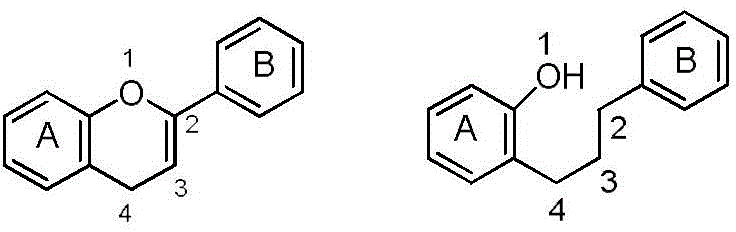
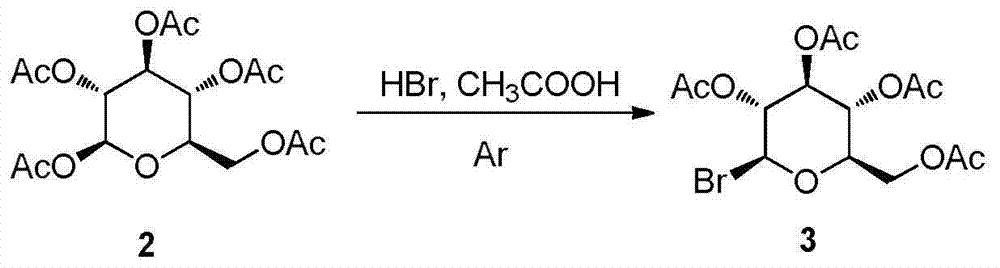
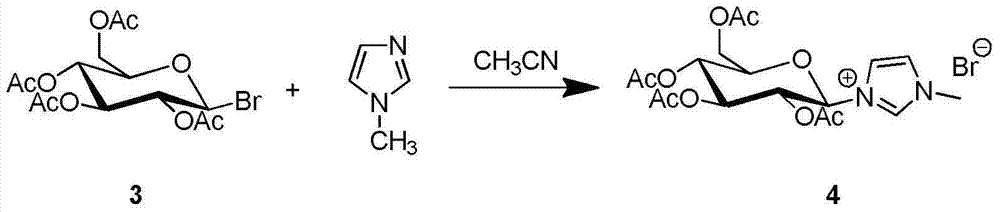
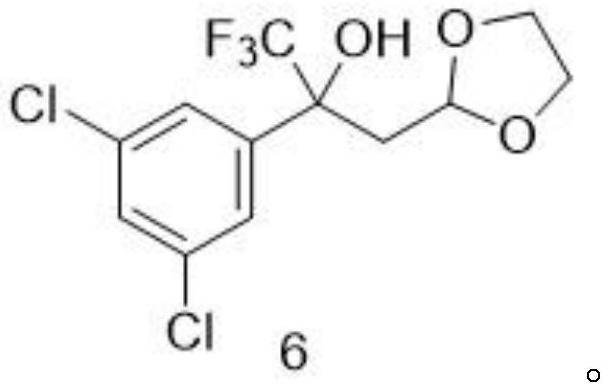
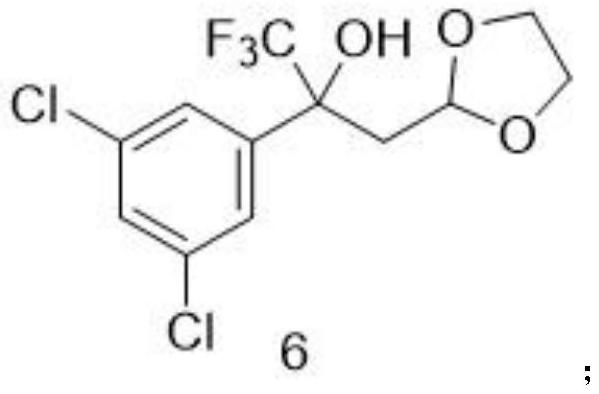
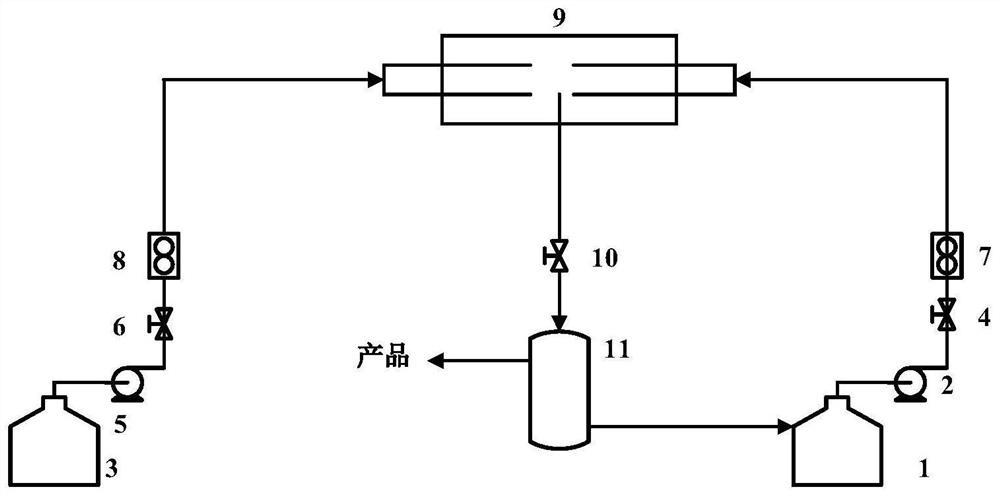
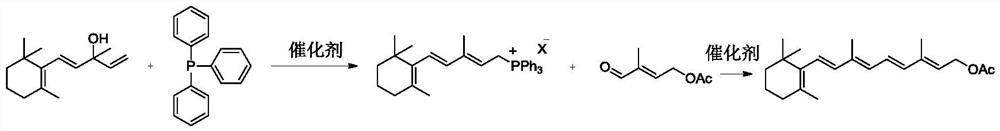
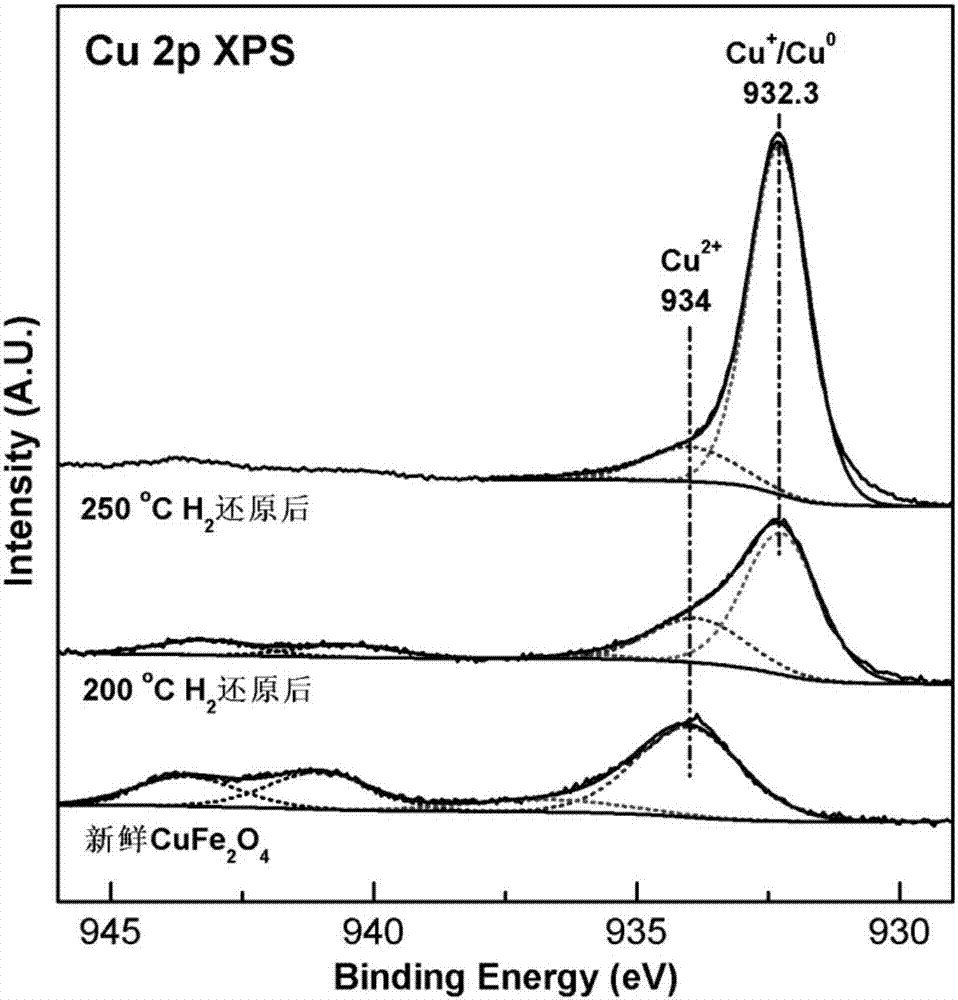
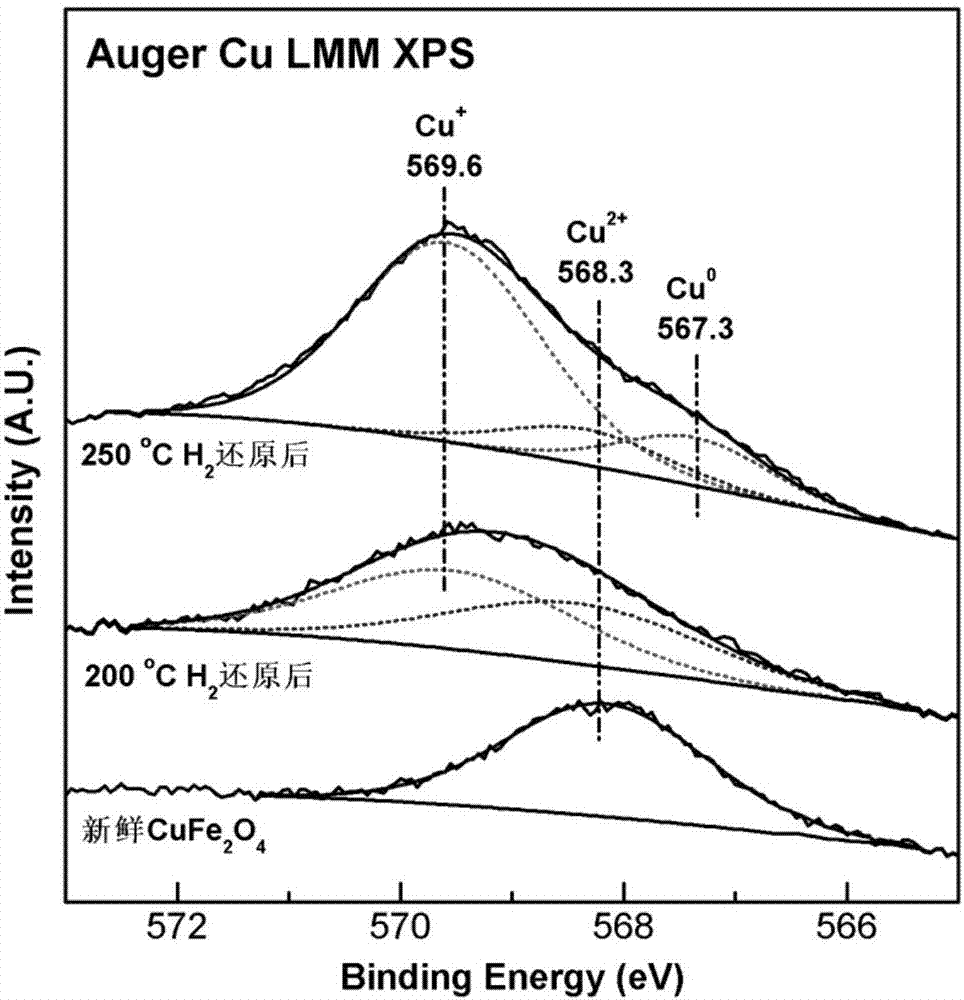
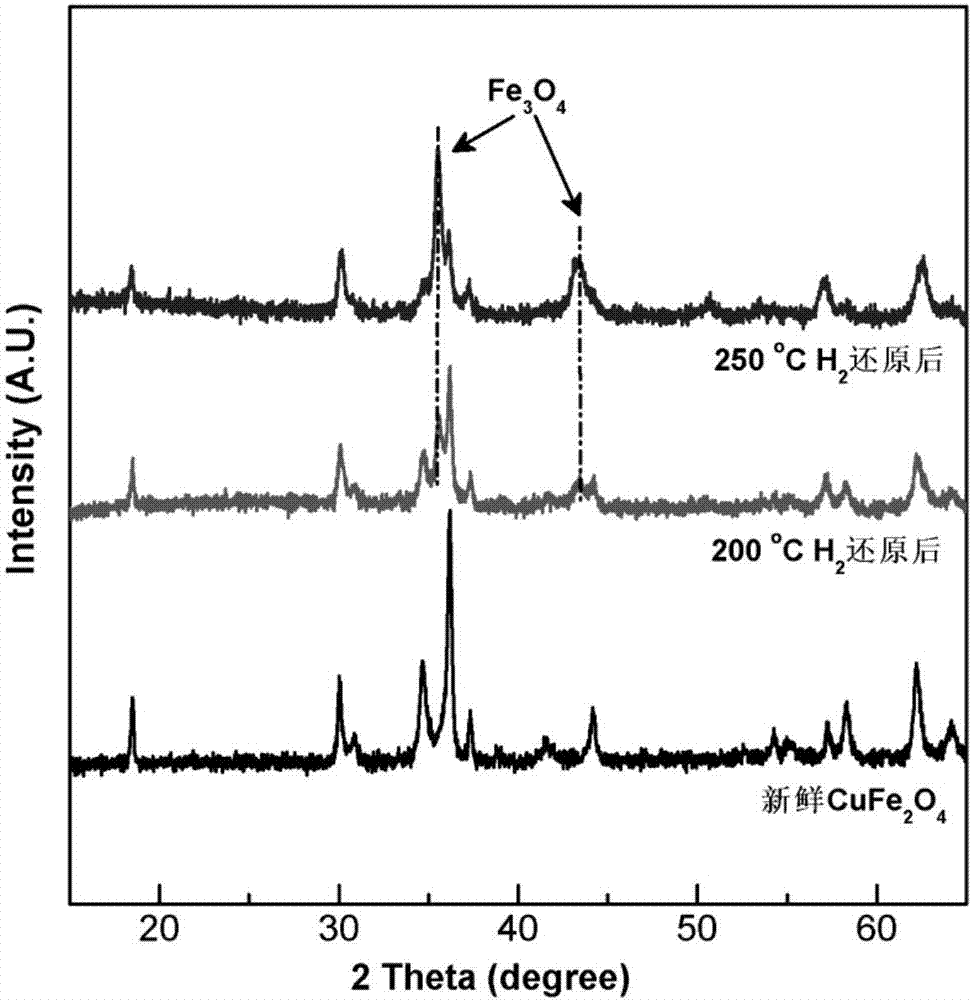
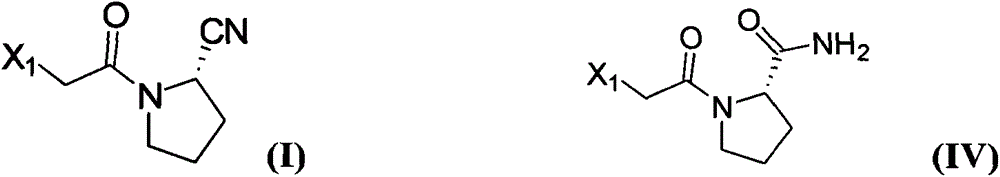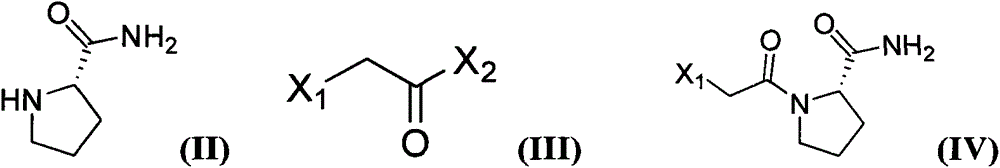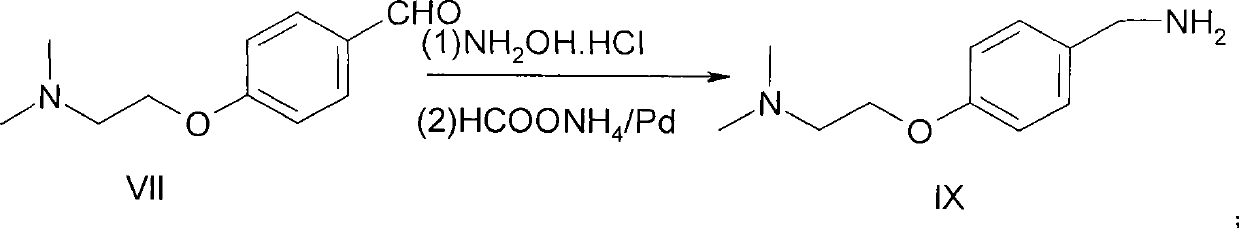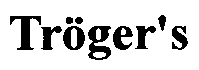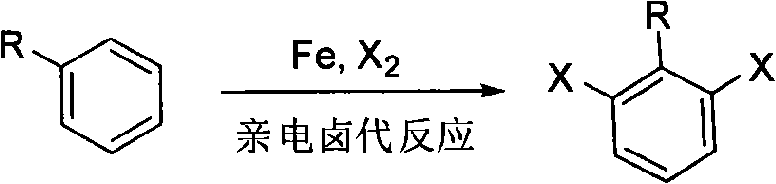Patents
Literature
88results about How to "Response to environmental protection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
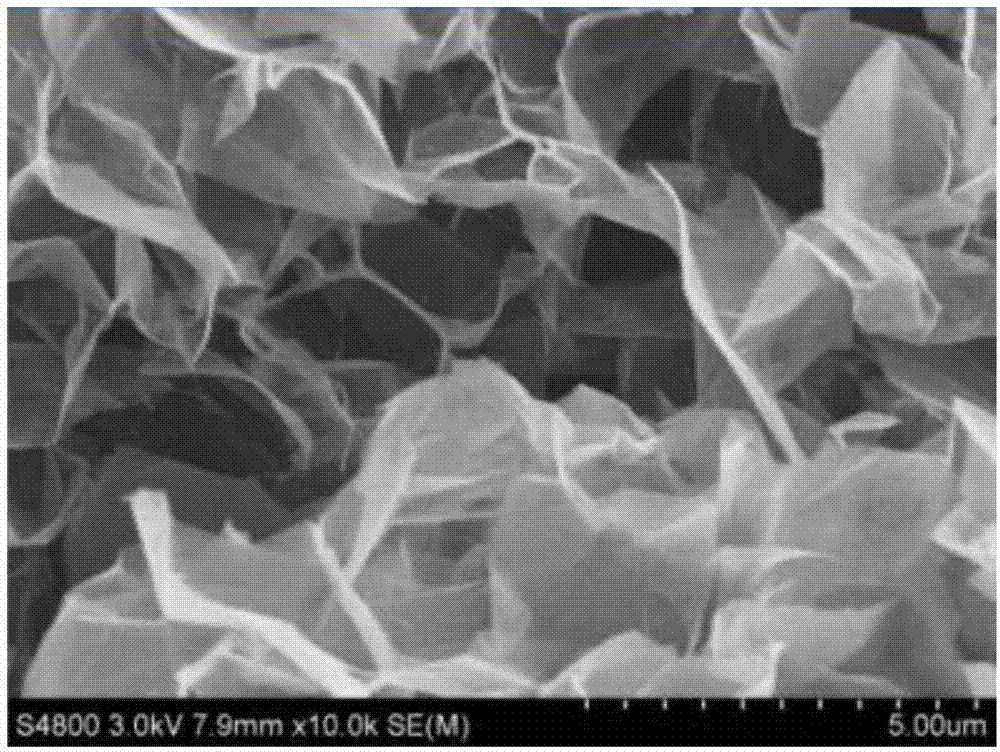
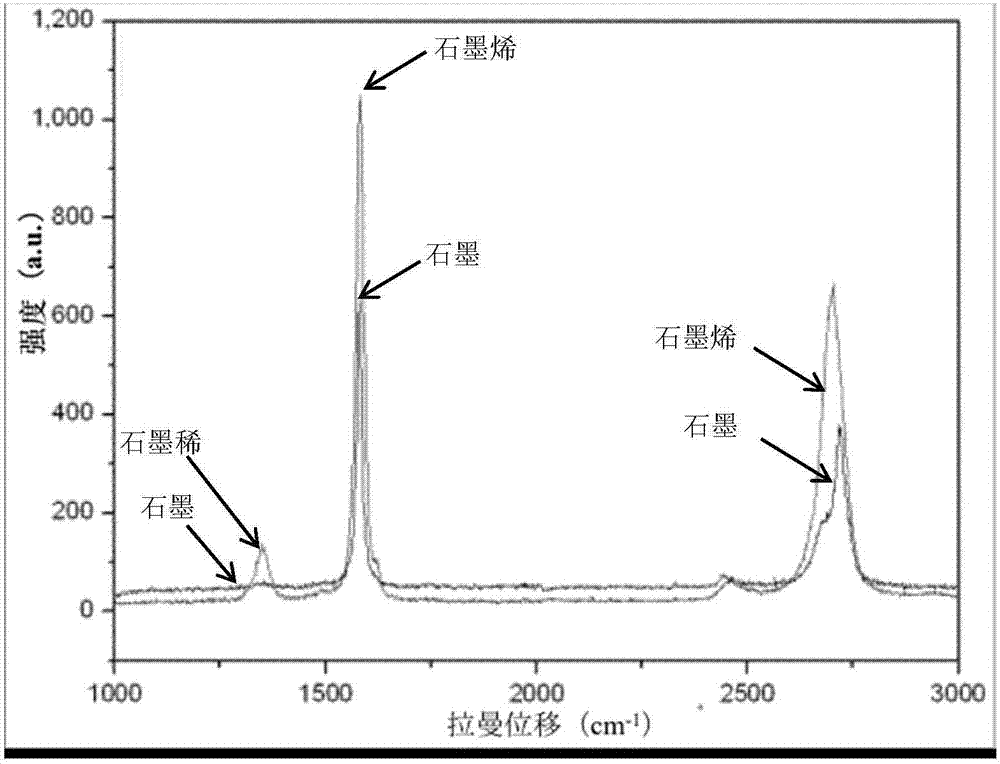
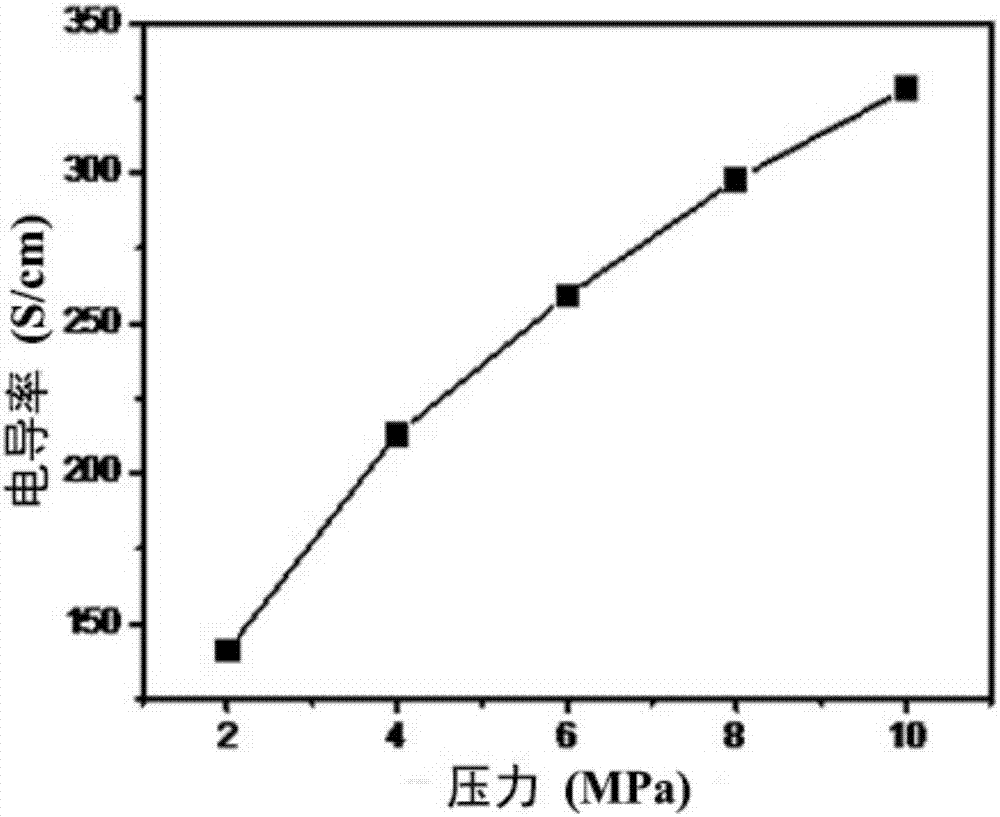
Peeling method of layered material and high-quality peeling material obtained by peeling
The invention discloses a peeling method of a layered material and a high-quality peeling material obtained by peeling. The method comprises the following steps: 1) mixing the layered material and anintercalating agent to achieve infiltration of the layered material by the intercalating agent; 2) adding an activating agent to allow the activating agent reacted with the intercalating agent, so that the physical impact of a generated gas flow on the layered material causes the sheet layers of the layered material to peel, thereby resulting in a dispersion solution of a peeling material precursor; and 3) mixing the obtained dispersion solution of the peeling material precursor with a dispersion medium, and performing physical disintegration on the obtained dispersion system so as to obtain adispersion solution of the peeling material. According to the peeling method, the gas flow compact generated by the violent reaction of the intercalating agent and the activating agent which are intercalated on the sheet layer surfaces and at the interlaminations is used to overcome a force between the sheet layers to realize expansion peeling, so that the peeling material with less layer and high quality is obtained; and the process is simple, the production cycle is short, and high efficiency mass production of the peeling material can be realized.
Owner:哈尔滨万鑫石墨谷科技有限公司
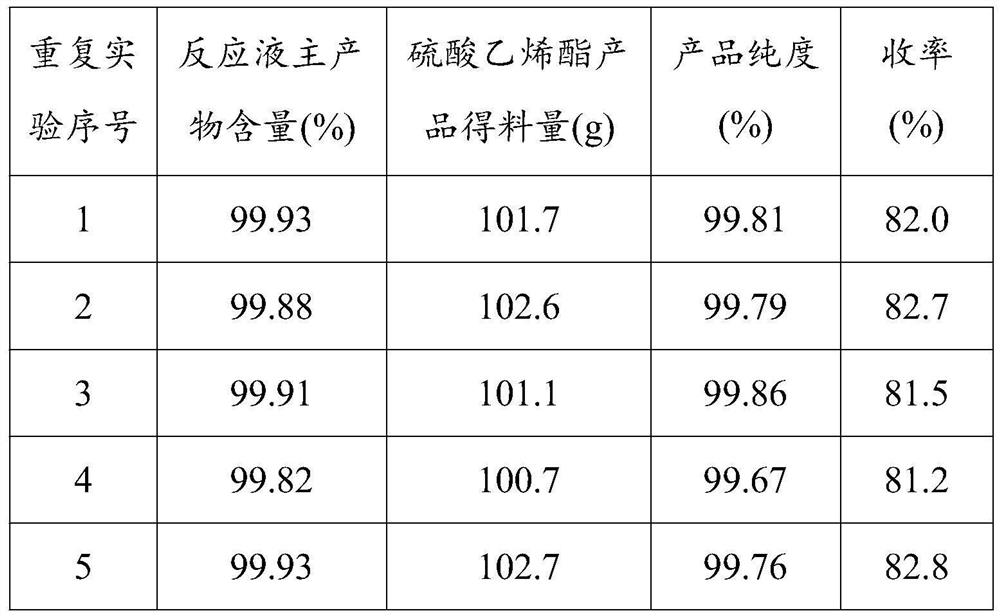
Method for preparing cyclic sulfate by directly oxidizing hydrogen peroxide
PendingCN111909129ALess impuritiesHigh purityOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsPtru catalystCatalytic oxidation
