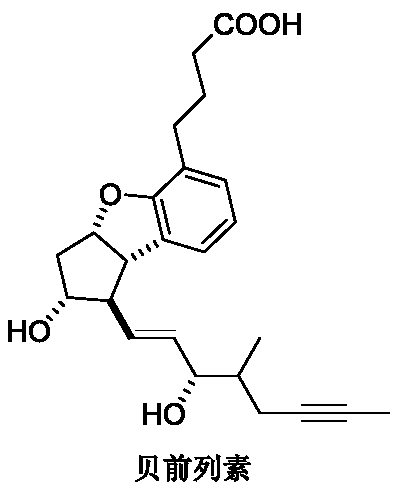
Synthetic method of beraprost
A synthesis method and beraprost technology, applied in organic chemistry methods, organic chemistry and other directions, can solve the problems of cumbersome operation, difficult industrialized production, and high equipment condition requirements, and achieve the advantages of shortening reaction steps, improving yield and shortening reaction time. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
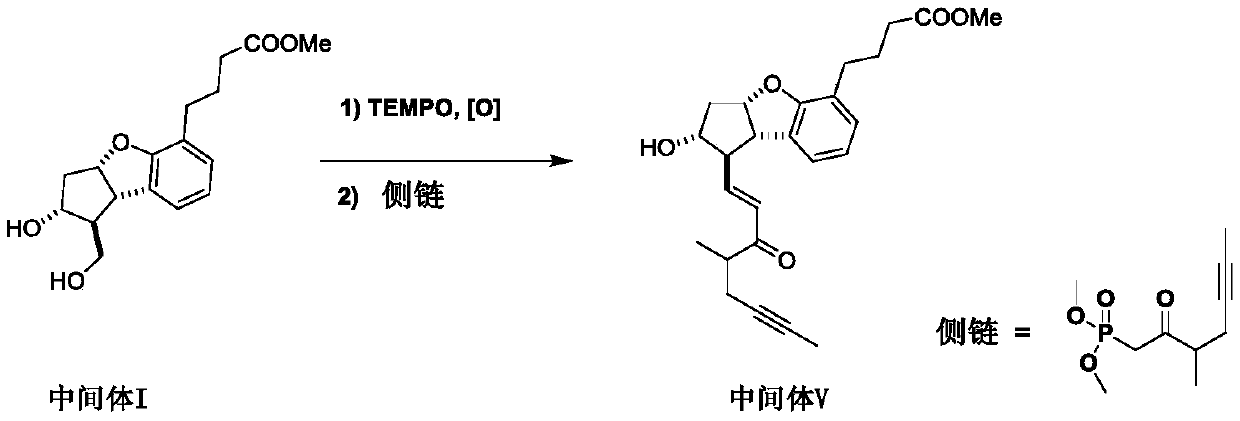
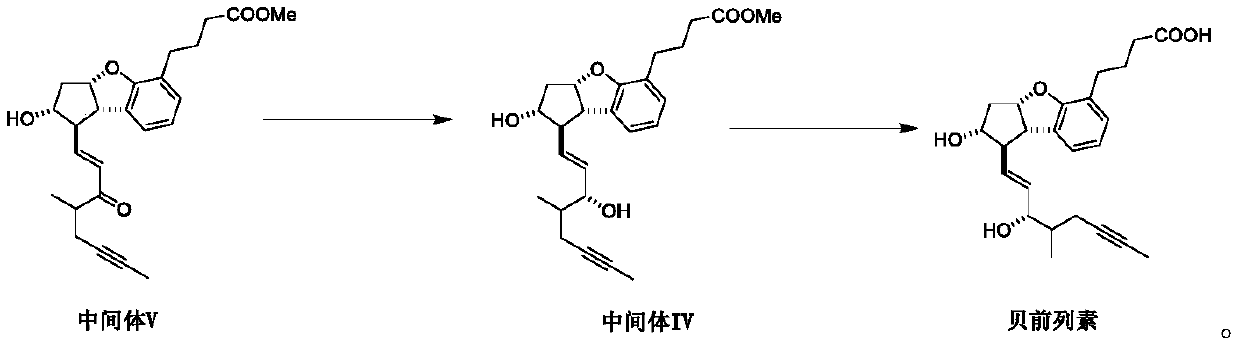
[0047] Embodiment one: the synthesis of intermediate V
[0048] Add 15g of intermediate I, a mixture of dichloromethane and N,N-dimethylacetamide (300:1), TEMPO, diacetoxy iodobenzene, in which dichloromethane and N,N- The mass ratio of the solution of the mixed solution of dimethylacetamide (300:1) to intermediate I is 20:1, the molar ratio of TEMPO to intermediate I is 0.05:1, and the ratio of diacetoxy iodobenzene to intermediate I The molar ratio is 1.2:1, reacted at 30-35°C for 5 hours, the reaction was complete, evaporated the solvent and the generated acetic acid to obtain 4-((1R,2R,3aS,8bS)-1-formyl-2-hydroxyl - 2,3,3a,8b-Tetrahydro-1H-cyclopenta[b]benzofuran-5-yl)butanoic acid methyl ester and its enantiomer crude, dissolved in dry THF, THF The mass ratio with intermediate I is 3:1, stand-by;
[0049] Add dry tetrahydrofuran and sodium hydride to another reaction flask, the mass ratio of tetrahydrofuran to intermediate I is 10:1, and the molar ratio of sodium hydrid...
Embodiment 2
[0050] Embodiment two: the synthesis of intermediate V
