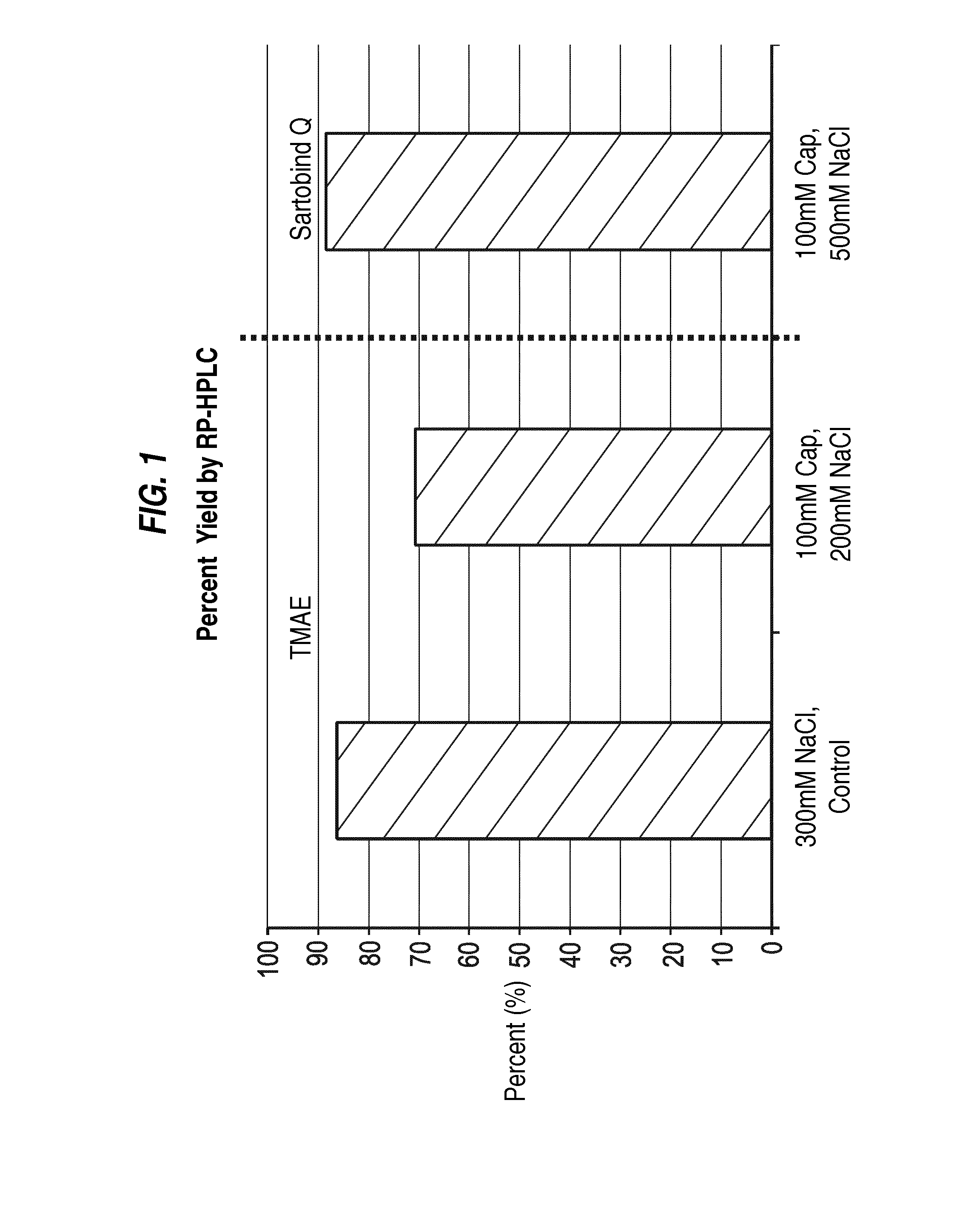
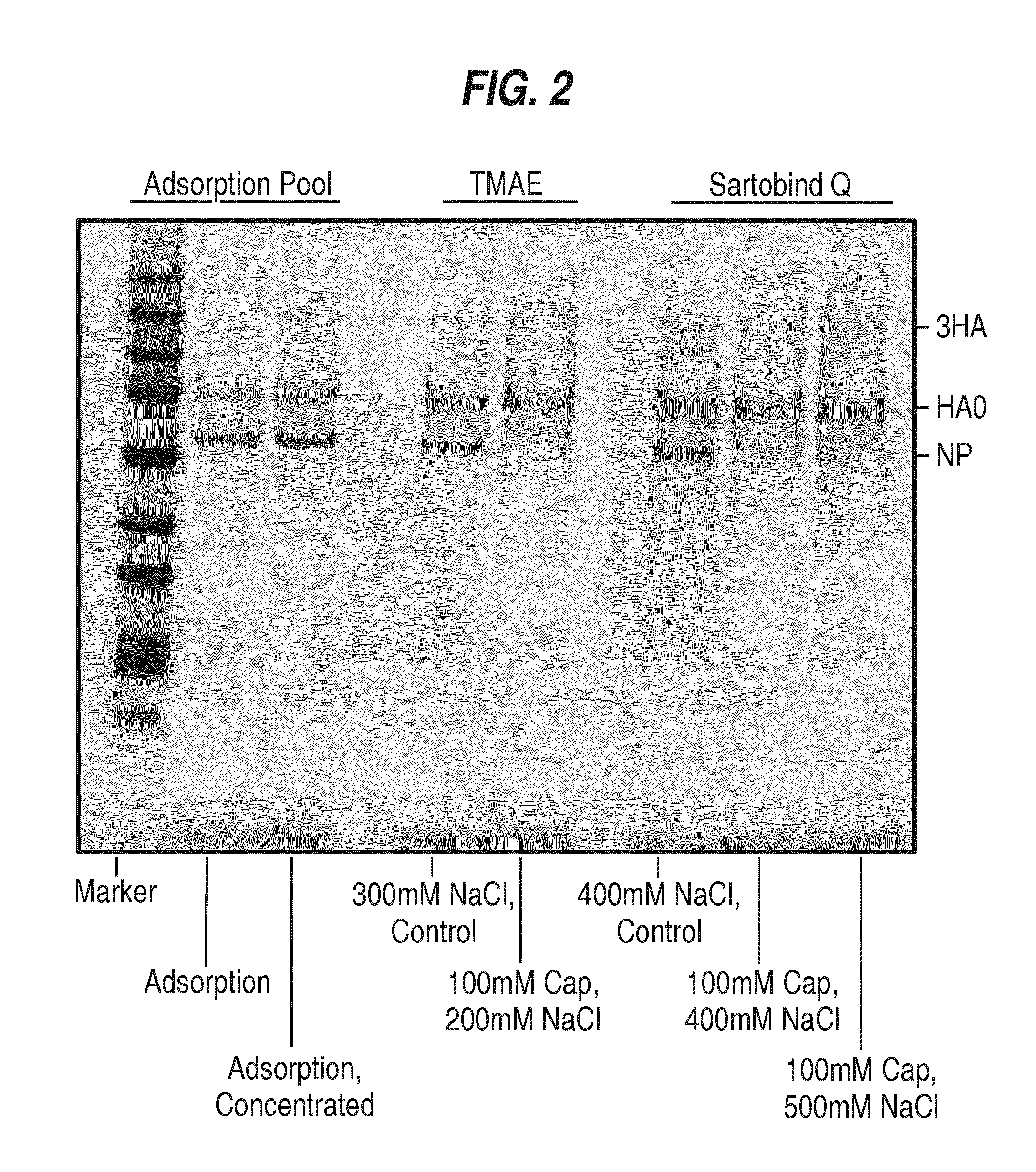
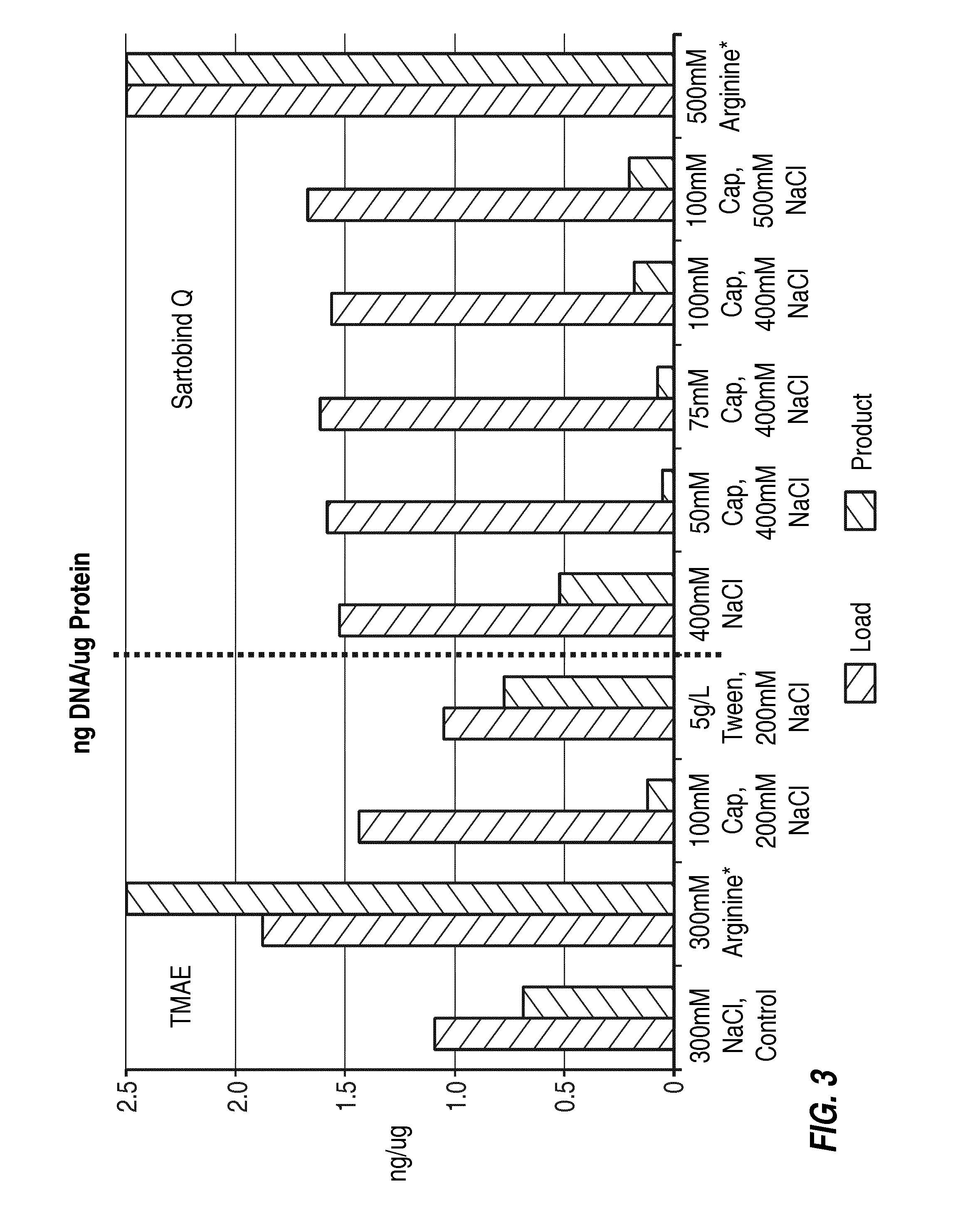
Removal of residual cell culture impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment 1
2. The method of embodiment 1, wherein the first solution is selected from the group consisting of: cell or tissue lysates, culture media, cell culture supernatants, plasma, and partially purified protein solutions.
3. The method of any one of the preceding embodiments, wherein the protein of interest is selected from the group consisting of: therapeutic proteins, immunogenic proteins (e.g., viral antigens), and antibodies or antigen-binding fragments thereof.
4. The method of any one of the preceding embodiments, wherein the anionic detergent is selected from the group consisting of: fatty acid detergents.