The method comprises the following steps: dropwise adding hydrogen peroxide into a mixture of cyclic sulfite, an organic solvent and a solid catalyst to carry out catalytic oxidation reaction, filtering out the solid catalyst after the reaction is finished, standing filtrate for layering, taking an organic layer, and performing distilling and concentrating to obtain a cyclic sulfate product. Cheaphydrogen peroxide is used for directly catalyzing and oxidizing cyclic sulfite to prepare cyclic sulfate, so that on one hand, the reaction is mild and easy to control, and the reaction conversion rate is high; on the other hand, no waste salt is generated, the evaporation capacity of water is small, energy consumption is low, generated waste water is little, and the production process is more environmentally friendly; the used solid catalyst contains an active component, an active auxiliary agent and an oxide carrier, and can be recycled, so that the consumption of noble metals is reduced, and the production cost is greatly reduced; the cyclic sulfate prepared by the method is few in impurities, high in purity and wide in market prospect.
Owner:CHANGSHU CHANGJI CHEM +1
One-step reaction method for preparing 4-nitropyridine-nitrogen oxide and halogenated-4-nitropyridine-nitrogen oxide
InactiveCN1743313AShort reaction timeShorten the oxidation reaction timeOrganic chemistryAcetic anhydrideNitration
This invention relates to a preparation method for 4-nitropyridines-nitrogenoxides and halogenated-4-nitropyridines-nitrogenoxides by one step, merging the separated oxidation and nitration into a single step. In the oxidation reaction, acetic anhydride, concentrated sulfuric acid, maleic anhydride and sodium bisulphate are added as catalyser; in nitration reaction, sodium nitrate is added to accelerate reaction. This method shortens the reaction time, especially the oxidation time reduced by half. After nitration reaction, 30~50% sodium hydroxide, instead of solid sodium carbonate, is used to neutralize the resultant solution to directly get the nitration resultants. In this method, the yield is more than 10% higher than that of routine methods and the production cost is cut down meanwhile.
Owner:ZHANJIANG NORMAL UNIV
Method for preparing composite fibers in ionic liquid medium
ActiveCN105544004ASimple preparation processResponse to environmental protectionConjugated cellulose/protein artificial filamentsVegetal fibresFiberFreeze-drying
