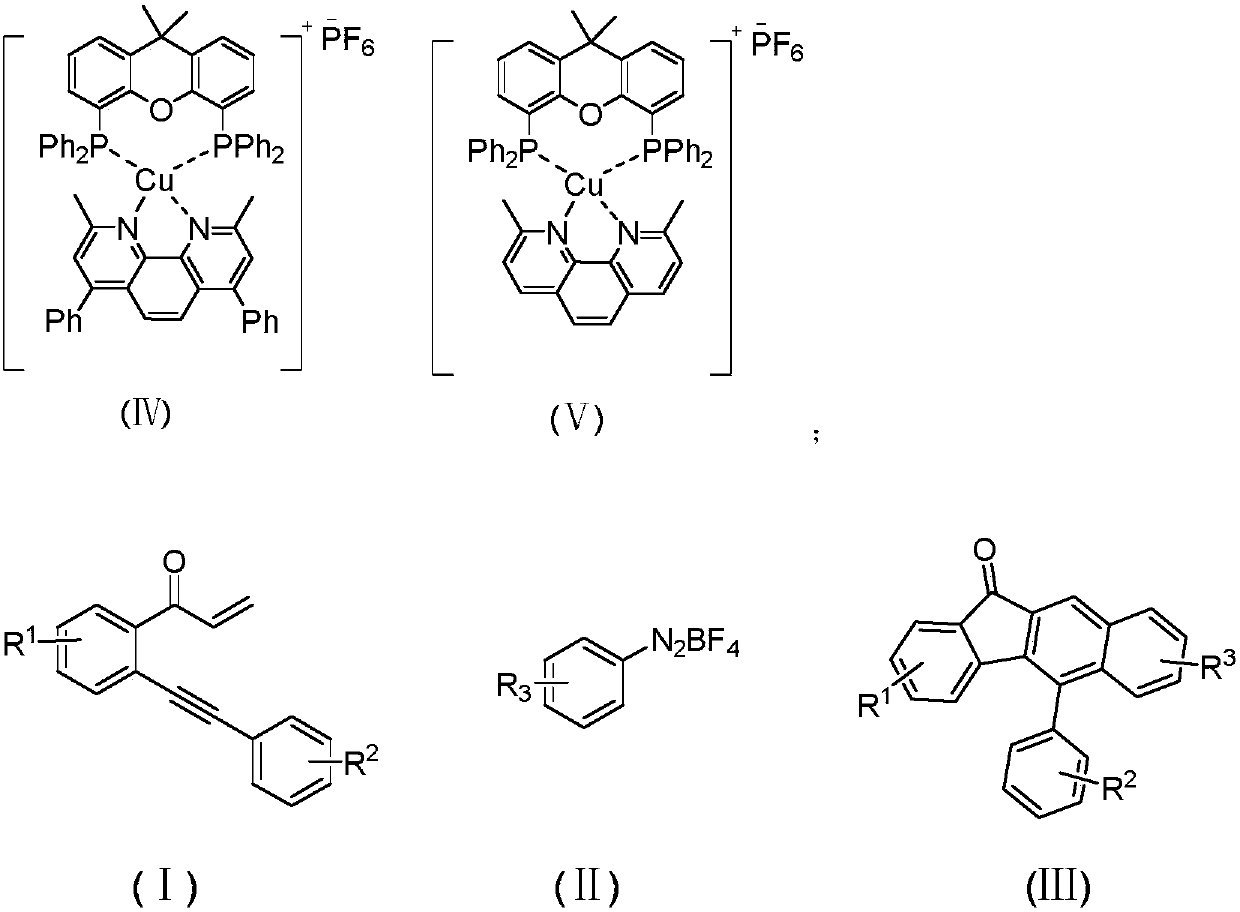
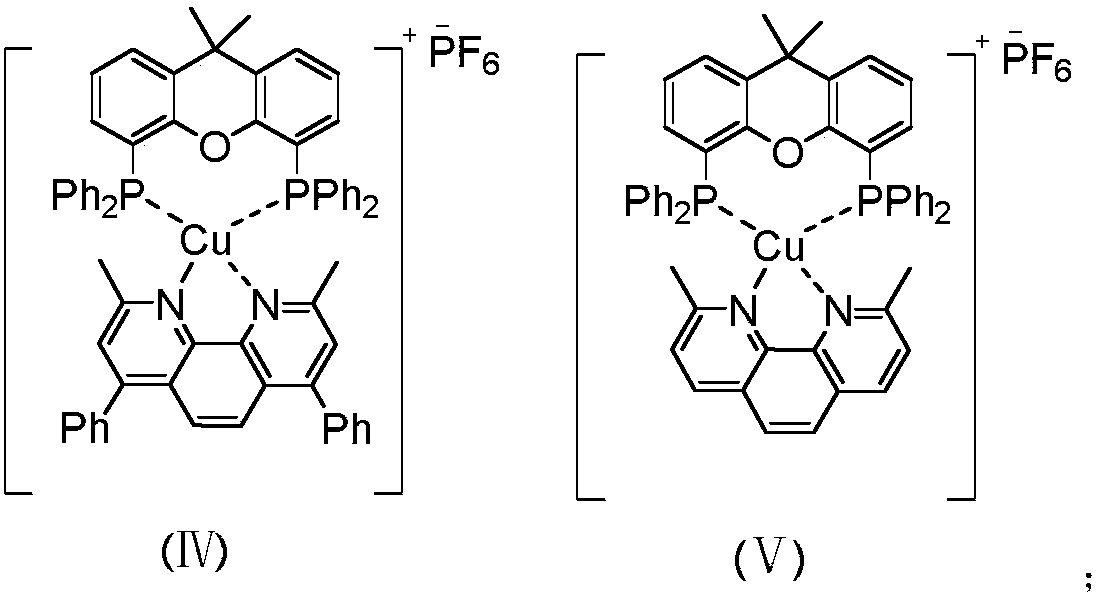
Method for synthesizing benzofluorenone compound through photocatalysis
ActiveCN110872219AAchieve synthesisReduce problems such as high energy consumptionOrganic compound preparationCarbonyl compound preparationPhotosensitizerPhoto irradiation
The invention relates to a method for synthesizing a benzofluorenone compound through photocatalysis. The method comprises the following steps: mixing a compound represented by a formula (I), a compound represented by a formula (II), a photosensitizer and a solvent, reacting for 1-3 h at a temperature of 30-50 DEG C under the irradiation of 15 W blue LED light, and performing post-treatment on thereaction liquid to obtain a benzofluorenone compound represented by a formula III. According to the invention, the method is safe, environment-friendly, free of waste gas and low in operation risk; the substrate adaptability is good, and oxidative cyclization of various substituent groups can be realized; reaction conditions are mild; and the benzofluorenone is synthesized by adopting a photocatalysis mode in the reaction, so that the method is more environment-friendly and is closer to the concept of green chemistry.
Owner:ZHEJIANG UNIV OF TECH
Single step enantioselective process for the preparation of 3-substituted chiral phthalides
The present invention discloses single step, highly enantioselective catalytic oxidative cyclization process for the synthesis of 3-substituted chiral phthalides. In particular, the invention discloses asymmetric synthesis of chiral phthalides via synergetic nitrile accelerated oxidative cyclization of o-cyano substituted aryl alkenes in high yield and enantiomeric excess (ee) in short reaction time. Also, disclosed herein is “one-pot” asymmetric synthesis of biologically important natural compounds having 3-substituted chiral phthalide structural framework in the molecule.
Owner:COUNCIL OF SCI & IND RES
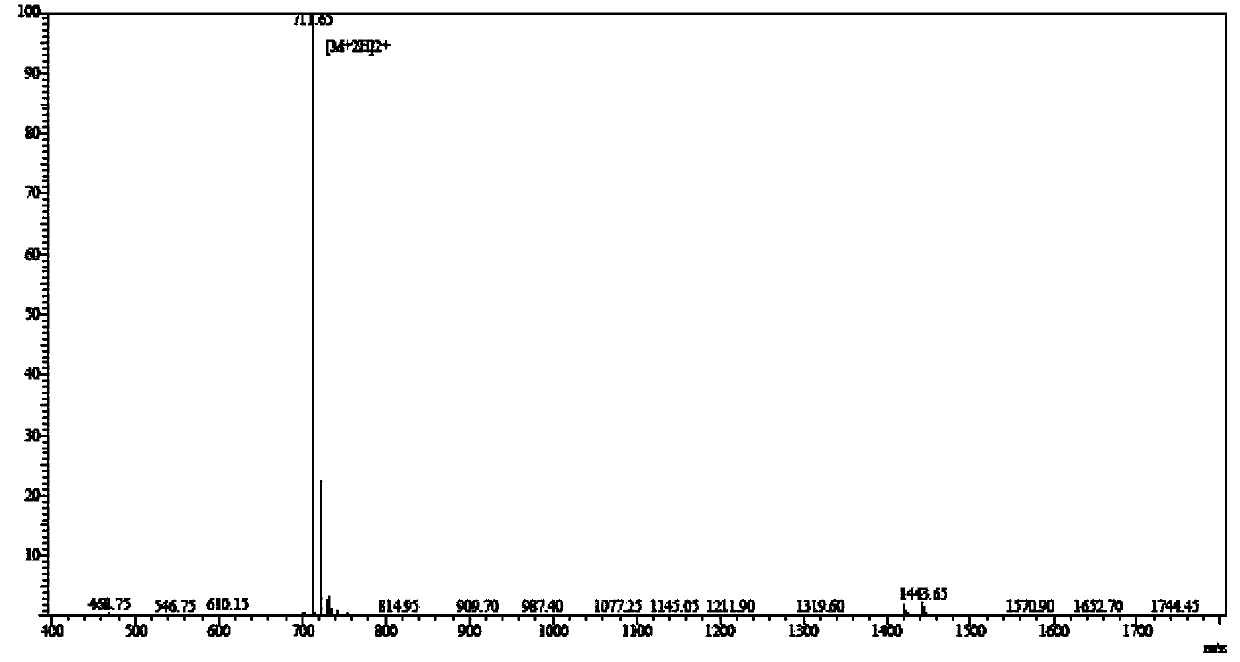
Solid-phase synthesis method for edotreotide
InactiveCN108676070AAvoid easy to causeMild reaction conditionsPeptide preparation methodsBulk chemical productionChemical synthesisSynthesis methods
The invention relates to a solid-phase synthesis method for edotreotide. The technical problem that an industrial synthesis method for the compound does not exist at present is mainly solved. The method disclosed by the invention mainly comprises the following steps: 1, coupling Fmoc-Thr(tbu)-Ol and dichlorotriphenyl chloride resin to obtain Fmoc-Thr(tbu)-Ol-2Cl-Trt Cl resin; 2, connecting remaining amino acids in sequence by virtue of a solid-phase synthesis method so as to obtain an edotreotide resin peptide I; 3, performing oxidative cyclization on the resin by using iodine so as to obtainan edotreotide resin peptide II; 4, performing Fmoc-removing protection, and coupling DOTA(Otbu)-3-COOH so as to obtain an edotreotide resin peptide III; 5, cracking to obtain crude edotreotide, purifying and refining through reversion phase chromatography, and freezing, so as to obtain the refined edotreotide. The invention provides a chemical synthesis method of edotreotide.
