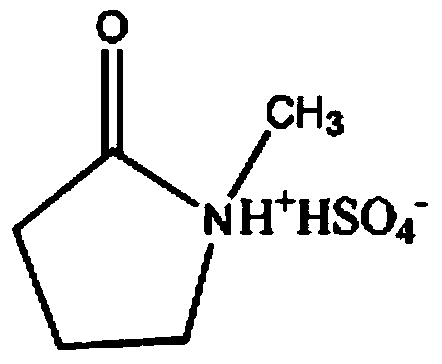
Method for synthesizing tributyl 2-acetylcitrate catalyzed by N-methyl-2-pyrrolidone bisulfate ionic liquid
A technology of acetyl tributyl citrate and methyl pyrrolidone is applied in chemical instruments and methods, preparation of organic compounds, organic compounds/hydrides/coordination complex catalysts, etc., and can solve the problem that tributyl citrate cannot be prepared integrally Ester and acetyl tributyl citrate, product stratification is not obvious, easy to produce side reactions and other problems, to achieve the effect of convenient separation, simple reaction and environmental protection, and simplified process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Preparation of tributyl citrate (TBC):
[0026] Take 0.1 mol of citric acid and 0.38 mol of n-butanol in a 250ml flask, add 15% (mass ratio) catalyst, and react at 130°C for 2.5 hours. After the reaction is over, observe the product, which is obviously divided into upper and lower layers, and the alcohol and water are distilled out under reduced pressure to prepare for the next step of reaction.
[0027] (2) Preparation of acetyl tributyl citrate (ATBC):
[0028] Add 0.6mol acetic anhydride to the reaction product of the previous step, react at 70°C for 2.5 hours, and cool down. The product is divided into upper and lower layers. Take the upper layer product (the lower layer is the catalyst, which is recycled), and distill off the excess acid and water under reduced pressure. Acetyl tributyl citrate with high purity was obtained, decolorized by activated carbon to obtain the finished product, and the yield was 99.4%. The ester content is ≥99.5% as detected by gas ...
Embodiment 2
[0030] (1) Preparation of tributyl citrate (TBC):
[0031] Take 0.1 mol of citric acid and 0.45 mol of n-butanol in a 250ml flask, add 15% (mass ratio) catalyst, and react at 120°C for 3 hours. After the reaction is over, observe the product, which is obviously divided into upper and lower layers, extract the alcohol and water, and prepare for the next step of reaction.
[0032] (2) Preparation of acetyl tributyl citrate (ATBC):
[0033] Add 0.8mol acetic anhydride to the reaction product of the previous step, react at 70-80°C for 2.5 hours, cool down, take the upper layer product, extract the excess acid and water in it, and obtain acetyl tributyl citrate with high purity, decolorize with activated carbon to obtain the finished product, Yield 99.1%. The ester content is ≥99.5% as detected by gas chromatography.
Embodiment 3
[0035] (1) Preparation of tributyl citrate (TBC):
[0036] Take 0.2mol of citric acid and 0.8mol of n-butanol in a 250ml flask, add 18% (mass ratio) catalyst, and react at 100°C for 2 hours. After the reaction is over, observe the product, which is obviously divided into upper and lower layers, extract the alcohol and water, and prepare for the next step of reaction.
[0037] (2) Preparation of acetyl tributyl citrate (ATBC):
[0038] Add 0.8mol acetic anhydride to the reaction product of the previous step, react at 80°C for 2.5 hours, cool down, take the upper product, extract the excess acid and water in it, and obtain acetyl tributyl citrate with high purity, decolorize with activated carbon to obtain the finished product, the yield 99.2%. The ester content is ≥99.5% as detected by gas chromatography.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com