[0051] Add 15g of intermediate I, a mixture of dichloromethane and N,N-dimethylacetamide (300:1), TEMPO, 5% NaClO solution, NaBr, in which dichloromethane and N,N- The mass ratio of the mixture of dimethylacetamide (300:1) to Intermediate I is 10:1, the molar ratio of TEMPO to Intermediate I is 0.05:1, and the molar ratio of NaClO to Intermediate I is 1.2:1 , the molar ratio of NaBr to intermediate I was 0.2:1, reacted at 25-30°C for 6 hours, the reaction was complete, the organic solvent was evaporated, the aqueous phase was extracted twice with ethyl acetate, the organic phases were combined, dried, filtered, and spun Evaporation afforded 4–((1R,2R,3aS,8bS)-1-formyl-2-hydroxy-2,3,3a,8b-tetrahydro-1H-cyclopenta[b]benzofuran-5 -yl) methyl butyrate and its enantiomer crude product, dissolved in dry tetrahydrofuran, the mass ratio of tetrahydrofuran and intermediate I is 5:1, stand-by;
[0052] Add dry tetrahydrofuran an...
Embodiment 3
[0053] Embodiment three: the synthesis of intermediate V
[0054] Add 15g of intermediate I, a mixture of dichloromethane and N,N-dimethylacetamide (300:1), TEMPO, diacetoxy iodobenzene, in which dichloromethane and N,N- The mass ratio of the mixed solution of dimethylacetamide (300:1) to intermediate I is 20:1, the molar ratio of TEMPO to intermediate I is 0.1:1, and the molar ratio of diacetoxy iodobenzene to intermediate I The ratio is 1.1:1, react at 25~30°C for 5 hours, the reaction is complete, evaporate the solvent and the generated acetic acid to obtain 4–((1R,2R,3aS,8bS)-1-formyl-2-hydroxyl-2 , 3,3a,8b-Tetrahydro-1H-cyclopenta[b]benzofuran-5-yl)butanoic acid methyl ester and its enantiomer crude, dissolved in tetrahydrofuran, tetrahydrofuran and intermediate I The mass ratio is 3:1, stand-by;
[0055] Add dry tetrahydrofuran and sodium hydride to another reaction bottle, the mass ratio of tetrahydrofuran to intermediate I is 6:1, and the molar ratio of sodium hydrid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com