Owner:滨海吉尔多肽有限公司 +1
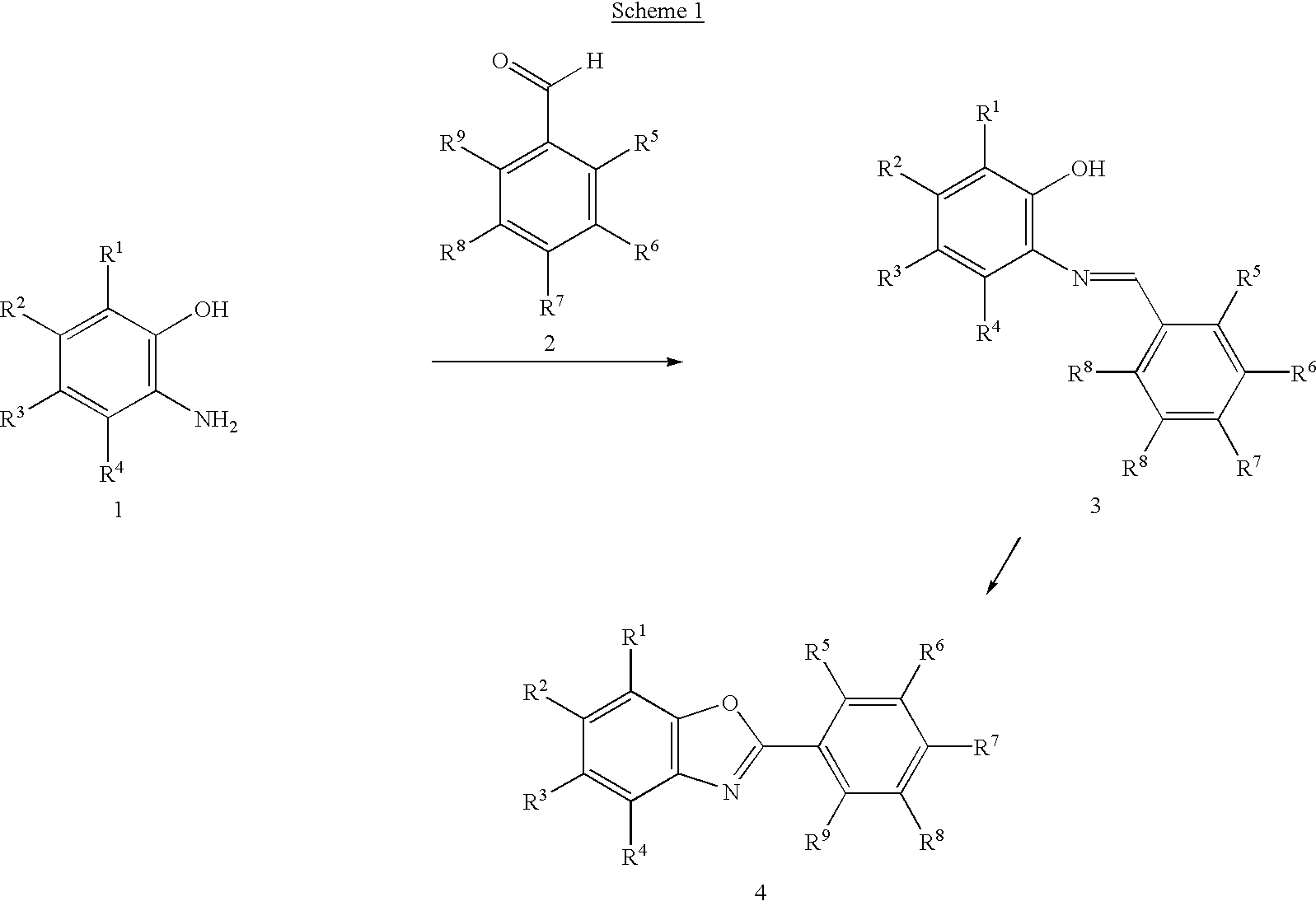
Solution phase sythesis of arylbenzoxazoles
This invention provides methods for the solution-phase synthesis of arylbenzoxazoles. The methods involve condensation of aminophenols with benzaldehydes to form a Schiff base. The Schiff base is then induced to undergo oxidative cyclization in the presence of DDQ. The resulting arylbenzoxazoles can be separated from the reduced DDQ byproduct by treatment of reaction mixture with a strongly basic ion exchange resin.
Owner:IRM
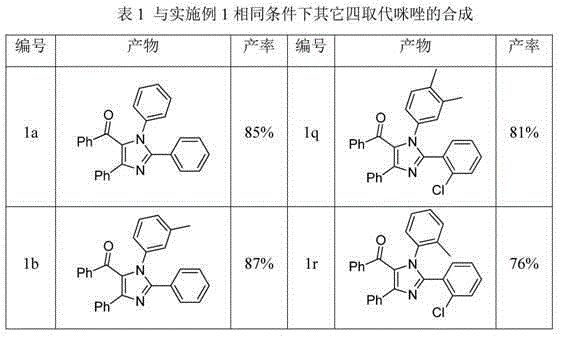
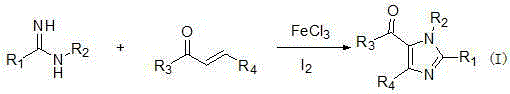
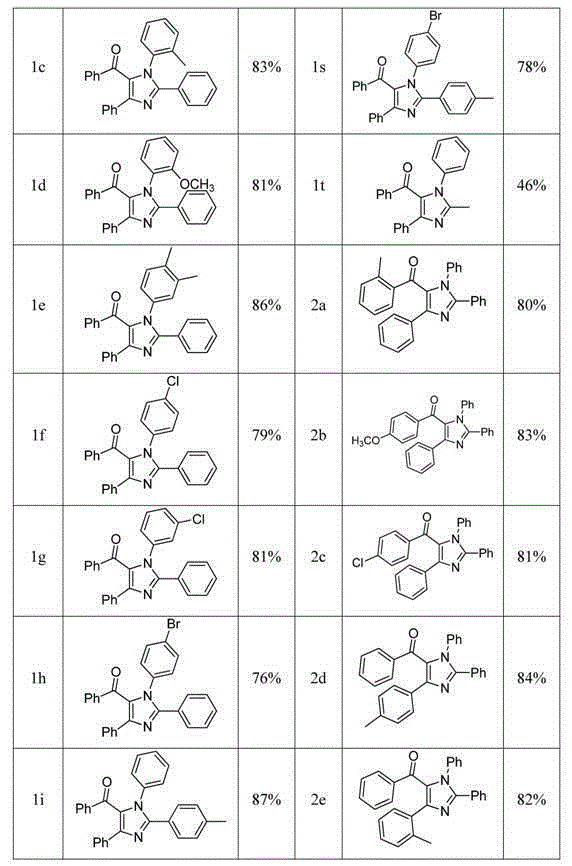
Tetra-substituted imidazole synthesis method
InactiveCN105001163ASimple processMild reaction conditionsOrganic chemistryChlorobenzeneSynthesis methods
The present invention discloses a tetra-substituted imidazole synthesis method, wherein amidine and chalcone derivatives are adopted as raw materials and chlorobenzene is adopted as a solvent. Meanwhile, ferric trichloride and iodine are adopted as catalysts. In this way, the tetra-substituted imidazole is synthesized through the oxidative cyclization reaction. The above raw materials of the method are readily available, and the operation of the method is simple. Meanwhile, the method is mild in reaction condition, high in yield and strong in practicality, thus being suitable for industrial production.
Owner:NORTHWEST UNIV(CN)
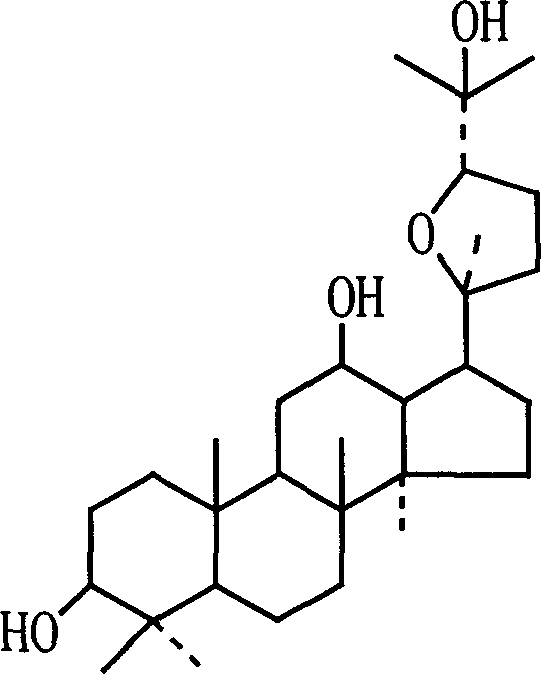
Use of 20(S)-protopanoxadiol derivatives and 20(S)-protopanaxatriol derivatives in preparation of antidepressant medicines
ActiveCN103211823AShorten immobility timeGood antidepressant activityOrganic active ingredientsNervous disorderEpoxyDisease
The invention relates to a new use of 20(S)-protopanoxadiol derivatives and 20(S)-protopanaxatriol derivatives, and mainly relates to a use in the preparation of medicines for treating or preventing depressive mental diseases. 20(S)-protopanoxadiol and 20(S)-protopanaxatriol are used as raw materials, and undergo oxidative cyclization to prepare dammara-20S-24(R)-epoxy-3beta,12beta,25-triol, dammara-20S-24(S)-epoxy-3beta,12beta,25-triol, dammara-20S-24(S)-epoxy-3beta,6beta,12beta,25-tetrol or dammara-20S-24(R)-epoxy-3beta,6beta,12beta,25-tetrol. Researches of pharmacological experiments show that dammara-20S-24(R / S)-epoxy-3beta,12beta,25-triol and dammara-20S-24(R / S)-epoxy-3beta,6beta,12beta,25-tetrol can obviously shorten the tail immobility time of mice in mouse tail suspension tests of a classic depression model, and can obviously shorten the swimming immobility time of the mice in mouse forced swimming model tests, so dammara-20S-24(R / S)-epoxy-3beta,12beta,25-triol and dammara-20S-24(R / S)-epoxy-3beta,6beta,12beta,25-tetraol have potential development values in the preparation of medicines for treating or preventing depressive mental diseases.