The invention discloses a method for preparing composite fibers in an ionic liquid medium. The method comprises the following steps: (1) oxidating cellulose into carboxyl cellulose in an oxidation system prepared from ionic liquid, nitric acid and NaNO2, and preparing a carboxyl cellulose solution; (2) subjecting silkworm cocoons to soda boiling, degumming, LiBr dissolving, dialysis and vacuum freeze drying so as to obtain silk fibroin, and then, preparing a silk fibroin solution in ionic liquid; (3) grafting the carboxyl cellulose to the silk fibroin in ionic liquid, thereby obtaining the composite fibers. According to the method, the ionic liquid serves as a solvent, cellulosic materials and the silkworm cocoons serve as raw materials, the chemical principle of amidation reaction is adopted, the preparation process is simple, the reaction is green, environment-friendly and pollution-free, the ionic liquid solvent can be recovered and reused through rotary-evaporation drying, and the recovery rate reaches 98%. The reaction system only needs an isothermal reaction of 1 to 2 hours, the percentage of grafting of the cellulosic materials reaches 21% to the maximum, and the composite fibers are excellent in mechanical properties, crinkle resistance, wear resistance and hygroscopic property and are degradable and reproducible.
Owner:JIANGSU UNIV OF SCI & TECH
Synthesis method of 2,8-diaryl(amino) Troger's base derivatives
ActiveCN104926819AAddressing a severe lack ofEasy and quick to getOrganic chemistryDigestive systemHepg2 cellsSynthesis methods
The invention discloses a synthesis method of 2,8-diaryl(amino) Troger's base derivatives, belonging to the synthesis method of the Troger's base derivatives. The 2,8-diaryl(amino) Troger's base derivatives comprise 2(8)-triarylpyridyl (tripyridyl) Troger's base (TB) derivative, 2,8-diaryl Troger's base (TB) derivative and 2-aryl-8-amino Troger's base (TB) derivative; the synthesis method adopts Suzuki reaction to respectively introduce triarylpyridyl, tripyridyl, phenyl, phenanthryl or anthryl fragment into the TB skeleton, and Ullmann reaction to introduce carbazole, diphenylamine or phenothiazine into the TB skeleton, to obtain the new 2,8-diaryl(amino) Troger's base (TB) derivatives. Four products with excellent human liver cancer HepG2 cell activity resistance are selected from all products with human liver cancer HepG2 cell activity resistance. The method introduces phenothiazine, carbazole and other fragments with physiological activities into the TB skeleton, to design and synthesize 2,8-diaryl(amino) Troger's base derivatives for the first time, so as to screen compounds with liver cancer activity resistance through the combination of both.
