Thiophene macrocyclic compound and preparation method for its derivant
A technology for macrocyclic compounds and cyclic compounds, applied in the direction of organic chemistry, etc., can solve the problem of no progress in the preparation method of thiophene twelve-membered cyclic compound 2, and achieve the effects of improving the preparation yield and increasing the solubility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0049] The preparation method of the present invention is as follows:
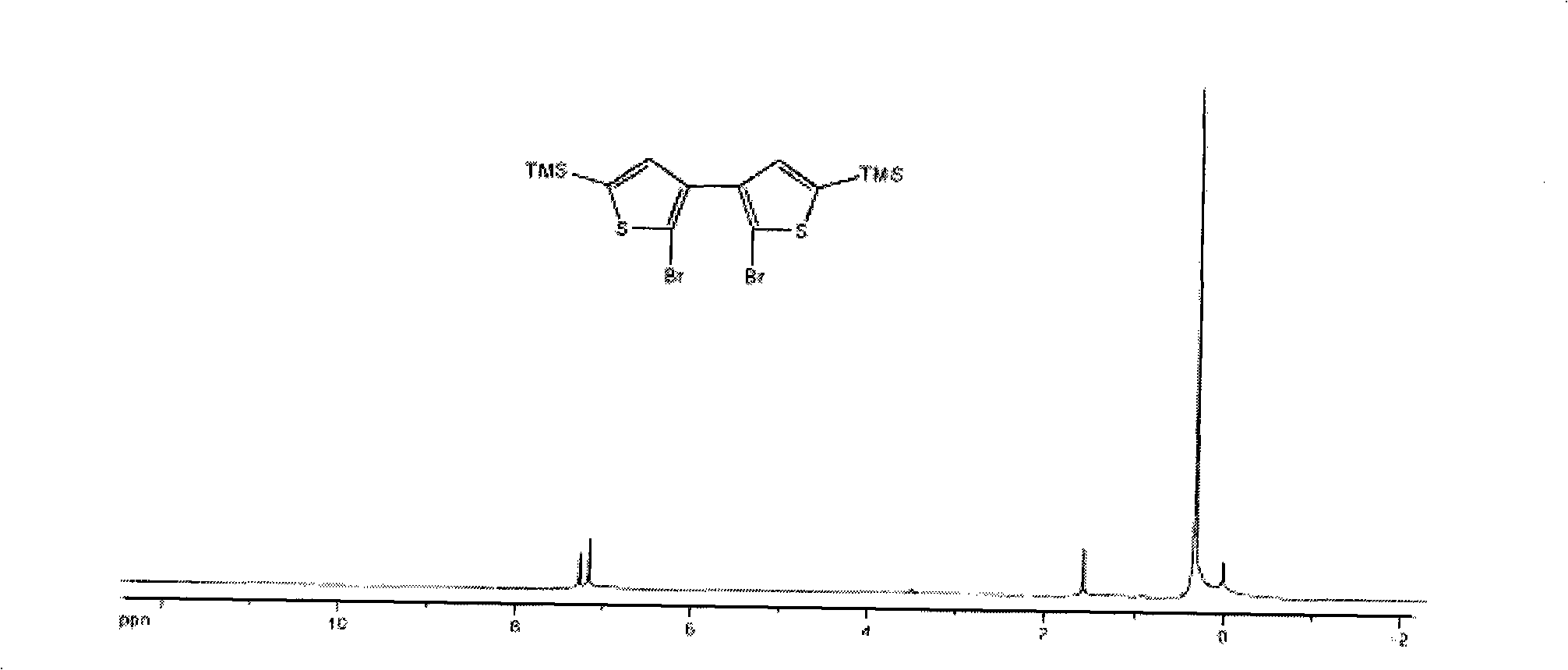