Owner:SHANGHAI INNOVATIVE RESEARCH CENTER OF TRADITIONAL CHINESE MEDICINE
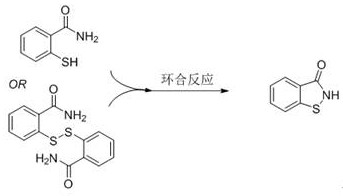
Method for preparing 1, 2-benzisothiazolin-3-one through catalytic oxidation
ActiveCN113200937AHigh catalytic activityImprove reaction efficiencyOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsPtru catalystThiazole
The invention provides a method for preparing 1, 2-benzisothiazoline-3-one through catalytic oxidation, which comprises the following steps of: by taking a metal manganese salt or a manganese complex as a catalyst, carrying out oxidative cyclization reaction on an amine compound in an oxygen or air environment to generate the 1, 2-benzisothiazoline-3-one. In the reaction, oxygen or air is used as an oxidant; the catalyst is simple, cheap, high in catalytic activity and high in reaction efficiency; the preparation process is simple, the product selectivity is high, and byproducts are few; and less waste is produced, and the method is environment-friendly, and has a relatively good industrial application prospect.
Owner:ZHENGZHOU UNIV
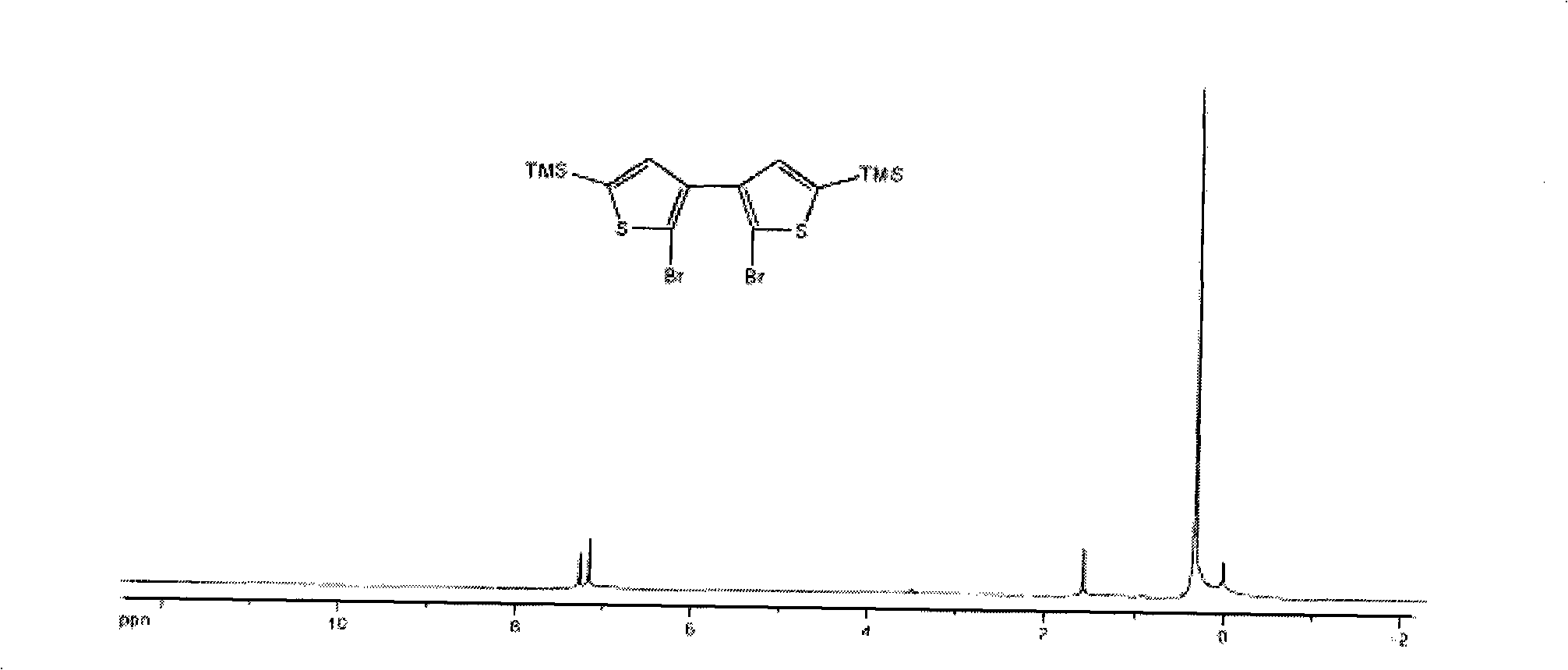
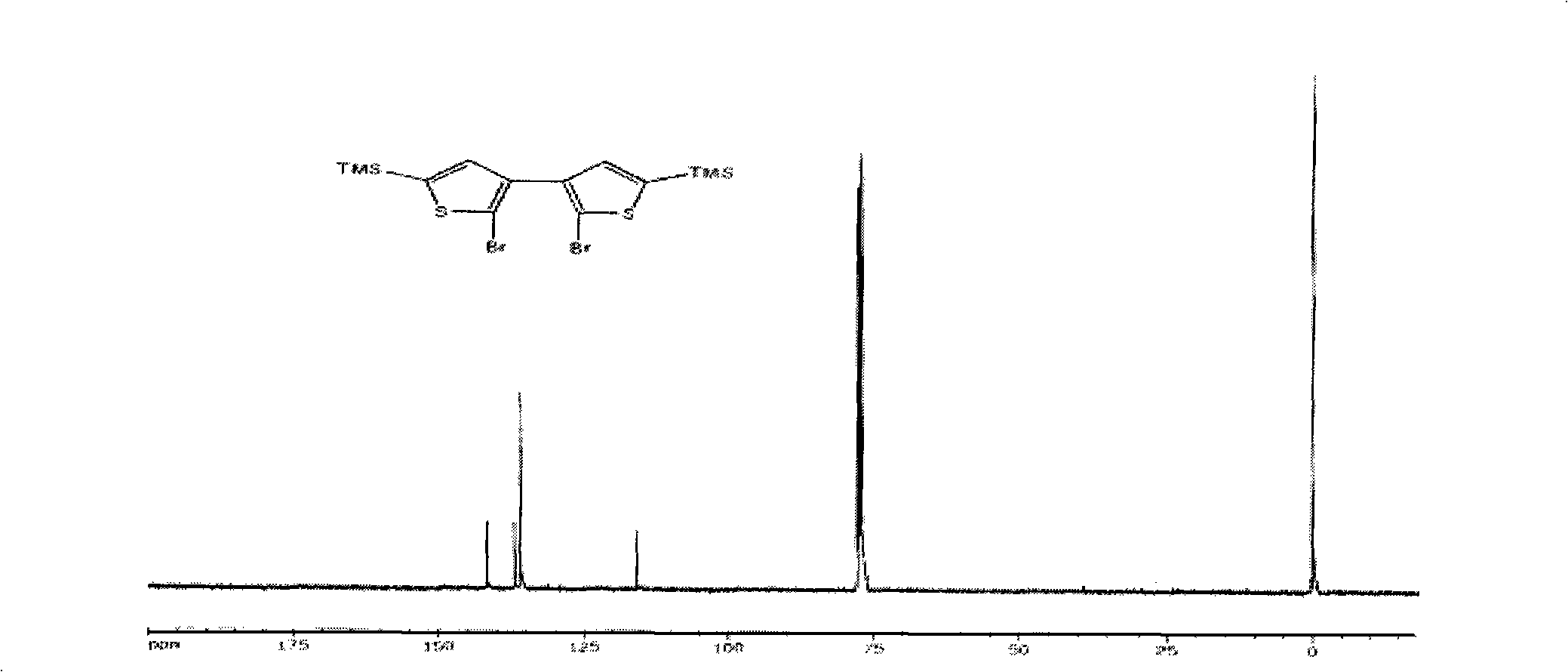
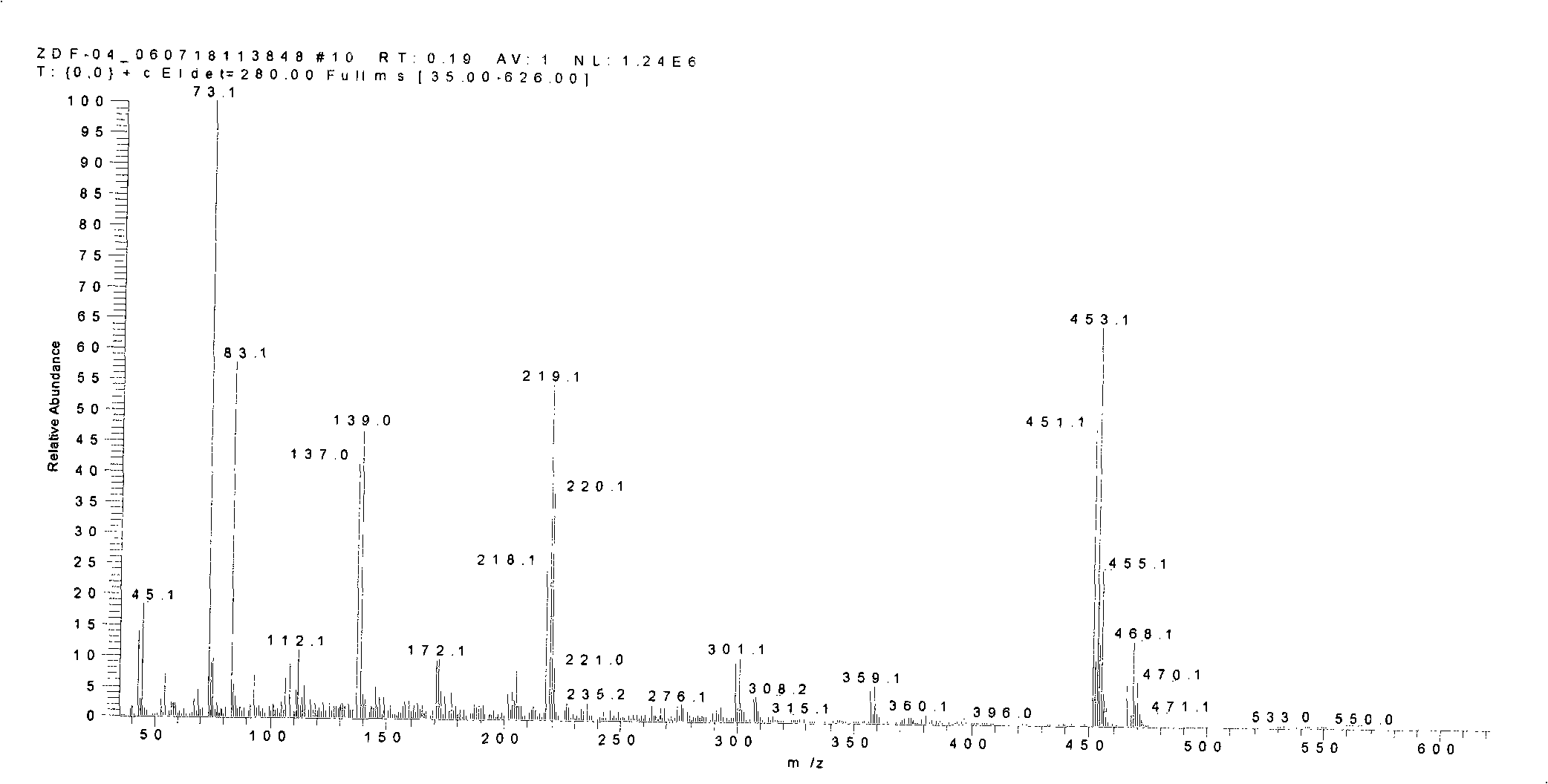