Owner:南京百鑫德诺生物科技有限公司
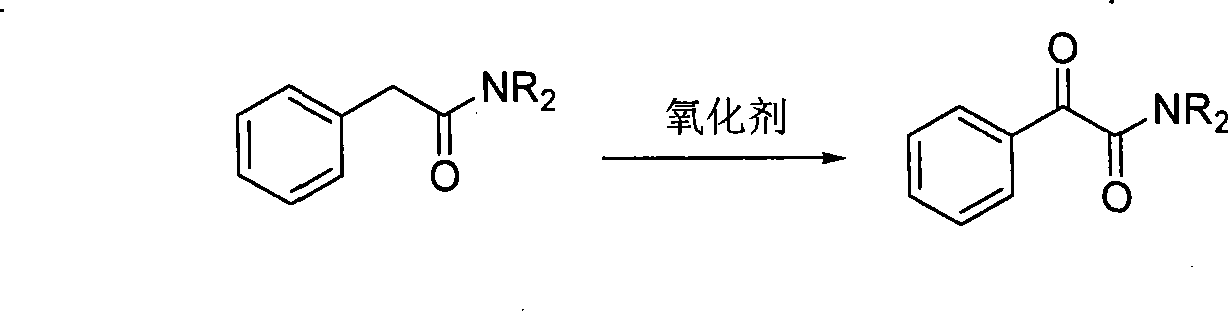
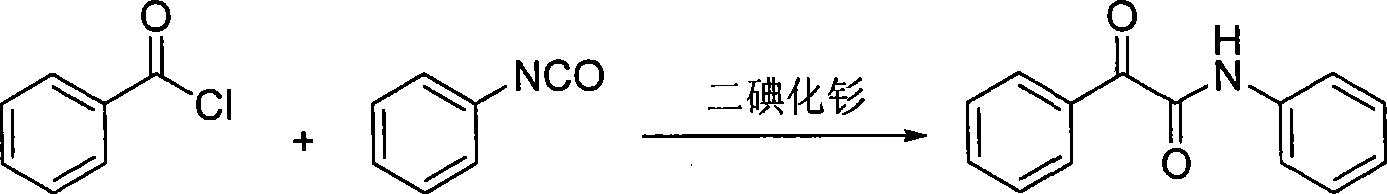
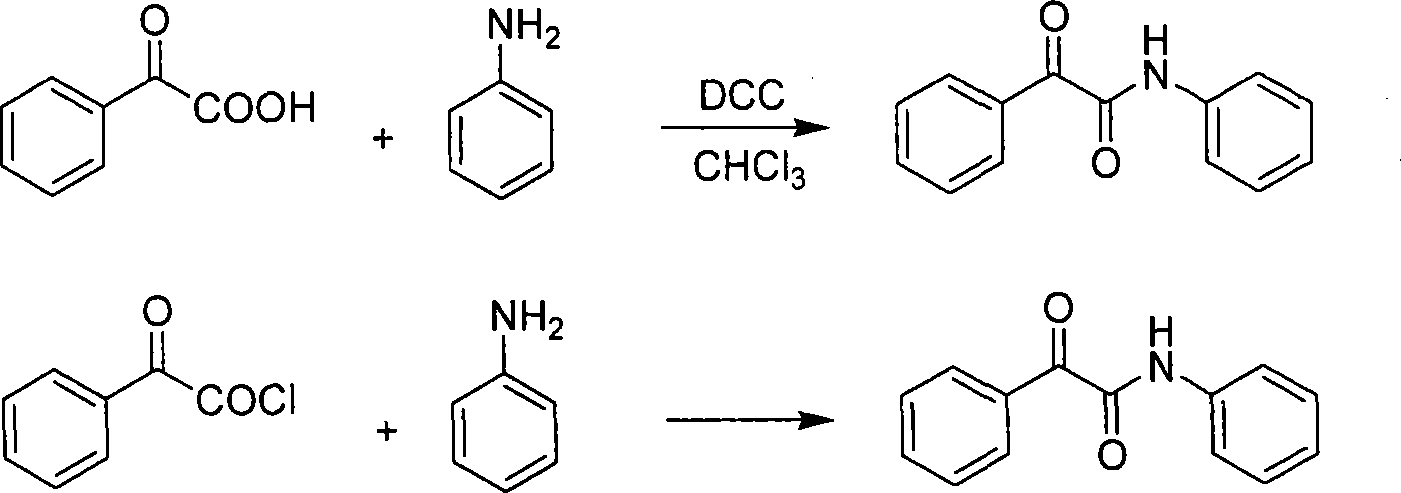
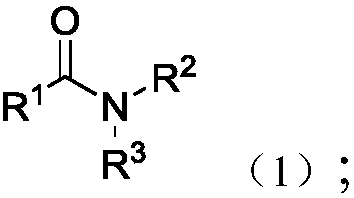
Synthesis method for alpha-carbonylamide compound
InactiveCN101121692ARaw materials are easy to getEasy to operateOrganic chemistryProduction rateAcetic acid
The invention relates to a synthesis method for the alpha-carbonyl amide compound. The specific steps of the method are: dissolve the amide, the carbonic cesium of the amount of the catalyst and the additive of the amount of the catalyst into the DMF and communicate the solution with the air through the drying pipe; at the temperature of 100 to 120 Celsius system, the material is heated and reacted until the raw material amide disappears; the ethyl acetate is added into the reaction system to dilute; then the method is to filter to remove the solvent in the filter liquid and to get the crude product; after the purification, the required alpha-carbonyl amide can be got. The raw material of the invention is easy to get; the operation is quite simple; the conditions are mild; the invention is suitable for the large-scale industrial production; the air is used as the green oxidant; the reaction is of the environmental protection, and highly efficient; the production rate can reach to 93 percent.
Owner:SHANGHAI UNIV
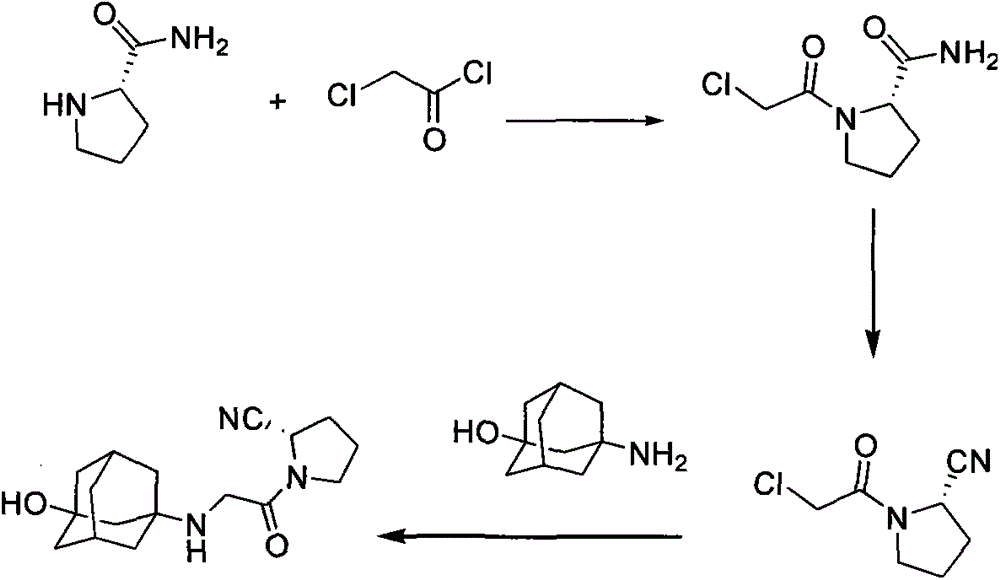
Method for preparing substituted (S)-pyrrolidine-2-formonitrile and vildagliptin
The invention provides a method for preparing (S)-1-(2-halogenated acetyl)pyrrolidine-2-formonitrile as shown in the formula (I). Optionally in the presence of a diluent, (S)-1-(2-halogenated acetyl)pyrrolidine-2-formamide and a dehydrating agent propanephosphonic acid cyclic anhydride (T3P) react with each other. The invention also provides a method for preparing vildagliptin by using S-prolinamide involving the above reaction. The method for preparing the compound as shown in the formula (I) has the following advantages: use of expensive trifluoroacetic anhydride is not required, yield is increased, and cost is reduced; use of cyanuric chloride prepared from highly toxic raw materials is not required, and the reaction is more environmentally friendly; and an improved method for preparing vildagliptin is then obtained. In the formula (I) and formula (II), X1 is halogen.
Owner:HYBIO PHARMA
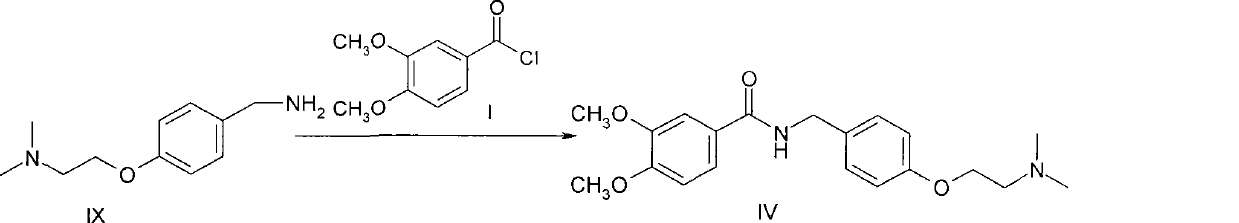
Preparation method of itopride hydrochloride
ActiveCN102993038ALow priceEasy to buyOrganic compound preparationCarboxylic acid amides preparationBenzoyl chlorideEther
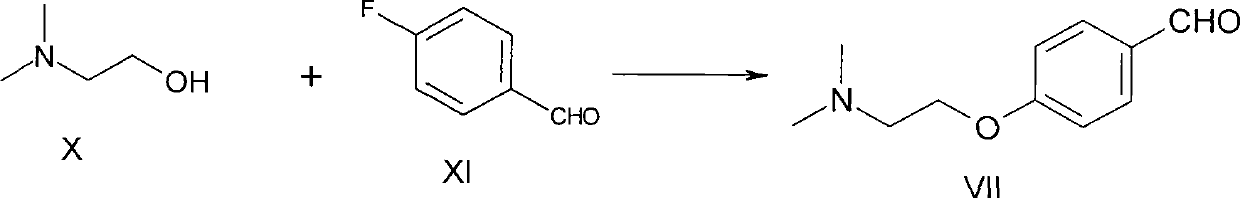
The invention relates to a preparation method of gastrointestinal crude drug itopride hydrochloride, and belongs to the field of medicines. The invention discloses a method for preparing itopride hydrochloride, which comprises the following steps of: taking cheap N,N-dimethylaminoethanol as an initial raw material; implementing an etherification reaction to obtain an intermediate product (VII) and implementing one-step reduction ammoniation to obtain a benzylamine product (IX); and reacting with 3,4-dimethoxy benzoyl chloride to generate hydrochloride. The preparation method of the itopride hydrochloride is cheap in raw material, moderate in reacting condition, and low in preparation cost of the itopride hydrochloride.
