Method for promoting acylation of amine or alcohol by carbon dioxide
A carbon dioxide and acylation technology, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of harsh reaction conditions, long reaction time, cumbersome operation, etc., and achieve mild reaction conditions, simple operation, and economical The effect of the preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
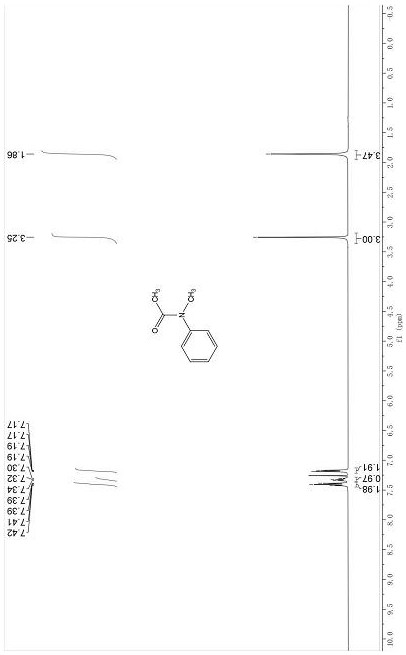
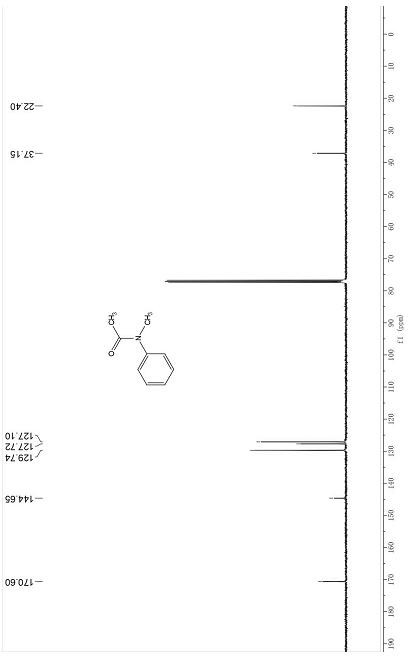
[0047] Dissolve 0.2 mmol of an amine reaction substrate such as N-methylaniline in 1 mL of ethylene glycol dimethyl ether, then add 3 equivalents of potassium carboxylate or potassium thiocarboxylate, and react under a carbon dioxide (1 atm) atmosphere React at 35°C for 8 h, cool to room temperature, pour the reaction mixture into 10 mL of water, extract three times with 10 mL of ethyl acetate, combine the organic phases and wash with 15 mL of saturated brine, dry the organic phase over anhydrous sodium sulfate, and remove the organic phase by rotary evaporation. Solvent, the crude product is purified through a section of about 10 cm long silica gel chromatography column (petroleum ether: ethyl acetate = 20:1~2:1) to obtain the corresponding amide compound 3a, compound 3b, compound 3c, and compound 3d respectively , Compound 3e, Compound 3f, Compound 3g, Compound 3h, Compound 3i, Compound 3j, Compound 3k, Compound 3l, Compound 3m, Compound 3n, Compound 3o, Compound 3p, Compound...
Embodiment 2
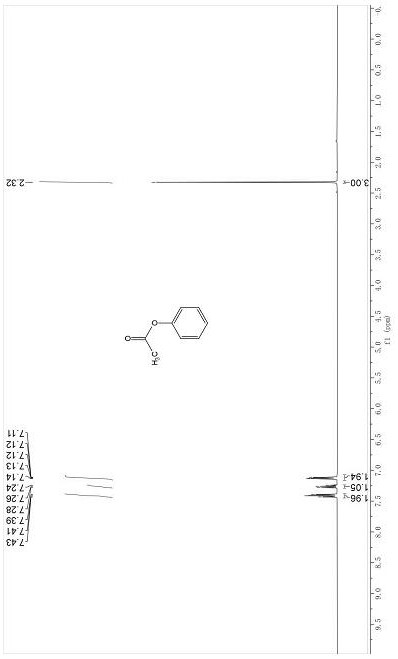
[0180] Dissolve 0.2 mmol of a phenolic reaction substrate such as phenol in 1 mL of γ-valerolactone, then add 3 equivalents of potassium thioacetate, react in a carbon dioxide (1 atm) atmosphere at 100oC for 14 hours, cool to room temperature, and The mixture was poured into 10 mL of water, extracted three times with 10 mL of ethyl acetate, the organic phases were combined and washed with 15 mL of saturated brine, after drying the organic phase with anhydrous sodium sulfate, the organic solvent was removed by rotary evaporation, and the crude product was passed through a section of about 10 cm long silica gel Chromatographic column (petroleum ether: ethyl acetate = 20:1 ~ 2:1) was purified to obtain the corresponding ester compound 5a, compound 5b, compound 5c, compound 5d, compound 5e, compound 5f, compound 5g, compound 5h , Compound 5i, Compound 5j, Compound 5k, Compound 5l, Compound 5m, Compound 5n.
[0181] Spectral data are as follows:
[0182]
[0183] o-tolyl acetat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com