Thiophene macrocyclic compound and preparation method for its derivant
Owner:HENAN UNIVERSITY
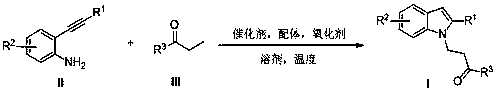
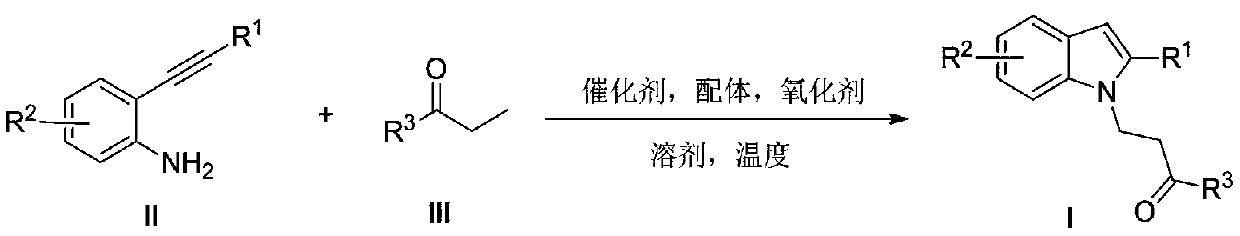
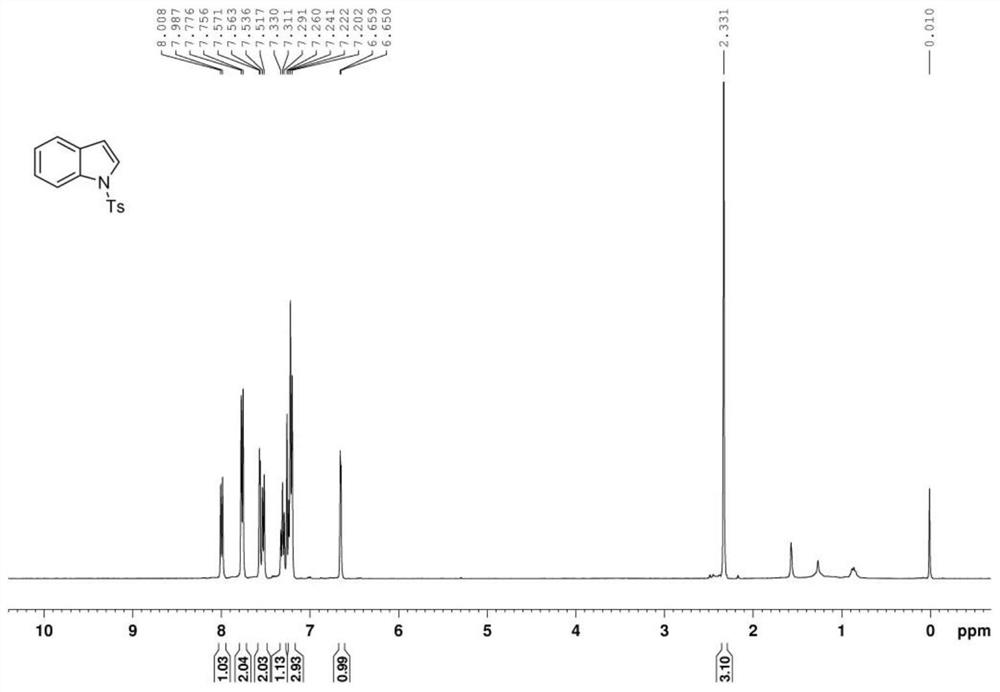
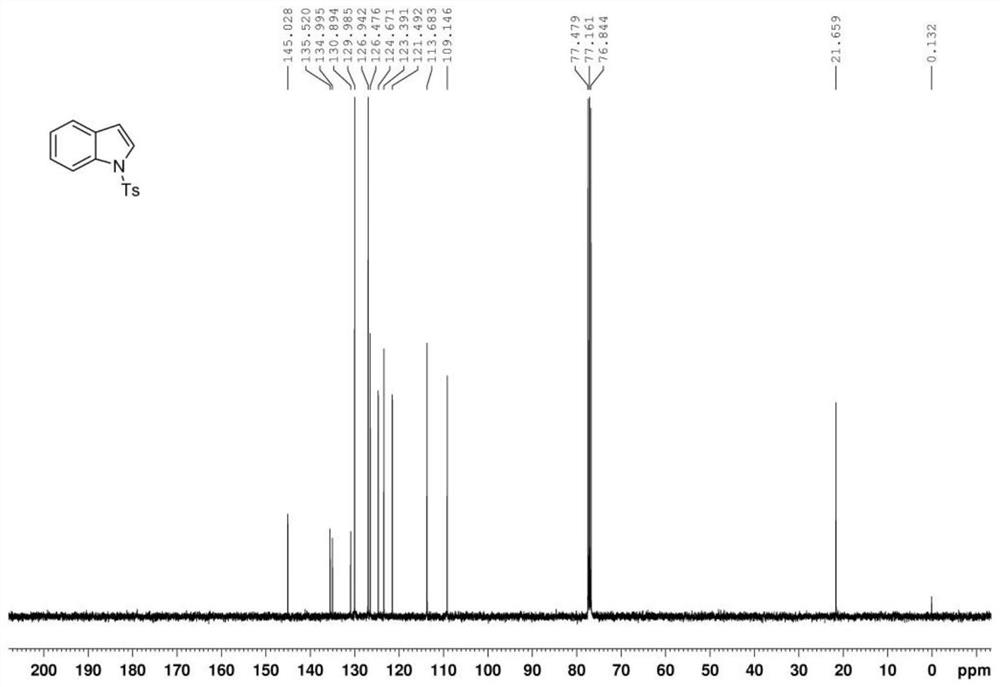
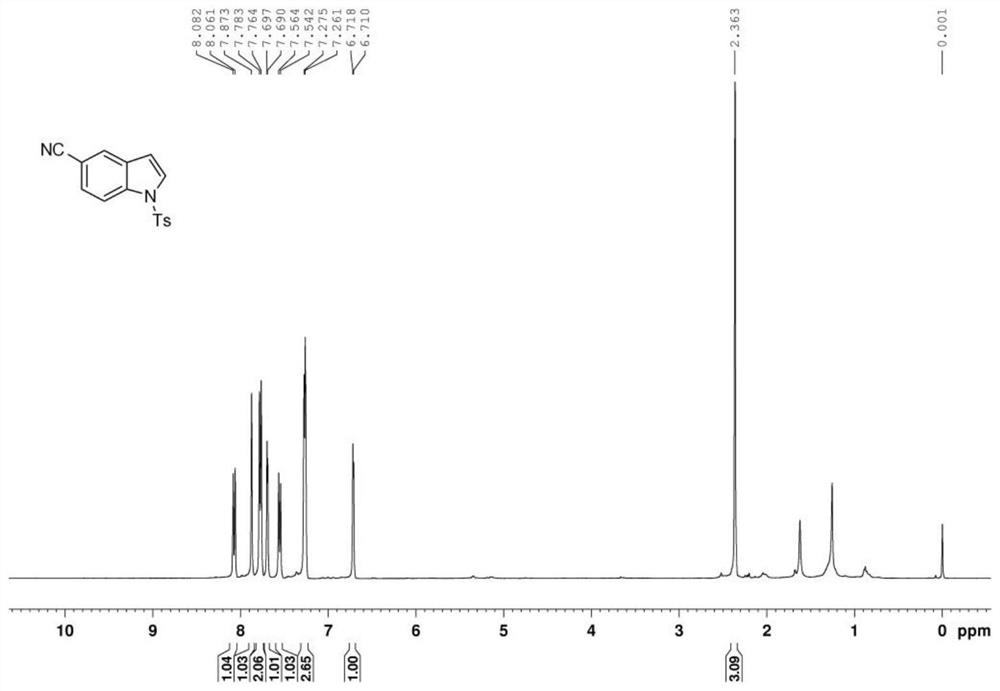
Preparation method of polysubstituted 2-aryl indole derivative
ActiveCN111004164AReaction raw materials are readily availableSimple and fast operationOrganic chemistryPtru catalystOxidative cyclization
The invention discloses a preparation method of a polysubstituted 2-aryl indole derivative. The method specifically comprises the following steps: by taking a 2-acetenyl aniline compound and a ketonecompound as raw materials, carrying out oxidative cyclization reaction on the 2-acetenyl aniline compound and the ketone compound under the action of a catalyst, a ligand and an oxidizing agent to prepare the polysubstituted 2-arylindole derivative. The method provided by the invention has the characteristics of easily available raw materials, simple operation, high chemical selectivity and regioselectivity, and the like, and has great implementation value and social and economic benefits.