Owner:迪嘉药业集团股份有限公司
Method for synthesizing chiral Tr*ger's base derivatives
InactiveCN104804006AMild reaction conditionsEasy to operateSugar derivativesOrganic-compounds/hydrides/coordination-complexes catalystsSolventD-Glucose
The invention relates to a new synthesis method of chiral Tr*ger's base derivatives. According to the synthesis method, a chiral ionic liquid, 1-pyridine-3,4,6-tri-acetyl glucopyranose tetrafluoroborate, with glucose being a chiral source, which is used as a catalyst, and a solvent undergo direct one-step catalytic synthesis to obtain the series of chiral Tr*ger's base derivatives. According to the invention, the novel sugar-containing chiral ionic liquid, 1-pyridine-3,4,6-tri-acetyl glucopyranose tetrafluoroborate, is used as a catalyst. The method provided by the invention is green and environmental friendly, is simple and economical, and can be adopted to efficiently prepare the Tr*ger's base derivatives with high yield and high optical purity by one step.
Owner:XUZHOU NORMAL UNIVERSITY +1
Synthesis method for TB derivatives with human liver cancer HepG2 cell resisting activity
ActiveCN104892616AAddressing a severe lack ofEasy and quick to getOrganic chemistryAntineoplastic agentsSynthesis methodsChalcone
The invention discloses a synthesis method for TB derivatives with human liver cancer HepG2 cell resisting activity, belonging to a synthesis method for TB derivatives. The synthesis method comprises the steps of carrying out aldol condensation reaction on different formyl-substituted TB derivatives and o-acetylphenol compounds to obtain TB-chalcone compounds; and carrying out condensation reaction on different formyl-substituted TB derivatives and diaminobenzene under the catalysis of silicon sulfoacid (SSA) to obtain TB-benzimidazole compounds, and controlling different reaction conditions by taking the TB-chalcone compounds as raw materials to obtain a series of TB-flavonoid compounds. The activities of two different types of TB derivatives resisting a human liver cancer HepG2 cell are primarily researched, and six products with favorable human liver cancer HepG2 cell resisting activity is screened, so that the basis is laid for developing the TB derivatives into novel anti-cancer drugs. The synthesis method has the advantages of relatively high reaction yield, mild reaction condition, simplicity in operation, very short reaction time, high yield, simplicity and convenience in aftertreatment, environment-friendly reaction, high economic efficiency, high efficiency and extremely high actual application value.
Owner:GUANGDONG CELL BIOTECHNOLOGY CO LTD
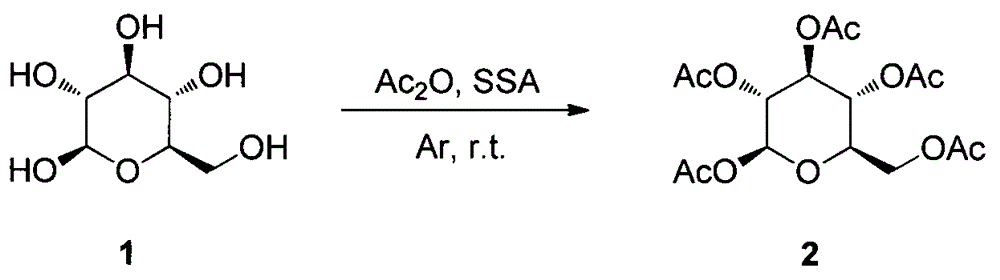
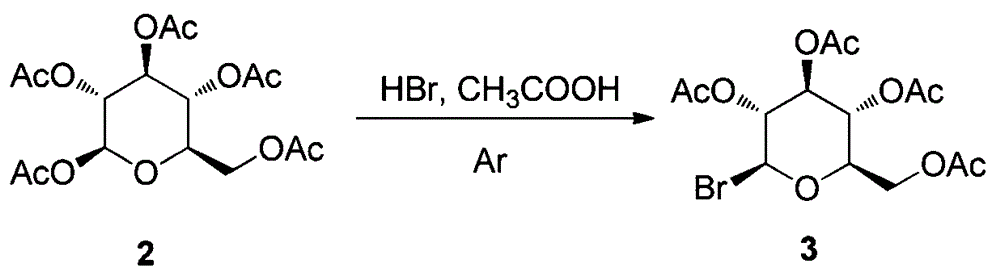
Synthesis and uses of beta-1-imidazole-2,3,4,6-tetrasulfo-D-glucopyranose hydrosulfate
ActiveCN104761601AEasy accessHigh catalytic activitySugar derivativesOrganic-compounds/hydrides/coordination-complexes catalystsChemical synthesisChlorosulfuric acid
The invention relates to synthesis and uses of beta-1-imidazole-2,3,4,6-tetrasulfo-D-glucopyranose hydrosulfate and belongs to hydrosulfate chemical synthesis by multi-component reactions and uses of the hydrosulfate. The synthesis includes: subjecting glucose 1 that is adopted as a raw material to acetylation to obtain a compound 2; performing bromination by adopting the compound 2 as a raw material to obtain a compound 3; reacting the compound 3 with 1-methylimidazole to obtain a compound 4; hydrolyzing the compound 4 under alkaline conditions to obtain a target compound 5; and reacting the compound 5 with chlorosulfonic acid to obtain a target product 6 that is the beta-1-imidazole-2,3,4,6-tetrasulfo-D-glucopyranose hydrosulfate. A five-component reaction of 2 equivalents of an aromatic aldehyde, 2 equivalents of an arylamine and 1 equivalent of ethyl acetoacetate is performed by adopting the target product 6 as a catalyst at 40 DEG C under mild conditions to synthesize an ethyl 4-phenylamino-1-phenyl-1,2,5,6-tetrahydro-2,6-diphenylpyridine-3-carboxylate derivative. The synthesis and the uses are characterized in that: (1) the product 10 is obtained rapidly with a high yield; (2) reaction conditions are mild, operation is simple, reaction time is short, the yield is high and after-treatment is simple and convenient; and (3) the synthesis is green, economical and efficient.
Owner:江西省德兴市百勤异VC钠有限公司
Method for preparing formamide compound by catalyzing carbon dioxide hydrogenation with porous material
ActiveCN111205198AStable in natureLarge specific surface areaIndium organic compoundsOrganic compound preparationPtru catalystOrganic synthesis
The invention belongs to the technical field of organic synthesis and heterogeneous catalysis, and particularly relates to a method for preparing a formamide compound by catalyzing carbon dioxide hydrogenation through a porous material. The method comprises the following steps: by taking a porous organic metal polymer as a catalyst, reacting an amine compound with carbon dioxide and hydrogen in anair atmosphere to prepare the formamide compound. The method has the advantages of high reaction efficiency, good selectivity, mild conditions, economy, environmental protection, simple operation andthe like; wherein a solid metal polymer material with large specific surface area, strong carbon dioxide adsorption, hierarchical pore channel distribution and highly dispersed metal centers is designed and synthesized as a reaction catalyst by changing a cross-linked copolymer proportion; the catalyst is especially used for catalytic synthesis of fine chemical N, N-dimethylformamide (DMF), doesnot need any additional solvent, alkali or other additives, and is convenient for separation and purification of DMF. The catalyst can be recycled; no special equipment is needed in the reaction, thereaction operation is simple, and further industrial application is facilitated.
Owner:FUDAN UNIV
CuO base composite material and preparation method thereof
InactiveCN108355655AExcellent ability to store electronsGood electron transport propertiesCatalyst activation/preparationMetal/metal-oxides/metal-hydroxide catalystsSolubilityPhotocatalytic reaction
The invention provides a CuO base composite material and a preparation method thereof. Since CQDs (Carbon Quantum Dots) have excellent electron storage ability and upconversion fluorescence property and can form water solubility protection on the surface of the material, a CuO nanometer structure can be prevented from conglobation in a preparation process; the CuO has an excellent electron transmission property and visible-light response, CQDs aqueous solution is used for carrying out product post-processing, and a stable CQDs protection and fluorescence conversion layer is formed on the surface of the CuO and is also favorable for improving the current carrier separation and transmission of the composite material in a light-catalyzed reaction process and reducing the recombination rate after the current carrier is separated; in addition, the pi-pi mutual action between the conjugated structure of the CQDs between benzene is favorable for enriching benzene on the surface of the composite material, two ingredients are cooperated to efficiently degrade organic gas pollutants (the degradation efficiency of benzene can be 93%) under visible light.
Owner:苏州宝澜环保科技有限公司
Microwave synthesis method for diarylamine compound
InactiveCN103214385ARealize sustainable development and utilizationShort reaction timeOrganic compound preparationAmino-carboxyl compound preparationMicrowave methodIsomerization
The invention discloses a microwave synthesis method for a diarylamine compound. The method comprises the following steps of: subjecting 3-methyl dehydroshikimic acid and a primary arylamine compound to a condensation reaction, an isomerization reaction and a dehydration reaction under the action of an organic solvent, a catalyst and microwaves to enable a hexatomic ring framework to be aromatized; and then, cooling a reaction liquid, pouring the reaction liquid into saturated brine, rapidly stirring the solution, separating out a solid, and carrying out suction filtration, drying and recrystallization to obtain a 3-amido-4-hydroxybenzoic acid methyl ester compound. The raw material 3-methyl dehydroshikimic acid adopted in the method is a non-aromatic compound and can be prepared from shikimic acid by using a simple and convenient method without depending on fossil resources; sustainable exploitation and utilization can be realized; in addition, the microwave synthesis method adopts a microwave method which is short in reaction time, simple and convenient in operation, convenient in after-treatment and high in yield; and the microwave synthesis method is clean in reaction, friendly to the environment and low in energy consumption.
Owner:GUANGZHOU CHEM CO LTD CHINESE ACADEMY OF SCI
Method for preparing fluralana intermediate, prepared intermediate and application of fluralana intermediate
ActiveCN113461653ALow costLow priceOrganic chemistryAgainst vector-borne diseasesPtru catalystGrignard reagent
A method for preparing a fluralana intermediate, provided by the invention, comprises the following steps: carrying out nucleophilic addition reaction on 1-(3, 5-dichlorophenyl)-2, 2, 2-trifluoroethanone, carrying out acidolysis, reacting with a Grignard reagent, carrying out oxidation, carrying out oxidation cyclization reaction, reacting with hydroxyl(toluenesulfonyloxy)benzene iodide, and reacting with a trimethoxyphosphorus solution to finally generate the fluralan. In the preparation process, a catalyst and dimethoxy zinc are not needed, ultralow-temperature reaction conditions of -78 DEG C are not needed, operation is easy and convenient, meanwhile, reagents such as ozone and dimethyl sulfide which are harmful to the environment are replaced, and the method is more environmentally friendly. The invention also provides the intermediate for preparing the fluralan.