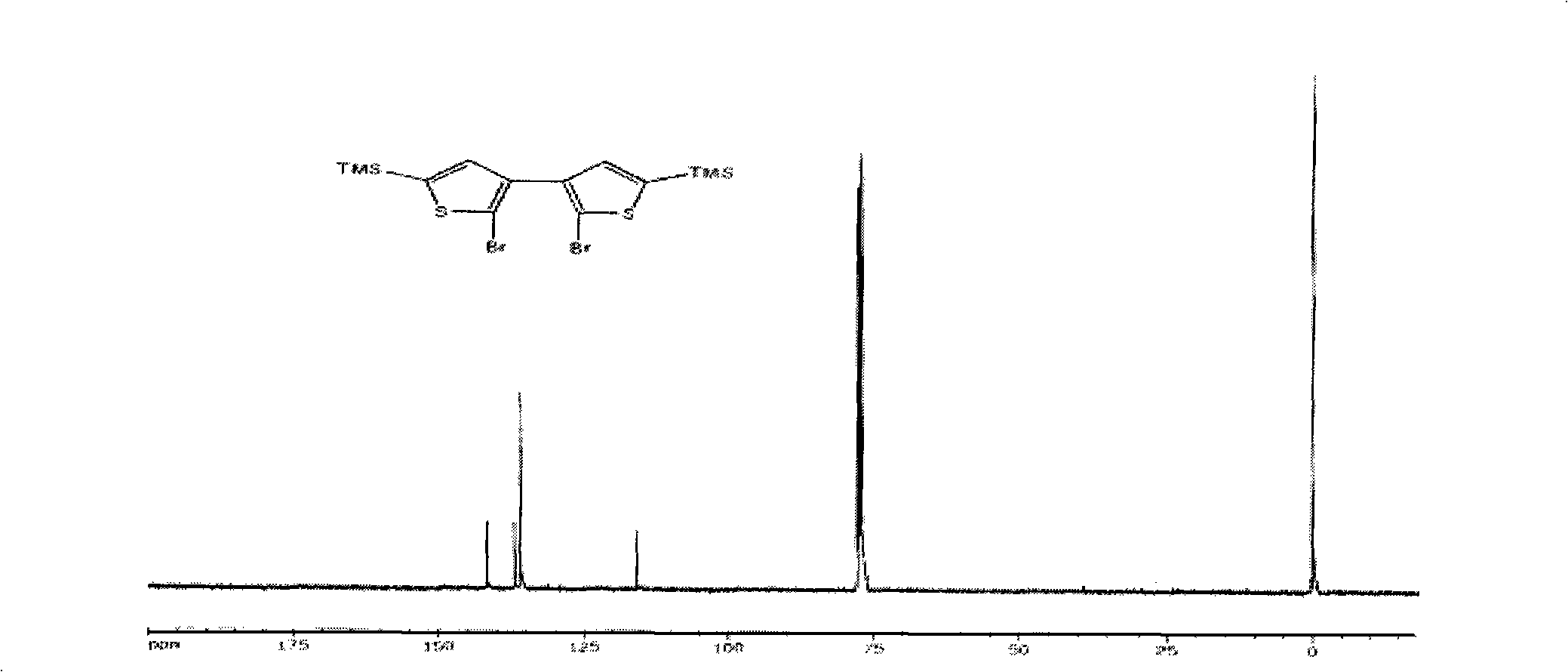
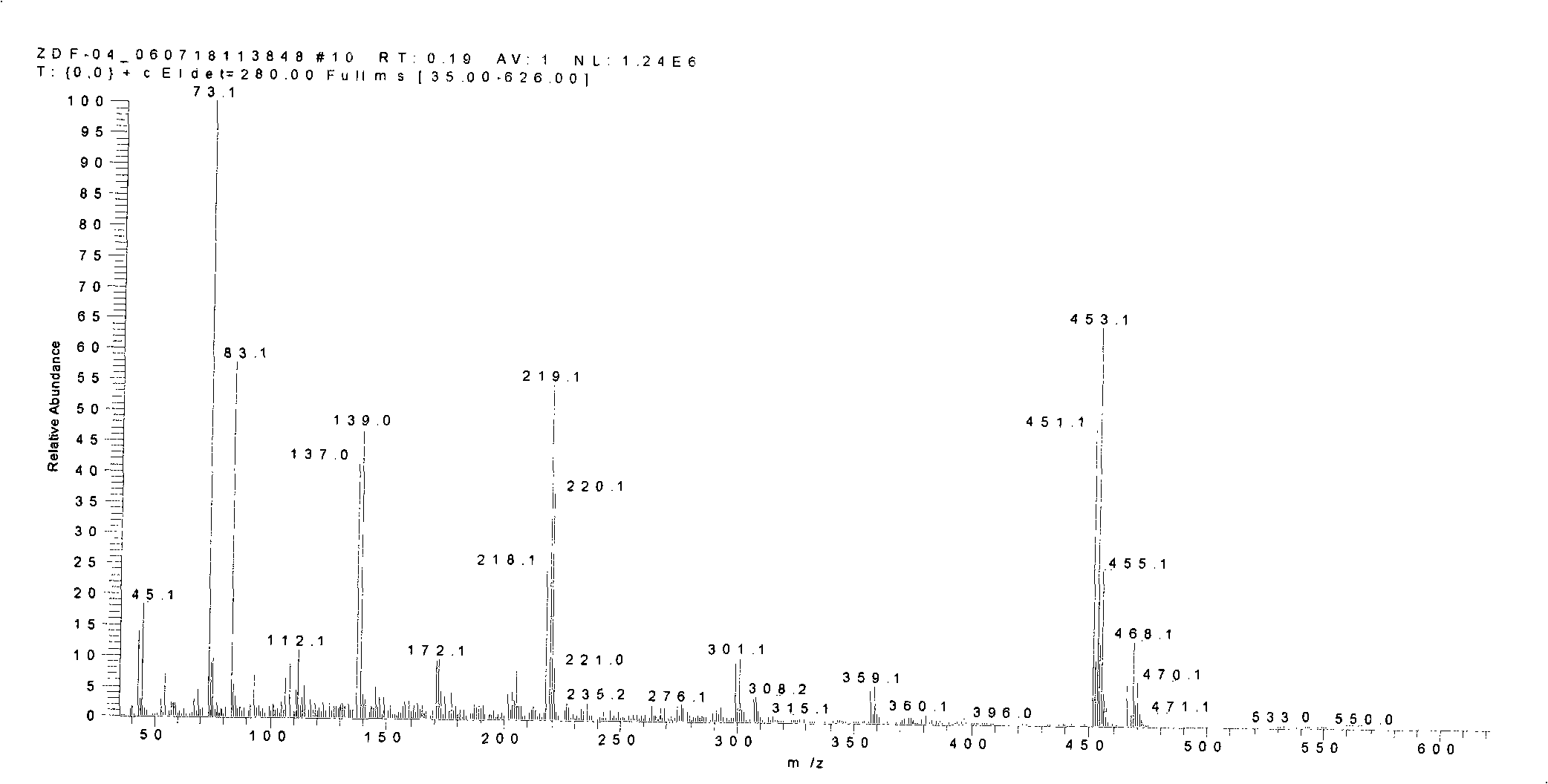
[0050] (1) Preparation of compound 3 (2,2'-dibromo-5,5'-bis(trimethylsilyl)-[3,3']-bithiophene)
[0051] First add 37.5mmol of n-butyllithium to 38.9mmol of diisopropylamine in ether solution, prepare LDA solution at 0℃, and add this LDA solution dropwise to 2,2'-dibromo-[ 3,3']-Bithiophene (5.44g, 16.8mmol) was reacted in ether solution for 2 hours. The temperature of the reaction solution was lowered to -70-80°C, 85.1 mmol of trimethylchlorosilane was dropped into the reaction solution, and the temperature was slowly raised to room temperature. The reaction system was quenched with water, extracted with ether, washed with water, and dried, and then the crude product was subjected to silica gel column chromatography using petroleum ether (60-90° C.) as the eluent to isolate compound 3 (7.00 g, 89.1%). The conditions of the other two parallel reactions are as follows: 4.44 g and 5.79 g of raw materials are use...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com