Owner:ZHEJIANG UNIV OF TECH
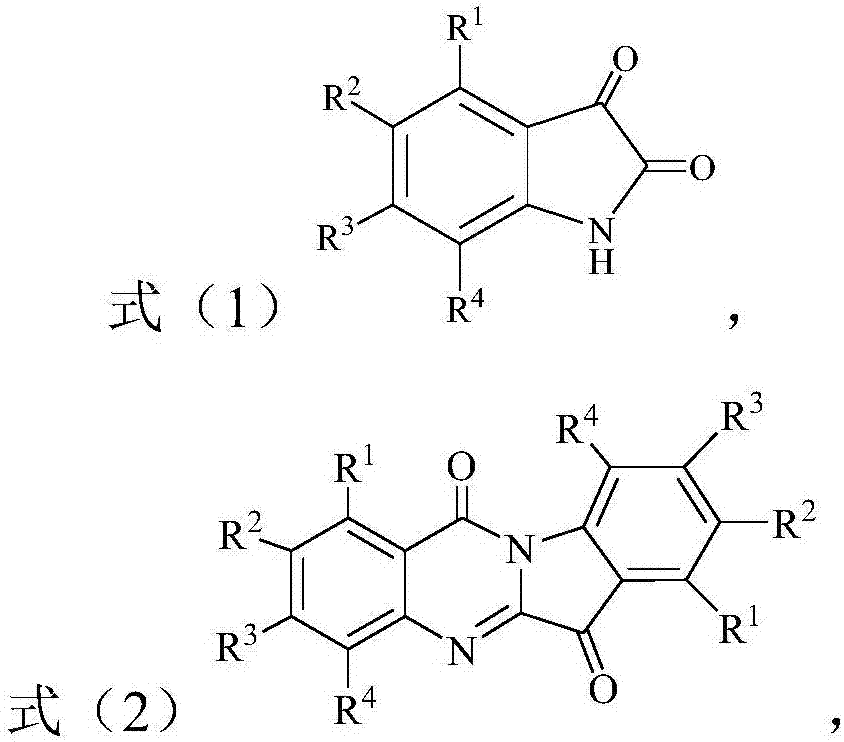
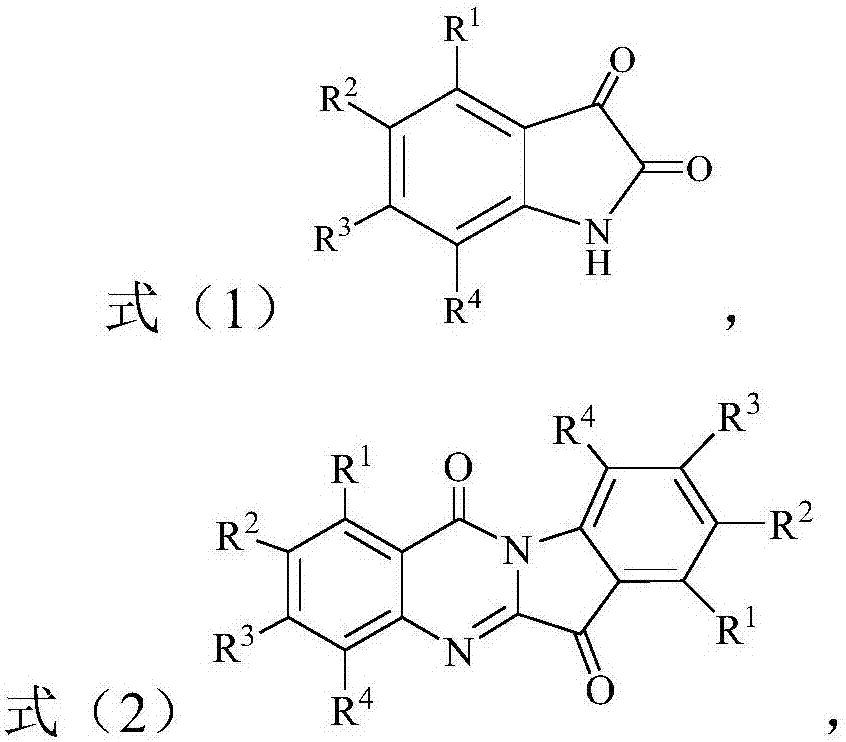
Synthesis method of benzo[e]pyridylimidazole[4,5-g]isoindole-1,3(2H)-diketone compound
The invention discloses a synthesis method of a benzo[e]pyridylimidazole[4,5-g]isoindole-1,3(2H)-diketone compound, and belongs to the technical field of organic synthesis. According to the technicalscheme, the preparation method is characterized by comprising the following steps: dissolving a 2-arylimidazole[1,2-a]pyridine compound and an N-substituted maleimide compound in a solvent, adding a catalyst and an oxidant, and reacting at 80 to 120 DEG C to obtain the target product benzo[e]pyridylimidazole[4,5-g]isoindole-1,3(2H)-diketone compound. The target product benzo[e]pyridylimidazole[4,5-g]isoindole-1,3(2H)-diketone compound is prepared from simple and easily prepared raw materials through transition metal catalyzed [4 + 2] oxidative cyclization reaction, so that heterocyclic structural units such as pyridylimidazole and maleimide are fused together to directly obtain a pentacyclic aromatic system; the whole synthesis process is simple, efficient, convenient to operate, mild in condition and wide in substrate application range.
Owner:HENAN NORMAL UNIV
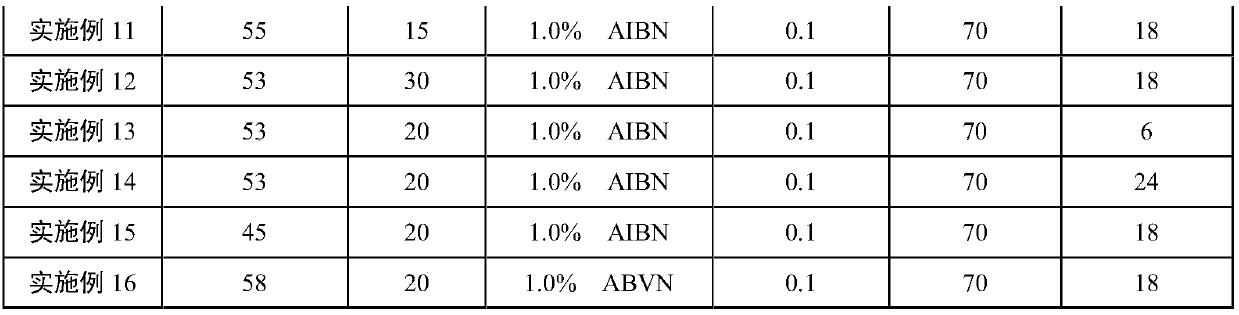
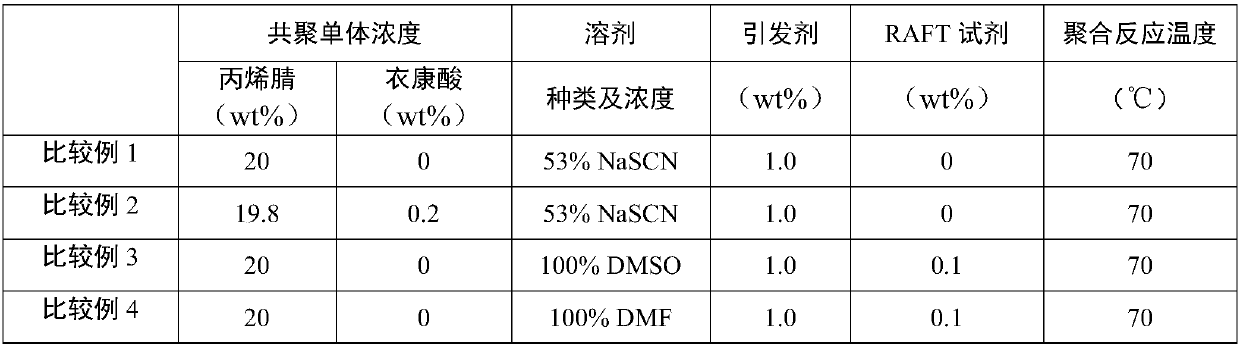
Polyacrylonitrile resin with controllable molecular weight and distribution and high regularity, and preparation method thereof
ActiveCN111100231ARealize living controllable polymerizationNarrow distributionPolymer scienceCarbon fibers
The invention discloses polyacrylonitrile resin with controllable molecular weight and distribution and high regularity, and a preparation method thereof. The polyacrylonitrile resin with controllablemolecular weight and distribution and high regularity is prepared through a reversible addition / fragmentation chain transfer (RAFT) free radical polymerization process and a polymerization reaction by taking trithiocarbonate (TRIT) containing carboxyl groups at two ends as an RAFT reagent. The polyacrylonitrile resin has the advantages of high molecular weight, narrow molecular weight distribution, high molecular chain structure regularity and few molecular structure defects. The hydrophilicity of the polyacrylonitrile resin prepared by the method is obviously improved, the oxidative cyclization activity is improved, and the polyacrylonitrile resin is suitable for preparing protofilaments for high-performance carbon fibers.
Owner:CHINA PETROLEUM & CHEM CORP +1
Process for conducting organic reactions in a standalone and affordable laboratory scale solar photo thermochemical reactor
InactiveUS20150196891A1Promote popularizationAffordable costSolar heating energyOrganic compound preparationOrganic synthesisLaboratory scale
A process conducts organic reactions in a standalone laboratory scale solar photo thermo chemical reactor. For organic reactions require elevated temperature, light and mechanical agitation, all three energy forms can be simultaneously derived from solar radiation. Organic synthesis, such as bromination of toluene derivatives (benzylic bromination), bromination of cyclic acyclic hydrocarbon and oxidative cyclization of N-phenylethyl benzamide through bromination were successfully conducted in such reactors.
Owner:COUNCIL OF SCI & IND RES
Synthesis technology of benthiavalicarb isopropyl
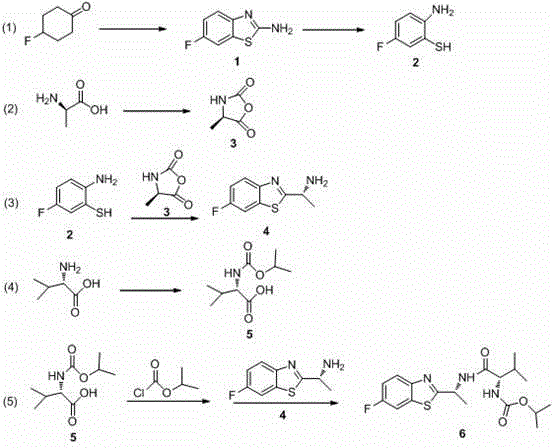
The invention discloses a new synthesis technology of benthiavalicarb isopropyl, and belongs to the field of pesticide chemistry. A method for implementing the synthesis technology comprises the steps as follows: fluorocyclohexanone is used as a raw material and catalyzed by iodine to generate 2-amino-6-fluorobenzothiazole through oxidative cyclization, and after hydrolysis and acidification, 2-amino-5-fluorothiophenol is prepared; meanwhile, D-alanine is used as a raw material and reacts with triphosgene to generate (R)-4-methyl-oxazolidine-2, 5-diketone, and an intermediate reacts with 2-amino-5-fluorothiophenol to prepare chiral (R)-1-(6-fluorine-benzothiazole-2-) ethylamine; and L-valine is used as a raw material and reacts with isopropyl chlorocarbonate to prepare N- Isopropoxy carbonyl-L-valine, carboxyl activation is performed simultaneously, and finally, the raw materials and (R)-1-(6-fluorine-benzothiazole-2-) ethylamine perform a one-pot reaction to directly generate benthiavalicarb isopropyl. The synthesis technology has short process route and high total yield, and purity degree is higher than 95%.