Owner:LUOYANG HUIZHONG ANIMAL MEDICINE
Preparation method of vitamin A acetate
ActiveCN112876395AEasy to operateReduce processOrganic chemistryOrganic-compounds/hydrides/coordination-complexes catalystsD-alpha-Tocopheryl AcetatePtru catalyst
The invention discloses a preparation method of vitamin A acetate. Thepreparation method is characterized in that raw materials including vinyl ionol, an organic phosphine compound and pentacarbonaldehyde react under the action of a catalyst to generate vitamin A acetate, and the vitamin A acetate is synthesized by adopting a one-step method. According to the invention, the problems of considerable byproducts, serious equipment corrosion and the like caused by a liquid acid catalyst in a reaction are avoided; and meanwhile, the defects of large dosage of a base catalyst, difficulty in waste alkali treatment and the like are overcome.
Owner:WANHUA CHEM GRP CO LTD
Visible light catalyst containing copper oxide and application thereof
ActiveCN107983349AThe preparation method is simple and diverseEasy to useOrganic compound preparationOrganic chemistry methodsAlkali freeOrganic synthesis
The invention belongs to the field of photocatalysis organic synthesis and more in particular relates to a visible light catalyst containing copper oxide and application thereof to catalytic synthesisof 1,3-butadiyne. The catalyst is AxCuyBzOn, wherein A is a non-valence-variable metal ion and B is a valence-variable metal ion; in a preparation process, hydrogen treatment is carried out for 0.5 to 5h at 150 to 300 DEG C so that a Cu<+> active center is enriched on the surface of the catalyst; under the irradiation of visible light, room-temperature, alkali-free and efficient photocatalysis terminal alkyne with the repeatedly usable catalyst is coupled to synthesize a 1,3-butadiyne compound. The method is simple to operate; when the photocatalyst is used, the terminal alkyne, which is cheap and easy to obtain, is subjected to photocatalytic oxidation to obtain the 1,3-butadiyne compound with high yield under conditions of room temperature, normal oxygen atmosphere, ethanol solvent andirradiation of the visible light or sunlight; alkali does not need to be additionally added in reaction so that the visible light catalyst is green and environmentally friendly, and large-scale production is easy to realize.
Owner:HUAZHONG UNIV OF SCI & TECH
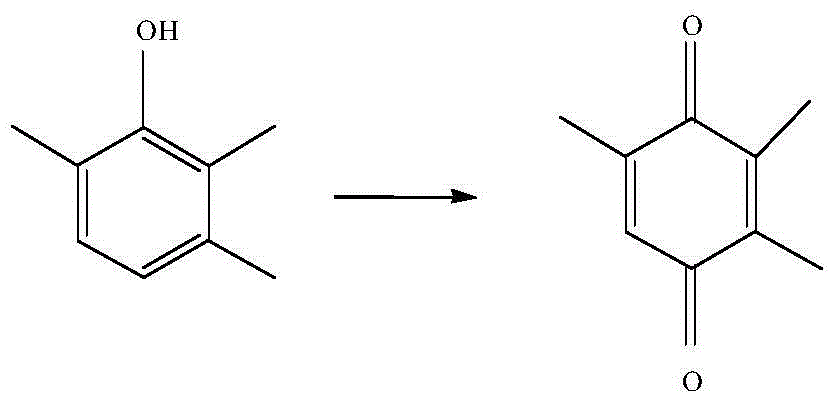
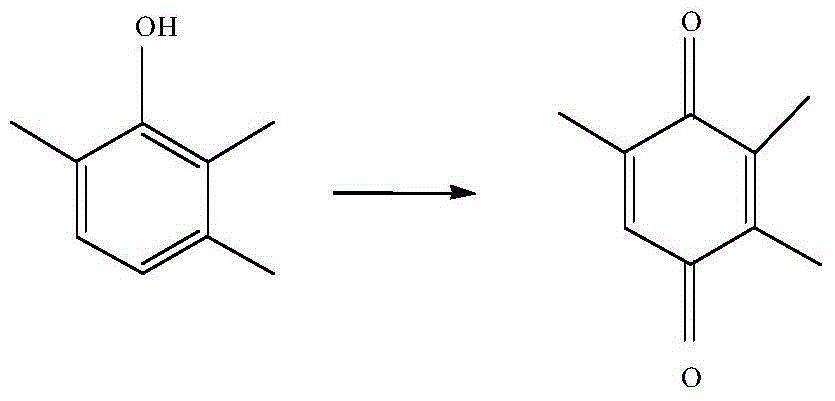
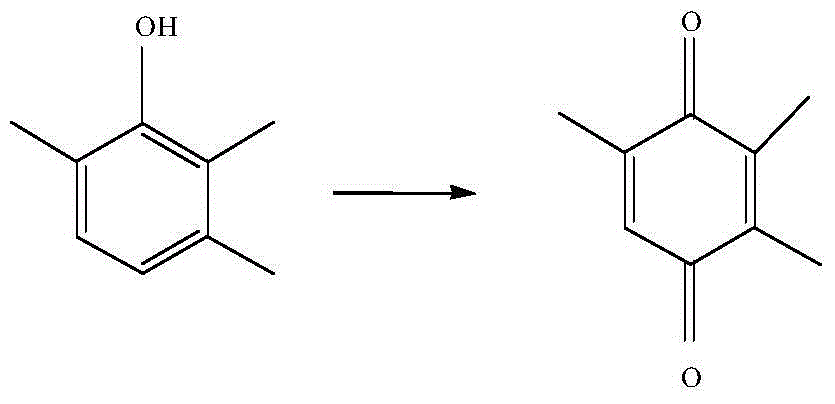
Vanadium catalyst and method utilizing vanadium catalyst to synthesize 2,3,5-trimethylbenzoquinone
InactiveCN104399507AHigh activityLow pricePhysical/chemical process catalystsQuinone preparation by oxidationVanadium CompoundsActivated carbon
The invention relates to a vanadium catalyst and a method utilizing the vanadium catalyst to synthesize 2,3,5-trimethylbenzoquionone. The carrier of the catalyst is active carbon, and the active component is metal vanadium, which is coated by a carbon-nitrogen material. The carbon-nitrogen material is formed by calcining a raw material namely dicyandiamide in an inert atmosphere. The preparation method of the catalyst comprises the following steps: dissolving a vanadium compound into a soluble solvent, then impregnating active carbon into the solvent, adding raw materials, drying, and then calcining in an inert atmosphere so as to obtain the catalyst coated by a carbon-nitrogen material. The catalytic synthesis of 2,3,5-trimethylbenzoquinone comprises the following steps: taking 2,3,6-trimethylphoenl as the raw material, adding an organic solvent, the vanadium catalyst, and hydrogen peroxide, and then carrying out reactions at a specific temperature so as to obtain the 2,3,5-trimethylbenzoquinone. The catalyst preparation method has the advantages of simpleness, cheap raw material, and suitability for industrial production. Moreover, the prepared catalyst has a very good activity and selectivity in the catalytic synthesis of 2,3,5-trimethylbenzoquinone, and can be easily recycled.
Owner:NANJING UNIV OF TECH
Method used for reduction of tertiary amide into alcohols and/or amines
ActiveCN110054538AHigh yieldSimple and safe operationOrganic reductionIsotope introduction to heterocyclic compoundsSilanesAlkali metal
The invention discloses a method used for reduction of tertiary amide into alcohols and / or amines. The method comprises following steps: tertiary amide, an alkali metal reagent, and a proton donor agent are added into an organic solvent for a following reaction selectively: when the proton donor agent is a raw material alcohol and / or inorganic salt aqueous solution, the reaction product is an alcohol compound and / or tertiary amine compound. The method is capable of realizing selective reduction of tertiary amide into alcohols and tertiary amine compounds, the yield is high, the suitable rangeis wide, operation is safe and simple, the adopted raw materials are cheap and easily available; no precious metal catalyst, toxic silanes, and flammable and combustible metal hydrides are adopted; notoxic by product is generated; reaction is more friendly to the environment; problems in the prior art that amide compound reducing method operation is complex, conditions are strict, and control ofproducts is difficult are solved.
Owner:CHINA AGRI UNIV
Quinone-catalyzed trifluoromethylation photocatalytic synthesis method
ActiveCN105503510AReduce pollutionEasy to makeOrganic-compounds/hydrides/coordination-complexes catalystsCatalytic reactionsTrifluoromethylationQuinone
The invention discloses a quinone-catalyzed trifluoromethylation photocatalytic synthesis method. According to the method, the using problem of highly-corrosive and highly-toxic fluorinating reagents such as hydrogen fluoride and sulfur tetrafluoride in an existing industrial trifluoromethylation reaction is solved, the heavy metal pollution problem of a catalyst in industry is avoided, and the good actual application prospect is achieved. The metal-free photocatalytic trifluoromethylation reaction is achieved by taking simplest and cheapest benzoquinone and derivatives thereof as a photocatalyst and taking a Langlois reagent-sodium trifluoromethanesulfinate (CF3SO2Na) as a trifluoromethyl source under the mild visible light condition, and a complete full-cycle reaction is achieved through a homemade fixed bed reactor. Trifluoromethyl modification can be further performed on organic molecules with the bioactivity and the pharmaceutical activity according to the actual needs. The method has the advantages that the synthesis condition is mild, the raw materials are cheap and easy to obtain, the environmental pollution is low, large-scale industrial production is facilitated, and the significant economic and social benefit is achieved.