5. The method of any one of the preceding embodiments, wherein the anionic detergent is different from any other detergent used in a process of protein purification.
6. The method of any one of the preceding embodiments, wherein the anionic detergent does not include deoxycholate and / or sodium lauryl sulfate.
7. The method of any one of the preceding embodiments, wherein the ion excha...
embodiment 17
18. The method of embodiment 17, wherein the sterile closed system is selected from the group consisting of: vials, syringes, and containers.
19. The method of embodiment 17 or 18, wherein the sterile closed system is plastic or glass.
20. The method of any one of embodiments 17-19, wherein the sterile closed system comprises a siliconized surface.
21. A use of the pharmaceutical composition of any one of embodiments 12-20, for the manufacture of a medicament for administering a subject in need thereof.
22. The pharmaceutical composition of any one of embodiments 12-20 for use as a medicament for administering to a subject.
23. A method comprising administering to a subject the pharmaceutical composition of any one of embodiments 12-20.
24. A viral vaccine comprising no more than 5 ng of residual DNA and no more than 1.0 μg nucleoprotein per dose.
embodiment 24
25. The viral vaccine of embodiment 24, comprising no more than 1 ng of residual DNA and no more than 0.5 μg nucleoprotein per dose.
26. The viral vaccine of embodiment 24, comprising no more than 1 ng of residual DNA and no more than 0.1 μg nucleoprotein per dose.
27. The viral vaccine of any one of embodiments 24-26, wherein the viral vaccine is an influenza vaccine.
28. The viral vaccine of any one of embodiments 24-27, further comprising an adjuvant.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
| Mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com