Owner:SHANGQIU NORMAL UNIVERSITY
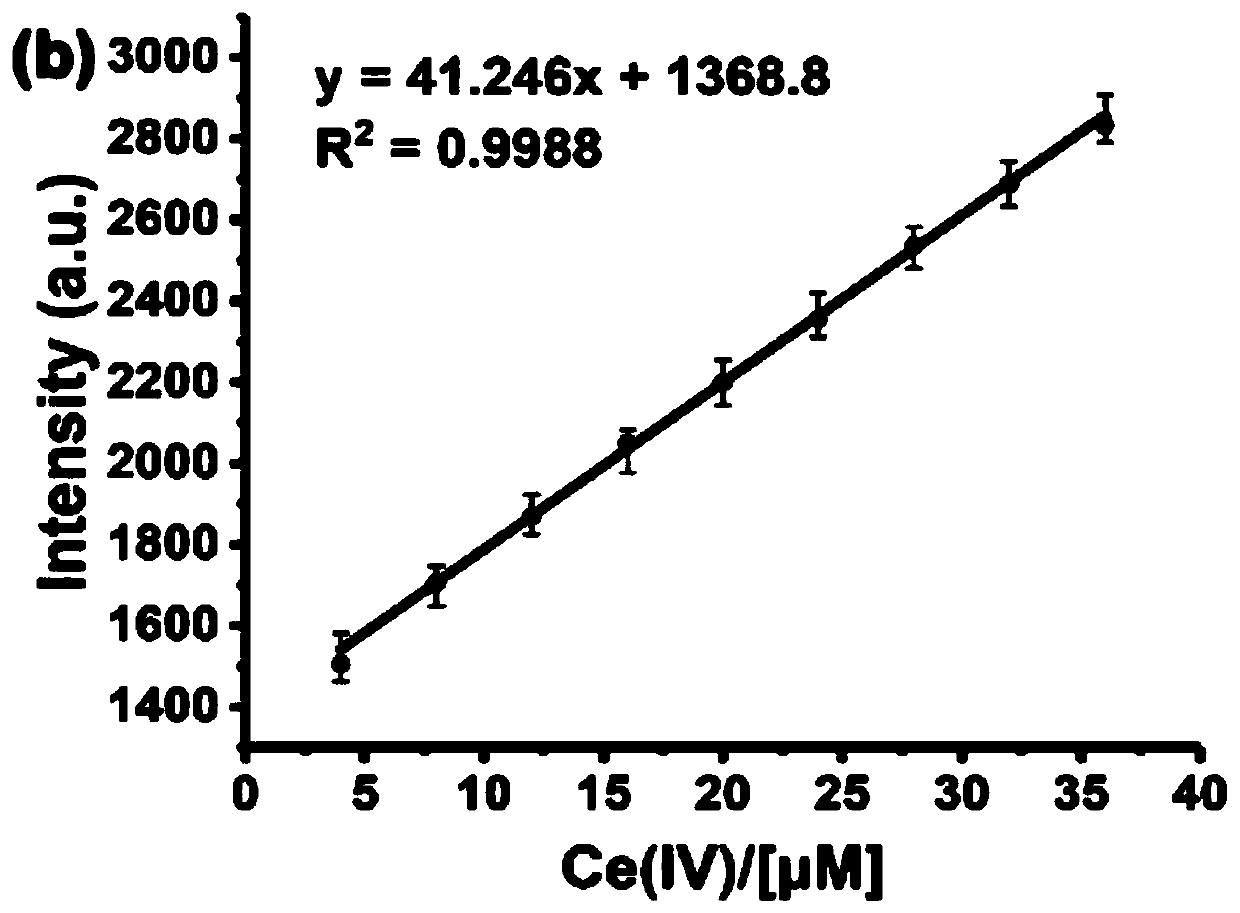
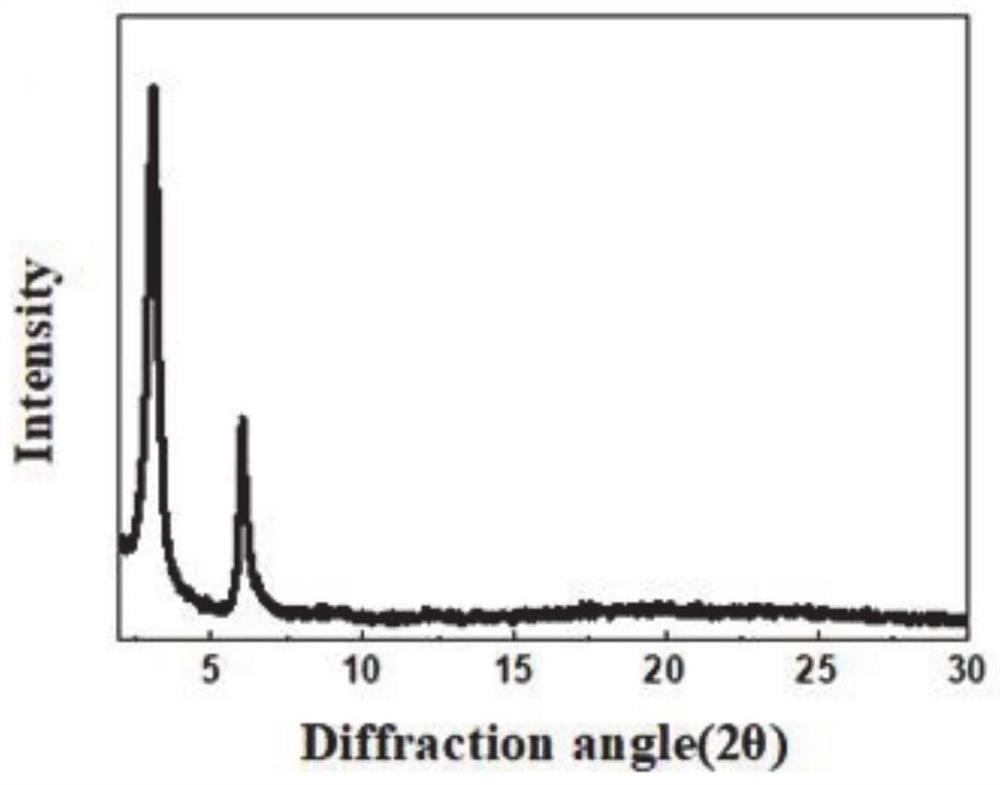
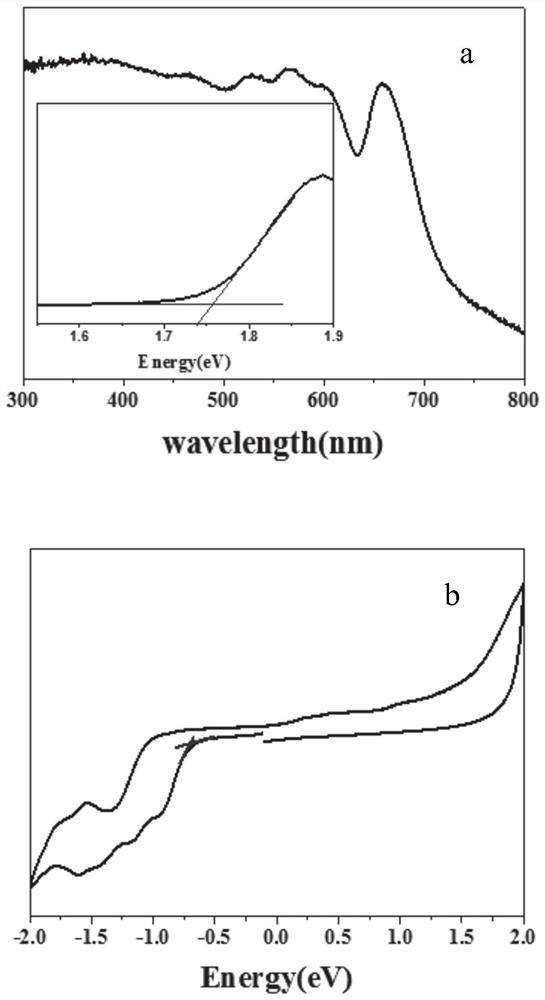
Fluorescence detection reagent for tetravalent cerium ions and fluorescence detection method thereof
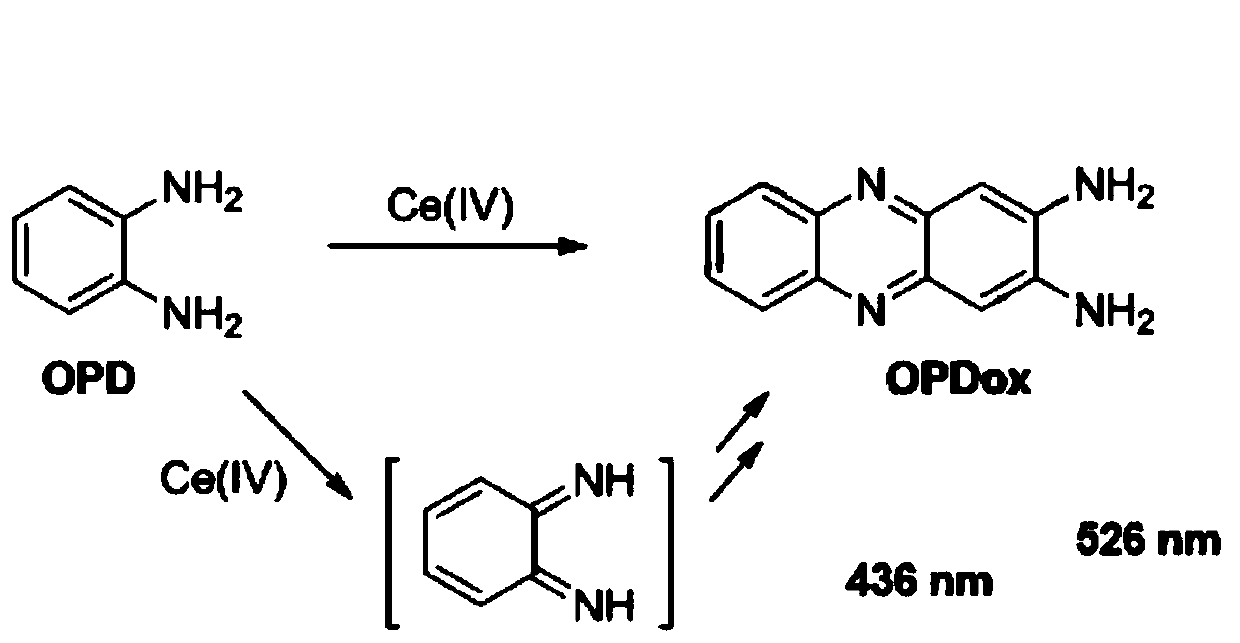
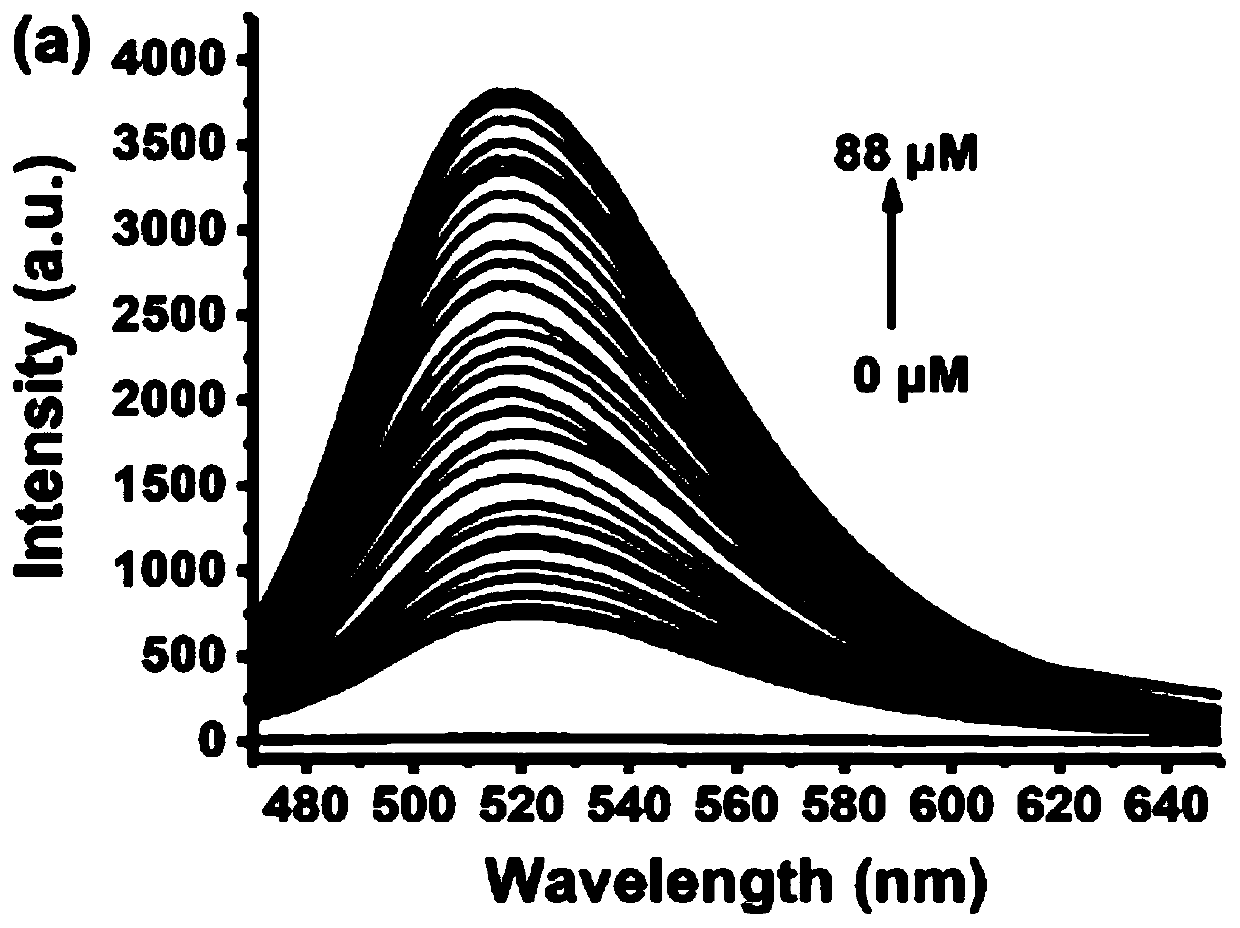
PendingCN111337467APromote oxidationStrong fluorescence responseFluorescence/phosphorescencePhenazineOxidative cyclization
The invention relates to a fluorescence detection reagent for tetravalent cerium ions and a fluorescence detection method thereof, tetravalent cerium ions are used as a single-electron oxidizing agentto promote o-phenylenediamine to be oxidized, cyclized and condensed to form a yellow fluorescence product 2, 3-diaminophenazine, so that a Ce (IV) ion fluorescence detection method is established. The fluorescence detection method of the tetravalent cerium ions has good selectivity and linear relation and strong fluorescence response, and can quantitatively detect the concentration of the tetravalent cerium ions.
Owner:JIANGXI UNIV OF SCI & TECH
Application of porphyrin-based covalent organic framework material in photocatalytic oxidation cyclization reaction
ActiveCN113546683AIncreased redox potentialIncrease costOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsDimethylaniline N-oxideMeth-
The invention relates to the technical field of catalytic synthesis, and concretely relates to application of a porphyrin-based covalent organic framework material in photocatalytic oxidation cyclization reaction. The structure of the porphyrin-based covalent organic framework material is as shown in formula I. A synthesis method of an N-methyltetrahydro-1H-pyrrolo[3, 4-c]-quinoline-1, 3(2H)-dione derivative comprises the following steps: adding an N, N-dimethylaniline derivative and a maleimide derivative into an organic solvent to obtain a solution A; adding the material in the formula I into the solution A to obtain a solution B; and irradiating the solution B with visible light to obtain a product. A metal-free covalent organic framework based on a porphyrin structure is used as a photosensitizer, oxygen is used as an oxidizing agent, an oxidative cyclization reaction of the N, N-dimethylaniline derivative and the maleimide derivative is realized under the irradiation of visible light, and the tetrahydroquinoline compound is efficiently constructed; and the method is green and mild in condition, the photosensitizer can be recycled, the utilization rate of the photosensitizer is increased, the cost is reduced, and industrial popularization and application are facilitated.
Owner:SHANDONG NORMAL UNIV
Preparation method of intermediate of antibacterial drug
ActiveCN112442084AHigh yieldHigh catalytic activityPhysical/chemical process catalystsOrganic compound preparationPhosphonomycinPtru catalyst