Owner:FUZHOU UNIV
Synthetic method of beraprost
InactiveCN111116530AImprove protectionAvoid reactionOrganic chemistry methodsCombinatorial chemistryHydrolysis
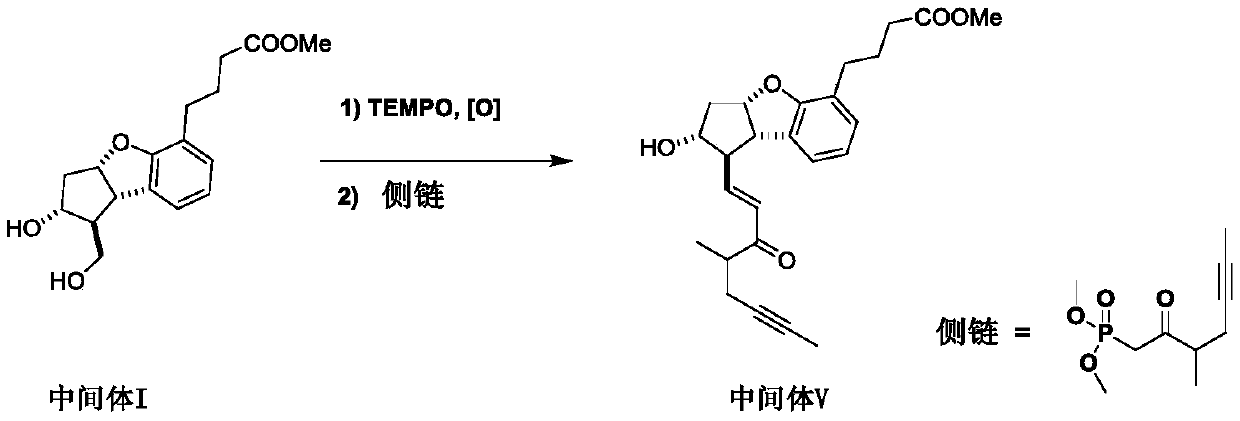
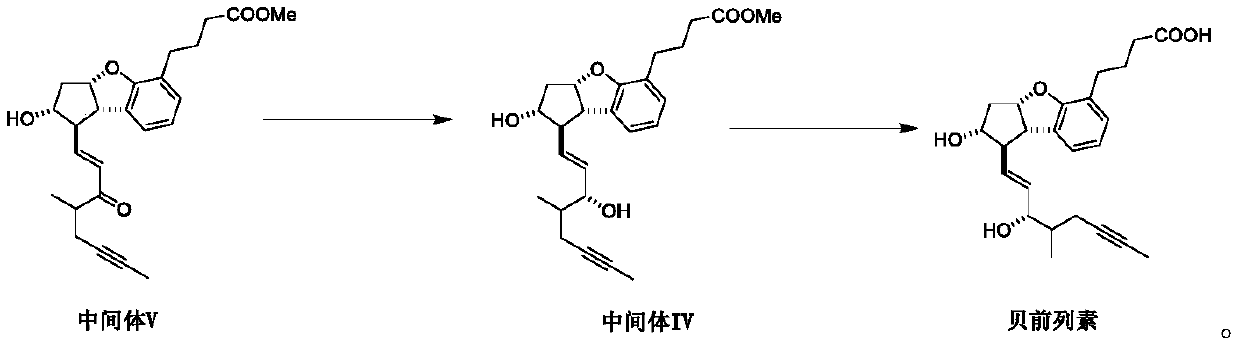
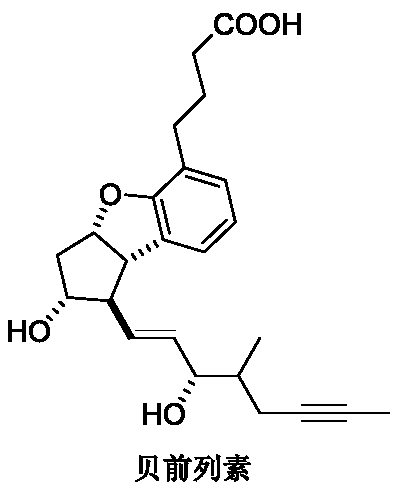
The invention relates to a synthetic method of beraprost. The synthetic method comprises the following steps: with an intermediate I as an initial raw material, carrying out selective primary alcoholoxidation and a Witting reaction to obtain an intermediate V; carrying out reduction and column chromatography purification on the intermediate V to obtain an intermediate IV; and hydrolyzing the intermediate IV to obtain beraprost. According to the synthetic method disclosed by the invention, two hydroxyl groups of the intermediate I are selectively oxidized, so a hydroxyl protection reagent is prevented from being used; in the oxidation step, ultralow-temperature (-60 to -80 DEG C) reactions and use of a reagent DCC with relatively high toxicity are avoided; in the reduction step, diisobutylaluminum hydride is prevented from being used; process operation units are greatly reduced, reaction steps are shortened, emission of three wastes is reduced, and the reactions are more efficient andenvironment-friendlier; and the main peak content of the prepared beraprost reaches 99.0% or above, and total process yield reaches 26% or above. The invention provides the synthetic method which ismore beneficial for industrial production.
Owner:JINAN KANGHE MEDICAL TECH
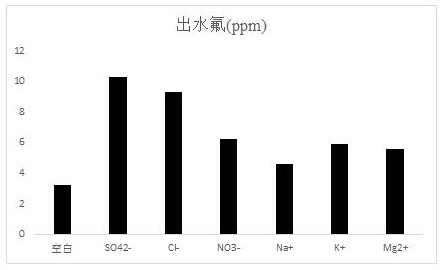
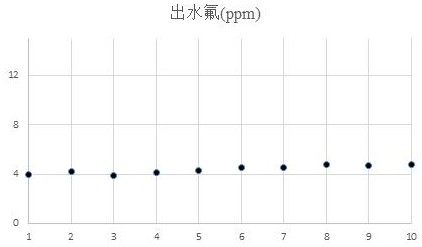
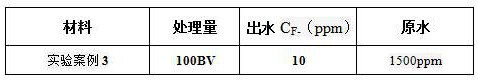
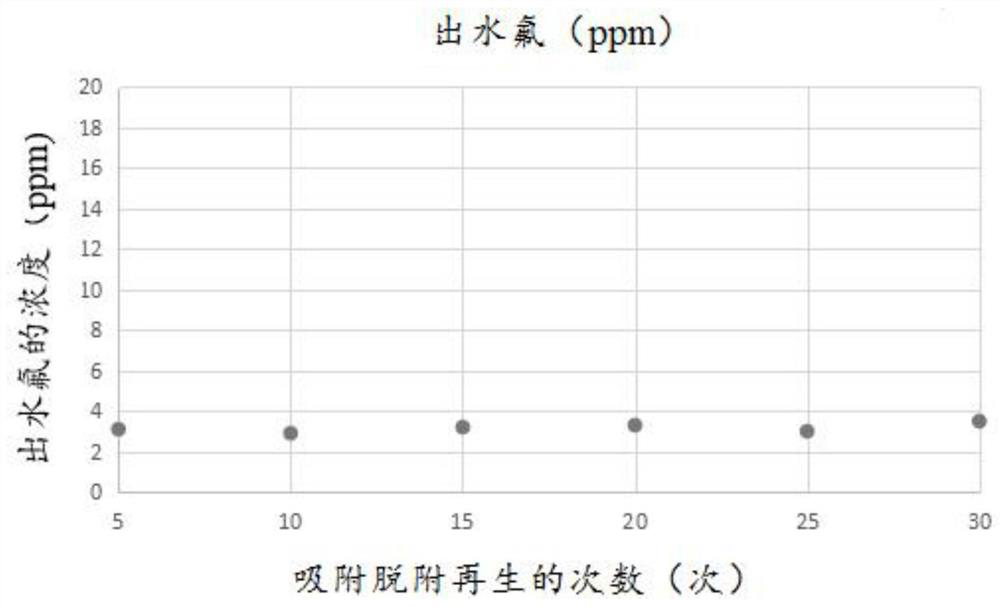
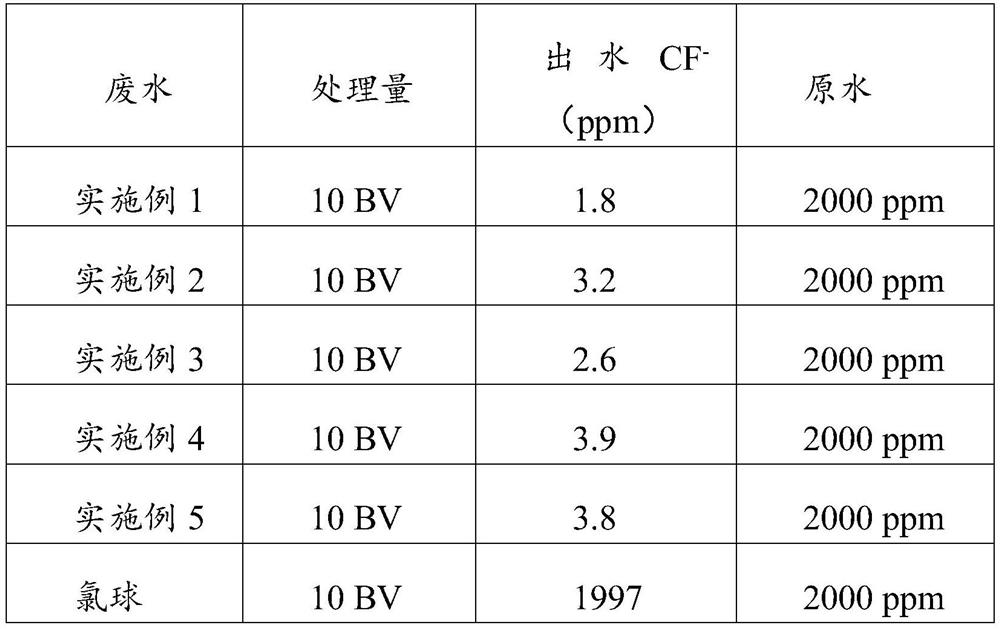
Preparation method of fluorine removal adsorbent modified by biopolymer composite material
ActiveCN113070046AIncrease surface areaRelative density is smallOther chemical processesWater contaminantsBiopolymerSorbent
A preparation method of a fluorine removal adsorbent modified by a biopolymer composite material comprises the following steps: respectively dissolving chitosan and pectin in deionized water or acid, mixing and heating the two solutions, and carrying out a biopolymer cross-linking reaction; adding a molecular sieve soaked in deionized water into the mixed solution, performing reduced pressure distillation, adding chloroacetic acid and a sodium hydroxide aqueous solution, and grafting the chloroacetic acid to modify the chitosan-pectin biopolymer composite material; adding metal nitrate dissolved in a mixed solution of ethanol and water into the modified molecular sieve, performing reduced pressure distillation, adding a sodium hydroxide solution, stirring at room temperature, and doping metal ions into crystal lattices of the modified polymer; and filtering redundant mixed solution, washing to be neutral with de-daubing water, and drying in a drying oven to obtain the modified defluorination adsorbent. The method is simple in preparation process, low in cost, easy to recycle and high in defluorination efficiency in wastewater.
Owner:JIANGSU HELPER FUNCTIONAL MATERIALS
Method for synthesizing tributyl 2-acetylcitrate catalyzed by N-methyl-2-pyrrolidone bisulfate ionic liquid
InactiveCN103739488AReduce dosageReduce generationOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAcetic anhydrideDistillation
The invention discloses a method for synthesizing tributyl 2-acetylcitrate catalyzed by N-methyl-2-pyrrolidone bisulfate ionic liquid, which comprises the following steps: 1) taking citric acid and n-butyl alcohol as raw materials, and N-methyl-2-pyrrolidone bisulfate ionic liquid as a catalyst, fully reacting and naturally layering a reaction mixture, directly performing reduced pressure distillation on the residual n-butyl alcohol and moisture to obtain the crude products; 2) adding acetic anhydride in the crude products obtained in the step 1), fully reacting and performing standing and layering, taking a substance at an upper level and performing reduced pressure distillation to obtain the crude products, and decolouring the crude products by active carbon to obtain the tributyl 2-acetylcitrate. The method has the advantages of high utilization rate of raw materials, high yield and good quality of products, low catalyst cost, mild reaction condition, repeatedly used catalyst, convenient products separation, environmental protection and no pollution.
