Method for preparing cyclic sulfate by directly oxidizing hydrogen peroxide
A technology of cyclic sulfate and cyclic sulfite, applied in the field of synthesis of new energy materials, can solve the problems of increased impurities, many side reactions, corrosion, etc., achieve high reaction conversion rate, clean production process, and broad market prospects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Preparation of solid catalyst
[0043] Commercially available neutral silica sol and neutral aluminum sol are respectively diluted with pure water according to the solid content into a 10% mass solid content solution, and 2000 g of 10% mass solid content silica sol and 10% mass solid content solution are respectively taken. Mix 850g of aluminum sol, then add 43.3g of bismuth nitrate, drop 10% sodium hydroxide into the mixture of silica sol and aluminum sol under stirring, until all the solids are precipitated, filter the solid, and filter the filter with pure water. Wash the cake until neutral, dry the filter cake in an oven at 120°C, and then pulverize it to obtain an oxide carrier.
[0044] Take 250 g of the oxide carrier, add 32.37 g of ruthenium trichloride trihydrate, 7.75 g of cerium nitrate hexahydrate, and 8.42 g of strontium carbonate, and add pure water under high-speed stirring to make a slurry. Vacuum filter the slurry and wash it with pure water until the ...
Embodiment 2
[0046] Synthesis of vinyl sulfate
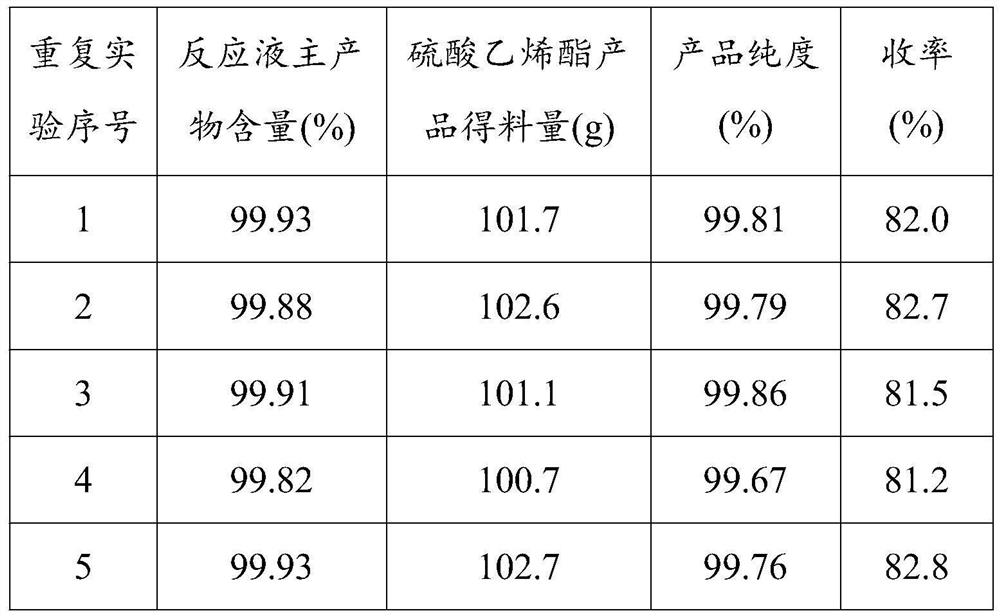
[0047] Add methylene chloride 972g, methyl tert-butyl ether 324g successively in reaction flask, the solid catalyst 56g that embodiment 1 prepares, structural formula is Vinyl sulfite 108g, stir and reactant temperature is raised to 40 ℃ with water bath of reaction system, then hydrogen peroxide mass concentration is 60% hydrogen peroxide 85g (S in the reactant: H 2 o 2 Molar ratio is 1.5) dropwise into the reaction system within two hours, keep the temperature at about 40°C and reflux during the dropwise addition. After the dropwise addition, continue to stir and reflux for 3 hours, cool to 5-10° C. with an ice-water bath, filter out the solid catalyst, wash it with a small amount of dichloromethane, and collect the recovered solid catalyst. The filtrate was allowed to stand to separate the organic layer, and after GC analysis, the mass percentage of the main product calculated by the area normalization method was 99.93% after deducting ...
Embodiment 3
[0049] Synthesis of vinyl dithionate
[0050] In the reaction bottle, add acetonitrile 856g, dimethyl carbonate 2140g, fluorobenzene 1284g successively, structural formula is 214g of vinyl dithionite, 90g of the solid catalyst prepared in Example 1, the reactant was heated to 80°C in a water bath under stirring, and then the mass concentration of hydrogen peroxide was 30% hydrogen peroxide 294.7g (S in the reactant: H 2 o 2 The molar ratio is 1:1.3) was added dropwise to the reaction system within 4 hours, and the temperature was kept at about 80°C to reflux during the dropwise addition. After the dropwise addition, the reaction was continued with stirring and heat preservation for 3 hours, cooled to room temperature, the solid catalyst was filtered off, and the filter cake was washed with a small amount of dichloromethane to collect and recover the solid catalyst. The filtrate was left standing at 5-10° C. to separate the organic layer. After GC analysis, the mass percenta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com