The invention provides a preparation method of phosphamycin levophosphorylamine. The preparation method comprises the following steps: dissolving cis-propenylphosphonic acid in an alcohol solvent at room temperature, slowly dropwise adding (+)-alpha-phenylethylamine, regulating the pH value of the formed system to 5.5-6, continuing stirring for 1-3 minutes, adding a silver catalyst, slowly and dropwise adding hydrogen peroxide, continuing stirring for 10-30 minutes, then rapidly heating the system to 50-55 DEG C, conducting filtering while the system is hot, and then cooling, crystallizing andwashing the filtrate successively to obtain the phosphamycin levophosphorylamine. According to the invention, silver carbonate is used as a catalyst, hydrogen peroxide is used as an oxidizing agent,heating is not needed in an oxidative cyclization process, and a reaction can be performed at normal temperature. The silver carbonate has very high catalytic activity in the invention, and compared with the prior art, the method has the advantages of small dosage, mild reactions, effective shortening of reaction time, simple post-treatment, and realization of separation of the catalyst from the system only through filtration of the system while the system is hot.
Owner:SINOPHARM ZHIJUN (SHENZHEN) PHARMA CO LTD
A kind of preparation method of quinazinone compound
ActiveCN106928215BHigh yieldGood effectOrganic chemistry methodsAntiviralsOxidative cyclizationSolvent
Owner:河南春风医药科技有限公司
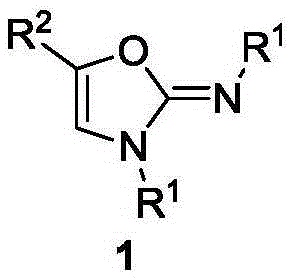
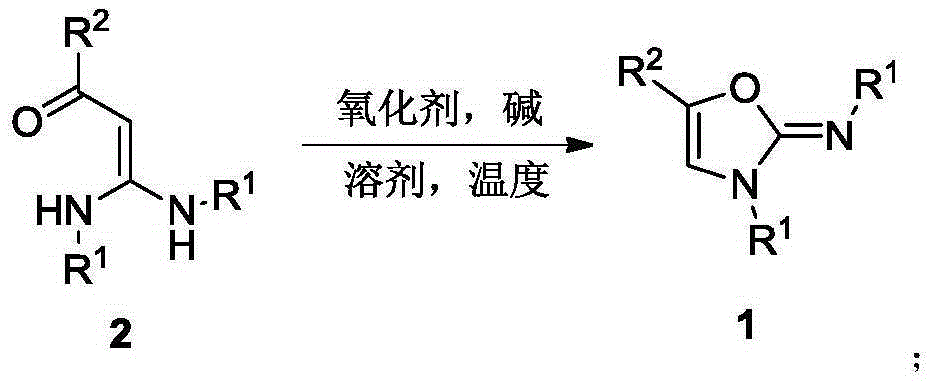
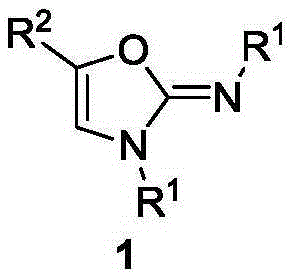
Method for synthesis of 2-imino oxazole
InactiveCN106749078AWith structural diversityEasy to manufactureOrganic chemistrySynthesis methodsKetone
The invention discloses a method for synthesis of 2-imino oxazole derivatives having potential bioactive activity. The method comprises that 3, 3-diarylamino-2-propen-1-one, which is easy to prepare and has structural diversity and multiple reaction centers, is used as a raw material and undergoes an oxidative cyclization-Baldwin rearrangement reaction in air to produce a 2-imino oxazole derivative. Compared with the reported synthesis methods of 2-imino oxazole derivatives, the method provided by the invention utilizes easily available raw materials, can be simply operated and has mild reaction conditions and a high yield.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Method for preparing Setmelanotide
PendingCN111718408AHigh yieldHigh purityPeptide preparation methodsDepsipeptidesFreeze-dryingOxidative cyclization
The invention provides a method for preparing Setmelanotide. According to the method, amino resins are used as starting resins, corresponding protecting amino acids are sequentially inoculated according to the reverse direction of the amino sequence of the Setmelanotide, so that Setmelanotide peptide resins are obtained; the Setmelanotide protecting peptide resins are subjected to acidolysis and oxidative cyclization to obtain Setmelanotide crude products; and the Setmelanotide crude products are subjected to purification and freeze drying to obtain Setmelanotide pure products. The technologyis simple to operate, and the products are high in total yield, and suitable for large-scale production.