Owner:ZHEJIANG UNIV OF TECH
Method for promoting acylation of amine or alcohol by carbon dioxide
ActiveCN112851538AMild reaction conditionsHigh toleranceOrganic compound preparationCarboxylic acid amides preparationCarboxylateThiocarboxylic acid
The invention relates to a method for promoting acylation of amine or alcohol by carbon dioxide, which comprises the following steps of: mixing an amine compound, carboxylate or thiocarboxylate compound and a reaction solvent under the action of carbon dioxide, and reacting to obtain an amide compound, or under the action of carbon dioxide, mixing the alcohol compound, the thiocarboxylate compound and the reaction solvent [gamma]-valerolactone, and reacting to obtain the ester compound. According to the invention, under the promotion action of carbon dioxide, carboxylate or thiocarboxylate is used as an acylation reagent, and amine and alcohol are converted into amide and ester compounds in the absence of a transition metal catalyst, so that acylation reagents such as acyl chloride or anhydride with irritation and corrosivity are avoided; and the method has the advantages of simple operation, mild reaction conditions, high tolerance of substrate functional groups, strong applicability and high yield, and provides an efficient, reliable and economical preparation method for synthesis of amide and ester compounds.
Owner:LANZHOU INST OF CHEM PHYSICS CHINESE ACAD OF SCI
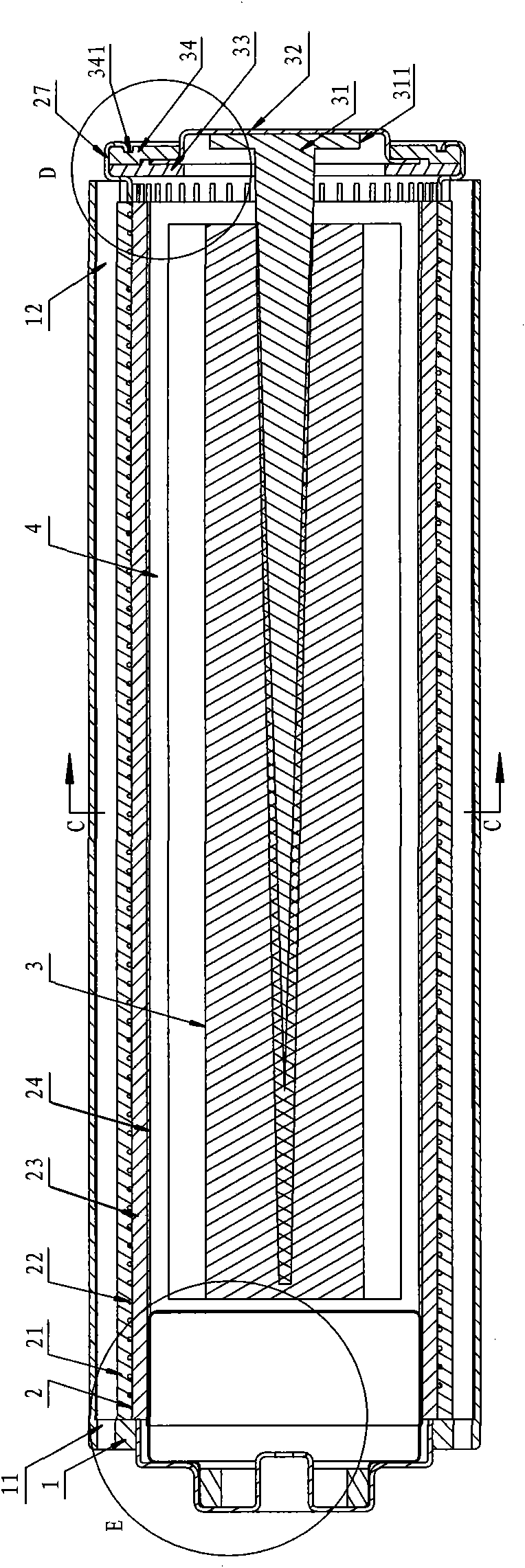
Disposable metal-air battery
The invention belongs to the technical field of chemical power supplies, in particular to a disposable metal-air battery which comprises a battery jacket, an air electrode and a metal anode, wherein the air electrode is positioned in the battery jacket, the metal anode is inserted into the air electrode, electrolyte is arranged between the metal anode and the air electrode, the battery jacket is provided with an air channel, and air cells communicated with air are arranged in the air electrode and between a cathode cap and the metal anode. The disposable metal-air battery is provided with a pressure-releasing air cell in the battery, thereby releasing pressure extruded by the electrolyte, preventing the pressure in the battery from increasing, preventing the electrolyte from leaking and better protecting the reaction environment of the battery. The invention has the advantages of simple and convenient assembly, firm sealing-in and good sealing.
Owner:哈峰
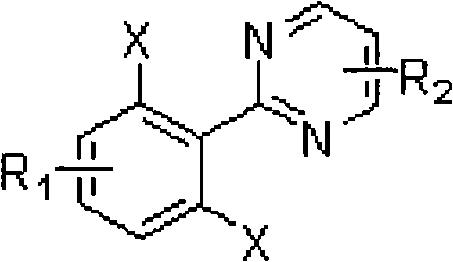
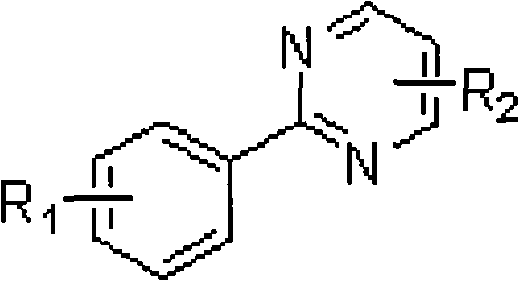
Ortho-position dihalogen substitution compound of aryl pyrimidine and preparing method thereof
InactiveCN101654439ARaw materials are easy to getEasy to operateOrganic chemistryOrtho positionOrganic synthesis
The invention relates to an ortho-position dihalogen substitution compound of aryl pyrimidine and a preparing method thereof. The compound has the following structural formula I: R1=H, p-Me, p-Cl, p-COOEt, p-CF3, m-Me, m-OMe, R2=H, 4-Ph, 5-Ph, X=Cl, Br. The invention comprises the following steps: dissolving aryl pyrimidine, palladium acetate, additive and halogenation calcium into acetic acid, stirring, and reacting until reaction raw material disappears; removing solvent, adding saturation sodium bicarbonate solution into a system, and extracting a product with ethyl acetate; obtaining a crude product by drying the organic phase and removing the solvent; purifying the product to obtain the corresponding ortho-position dihalogen substitution compound of aryl pyrimidine. The compound of the invention is a kind of important organic synthesis intermediate. The invention has easily obtained raw material, high reaction selectivity, and adopts calcium chloride and calcium bromide with low price as a halogen source, uses common solvent in reaction, and has simple operation, moderate reaction condition and environmentally friendly reaction, maximum reaction yield can reach 95%, so that the invention is very suitable for industrial production.
Owner:SHANGHAI UNIV
Preparation method of 2-pyrrolyl-1,3-oxazacyclohexane compound
InactiveCN108727360AReduce pollutionImprove economyOrganic chemistry methodsAntineoplastic agentsChemical synthesisNitrogen
The invention belongs to the technical field of organic chemical synthesis, and particularly relates to a preparation method of a 2-pyrrolyl-1,3-oxazacyclohexane compound. The method comprises the following steps of mixing 2-pyrrolecarboxaldehyde and a solvent dichloromethane uniformly, adding a compound 1 and anhydrous magnesium sulfate under a stirring condition, reacting for 3-5 hours at 20-30DEG C, and removing impurities after reaction to obtain the 2-pyrrolyl-1,3-oxazacyclohexane compound. The invention provides a new synthetic strategy and method for a 2-pyrrolyl-1,3-oxazacyclohexane skeleton compound; the method lays a theoretical foundation for synthesis of NSCLC (non-small cell lung cancer)-resistant Chinese Bittersweet Alkaloid (II), which has an important application value, and other 1,3-oxazacyclohexane skeleton compound, and further provides a material and raw material guarantee for carrying out physiological and pharmacological activity research on 1,3-oxazacyclohexanealkaloid.
Owner:HENAN UNIV OF SCI & TECH
One-step reaction method for preparing 4-nitropyridine-nitrogen oxide and halogenated-4-nitropyridine-nitrogen oxide
InactiveCN100348582CShort reaction timeResponse to environmental protectionOrganic chemistryAcetic anhydrideNitration
Owner:ZHANJIANG NORMAL UNIV
Environment-friendly polyisocyanate synthesis method suitable for industrial production
InactiveCN111333547AGood choiceMethod environmentally friendlyPreparation from carboxylic acid nitrogen analoguesToxic gasPtru catalyst
The invention provides a method for synthesizing polyisocyanate. The method comprises the following steps: carrying out a reaction on R1OOC-R-COOR1 in the presence of NaOH and NH2HCl, and neutralizingto obtain dihydroxamic acid HO-NH-CO-R-CO-NH-OH, wherein R1 is selected from C1-C15 aliphatic alkyl, and R is selected from one or more of phenylene, substituent-containing C6-C30 arylene, unsubstituted C6-C30 arylene, C6-C20 sub-aliphatic hydrocarbon group, C6-C20 cycloaliphatic hydrocarbon group and a naphthylene group; and rearranging the dihydroxamic acid under an alkaline condition to obtainthe polyisocyanate OCN-R-NCO. According to the method, highly toxic gases are not adopted, corrosive gases or intermediates are not generated in the reaction process, and noble metal catalysts are not needed, so that the method is environment-friendly and simple, the product selectivity in the reaction process is good, side reactions are few, and the yield is high.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Synthesis method of adsorbent for removing fluorine in high salt
PendingCN114082407AGood chemical stabilityLow costOther chemical processesWater contaminantsSorbentNitrate salts
The invention relates to a synthesis method of an adsorbent for removing fluorine in high salt, and belongs to the technical field of separation of fluorine-containing wastewater. The synthesis method of the adsorbent comprises the following steps: (1) swelling chloromethylated resin in an organic solvent, respectively adding an alkali and a quinoline amide derivative, stirring and reacting to obtain a mixture containing resin, and carrying out solid-liquid separation to take a solid phase which is modified chloromethylated bead resin 1; (2) adding a quinoline derivative and the organic solvent into the modified chloromethylated bead resin 1 in the step (1), stirring and mixing, and carrying out heating reflux reaction to obtain chloromethylated bead modified resin 2; and (3) adding a nitrate solution into the chloromethylated bead modified resin 2 obtained in the step (2), and reacting to obtain the defluorination adsorbent after the reaction is finished. The prepared modified defluorination material is simple in preparation process, low in cost, easy to recycle and high in defluorination efficiency in wastewater.
Owner:JIANGSU HELPER FUNCTIONAL MATERIALS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com