Owner:CHENGDU SHENGNUO BIOPHARM
Synthetic method of naphtho [2, 1-d] oxazole compound
The invention provides a method for synthesizing a naphthol [2, 1-d] oxazole compound through oxidative cyclization of a naphthol compound and an amine compound under a metal-free condition. Many functional groups, such as Me, OMe, tBu, F, Cl, Br, I, CF3, CN, NO2, CHO, ester and anhydride groups, furfuryl, thienyl and pyridyl, are easily transferred into the desired product, which is not achieved by other methods. Based on the advantage, the method can provide a variety of new naphtho [2, 1-d] oxazole compounds. The method has the advantages of being high in universality, high in efficiency, good in selectivity, good in atom and step economy, easy in substrate obtaining, mild in condition, easy and convenient to operate and the like, and therefore the reaction can be widely applied to the fields of chemistry, biology, medicine and material science.
Owner:HUNAN FIRST NORMAL UNIV
Method for constructing 3,5-disubstituted pyridine by utilizing mixed styrene derivative and N,N-dimethylformamide
ActiveCN111518021AWide range of raw materialsExtensive sources of raw materialsOrganic chemistryPolymer chemistryOxidative cyclization
The invention discloses a method for constructing 3,5-disubstituted pyridine by utilizing mixed styrene derivative and N,N-dimethylformamide. The method comprises the following step: subjecting the mixed styrene derivative, N,N-dimethylformamide and peroxydisulfate to a cyclization reaction under the catalytic action of iodized salt so as to obtain symmetrical and asymmetrical mixed 3,5-disubstituted pyridine products at the same time. According to the method, 3,5-disubstituted pyridine is synthesized from the mixed styrene derivative and DMF through one-step oxidative cyclization under the catalysis of iodate; and the method has the advantages of low raw material and catalyst cost, mild reaction conditions, capability of realizing high-yield preparation of the symmetrical and asymmetrical3,5-disubstituted pyridine products at the same time and the like.
Owner:YUANJIANG HUALONG CATALYST TECH
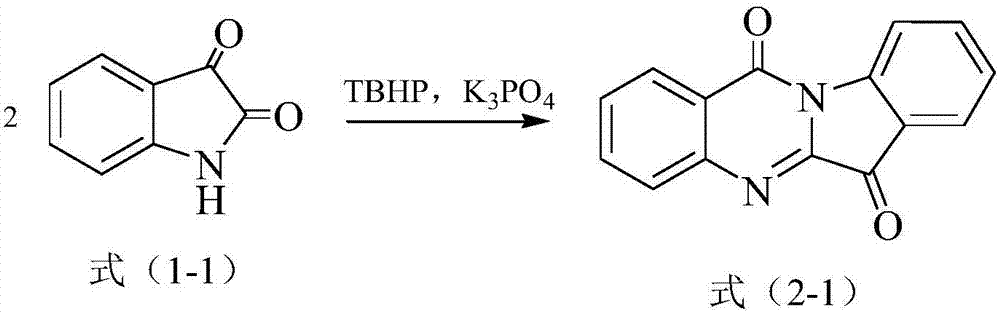
Preparation method of tryptanthrin compound
ActiveCN107141292ALow toxicityMild conditionsOrganic chemistryEnvironmental resistanceOrganic solvent
The invention relates to the field of synthesis of tryptanthrin and discloses a preparation method of a tryptanthrin compound. The tryptanthrin compound is a compound having a structure shown in formula (2); the preparation method comprises a step of performing oxidative cyclization reaction on a compound having a structure shown in formula (1) in an organic solvent in the presence of a peroxide and an alkaline compound to obtain the compound having the structure shown in formula (2); the peroxide is selected from tert-butyl hydroperoxide and / or hydrogen peroxide. Raw materials used in the preparation method provided by the invention are simple, low in toxicity, green and environment-friendly; the preparation method realizes reaction at room temperature basically, are mild in condition, and can be used for implementing industrial production of the tryptanthrin compound.
Owner:HUAZHONG NORMAL UNIV
Method for electrochemical synthesis of indole compounds
ActiveCN112708902AResponse to environmental protectionElectrolysis componentsElectrolytic organic productionChemical synthesisOxidative cyclization
The invention provides a method for synthesizing an indole compound by iodine-induced electrocatalytic intramolecular C(sp2)-H oxidative cyclization. The preparation method comprises the following steps: mixing iodized salt electrolyte, a 2-vinyl aniline compound and thiocyanate, and performing an intramolecular oxidative cyclization reaction under electrochemical conditions to obtain the indole compound. The invention provides a method for non-metallic catalytic electrochemical synthesis of indole compounds, so that a plurality of important drug intermediates can be derived. According to the method, no metal or chemical oxidizing agent is used, no iodobenzene or other by-products are generated, the reaction is green and environmentally friendly, the atom economy is high, and the gram-scale experiment yield is kept.
Owner:UNIV OF SCI & TECH OF CHINA
Application of silver catalyst in preparation of antibacterial drug intermediate
InactiveCN112409410AHigh yieldHigh catalytic activityAmino preparation from aminesGroup 5/15 element organic compoundsPhosphonomycinPtru catalyst
The invention provides an application of a silver catalyst in preparation of an antibacterial drug intermediate fosfomycin levoforight amine salt. The application is characterized by comprising the following steps: at room temperature, dissolving cis-propenylphosphonic acid in an alcohol solvent, slowly dropwise adding (+) alpha phenylethylamine, regulating the pH value of the system to 5.5-6 after dropwise adding, continuing stirring for 1-3 minutes, and adding the silver catalyst, continuously and slowly dropwise adding hydrogen peroxide, then continuously stirring for 10-30 minutes, quicklyheating the system to 50-55 DEG C, filtering while the system is hot, and cooling, crystallizing and washing the filtrate to obtain the fosfomycin levoforight amine salt. Silver carbonate is used asthe catalyst, hydrogen peroxide is used as an oxidizing agent, heating is not needed in the oxidative cyclization process, and the reaction can be performed at normal temperature. Silver carbonate hasvery high catalytic activity in the invention, and compared with the prior art, the application has the advantages of small dosage, mild reaction, effective shortening of the reaction time, simple post-treatment, and realization of separation of the catalyst from the system only through filtration of the system while the system is hot.
Owner:商河探荣新技术开发中心
A kind of synthetic method of myrtle alkenyl imidazo[2,1-b][1,3,4]thiadiazole compound
A method for synthesizing a myrtleenyl imidazo[2,1-b][1,3,4]thiadiazole compound, comprising the steps of: first selectively oxidizing α-pinene to myrtenene Aldehyde, then condensed with thiosemicarbazide to obtain myrtleenyl thiosemicarbazide, further oxidized and cyclized to obtain myrtleenyl thiadiazole, and then reacted with a series of 2-bromostyrene under microwave irradiation The ketone was subjected to cyclization reaction, and the myrtenyl imidazo[2,1-b][1,3,4]thiadiazole compound was synthesized. The present invention realizes the synthesis of myrtene aldoimidazo[2,1-b][1,3,4]thiadiazole compound for the first time. The antibacterial activity test showed that myrtenyl imidazo[2,1‑b][1,3,4]thiadiazole compound had certain antibacterial activity, which expanded the application range of α‑pinene.
Owner:GUANGXI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

![Synthesis method of benzo[e]pyridylimidazole[4,5-g]isoindole-1,3(2H)-diketone compound Synthesis method of benzo[e]pyridylimidazole[4,5-g]isoindole-1,3(2H)-diketone compound](https://images-eureka.patsnap.com/patent_img/30cb4ee4-c5c1-4ea1-9928-05f448ea955b/FDA0002746304620000011.png)
![Synthesis method of benzo[e]pyridylimidazole[4,5-g]isoindole-1,3(2H)-diketone compound Synthesis method of benzo[e]pyridylimidazole[4,5-g]isoindole-1,3(2H)-diketone compound](https://images-eureka.patsnap.com/patent_img/30cb4ee4-c5c1-4ea1-9928-05f448ea955b/BDA0002746304630000021.png)
![Synthesis method of benzo[e]pyridylimidazole[4,5-g]isoindole-1,3(2H)-diketone compound Synthesis method of benzo[e]pyridylimidazole[4,5-g]isoindole-1,3(2H)-diketone compound](https://images-eureka.patsnap.com/patent_img/30cb4ee4-c5c1-4ea1-9928-05f448ea955b/BDA0002746304630000022.png)

![Synthetic method of naphtho [2, 1-d] oxazole compound Synthetic method of naphtho [2, 1-d] oxazole compound](https://images-eureka.patsnap.com/patent_img/8cfc73d5-1714-4d2b-b5d4-b848e1abeffb/HDA0003659669950000011.png)
![Synthetic method of naphtho [2, 1-d] oxazole compound Synthetic method of naphtho [2, 1-d] oxazole compound](https://images-eureka.patsnap.com/patent_img/8cfc73d5-1714-4d2b-b5d4-b848e1abeffb/HDA0003659669950000012.png)
![Synthetic method of naphtho [2, 1-d] oxazole compound Synthetic method of naphtho [2, 1-d] oxazole compound](https://images-eureka.patsnap.com/patent_img/8cfc73d5-1714-4d2b-b5d4-b848e1abeffb/HDA0003659669950000021.png)
![A kind of synthetic method of myrtle alkenyl imidazo[2,1-b][1,3,4]thiadiazole compound A kind of synthetic method of myrtle alkenyl imidazo[2,1-b][1,3,4]thiadiazole compound](https://images-eureka.patsnap.com/patent_img/031e23d0-e4c8-4931-a296-a6eb3eda5917/BDA0001658495900000021.png)
![A kind of synthetic method of myrtle alkenyl imidazo[2,1-b][1,3,4]thiadiazole compound A kind of synthetic method of myrtle alkenyl imidazo[2,1-b][1,3,4]thiadiazole compound](https://images-eureka.patsnap.com/patent_img/031e23d0-e4c8-4931-a296-a6eb3eda5917/BDA0001658495900000022.png)
![A kind of synthetic method of myrtle alkenyl imidazo[2,1-b][1,3,4]thiadiazole compound A kind of synthetic method of myrtle alkenyl imidazo[2,1-b][1,3,4]thiadiazole compound](https://images-eureka.patsnap.com/patent_img/031e23d0-e4c8-4931-a296-a6eb3eda5917/BDA0001658495900000041.png)