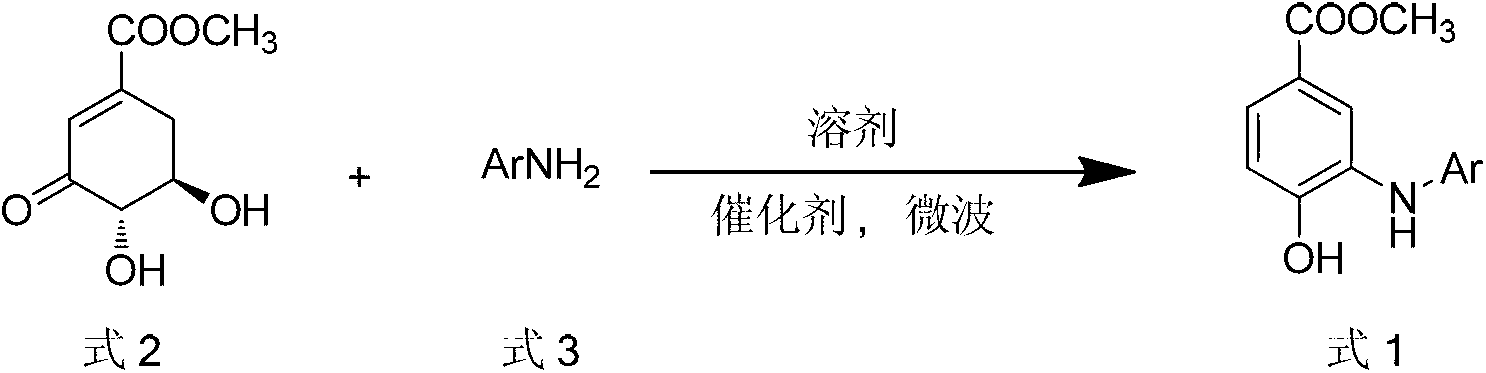
Microwave synthesis method for diarylamine compound
A diarylamine and microwave synthesis technology, applied in the chemical industry, can solve the problems of harsh reaction conditions, high price, instability, etc., and achieve the effects of convenient post-processing, short reaction time, and clean reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: Preparation of methyl 3-anilino-4-hydroxybenzoate
[0021] Methyl 3-dehydroshikimate (1.02 g, 5.5 mmol), aniline (0.46 ml, 5.0 mmol), p-toluenesulfonic acid (0.01 g, 0.05 mmol), 5 ml N,N-dimethylformamide in one pass into the microwave reaction vial. The reaction system was reacted in a microwave reactor at 130 °C for 8 min. The reaction was monitored by TLC. After the reaction was completed, after cooling, the reaction solution was poured into 80ml of saturated brine, stirred continuously, and a solid was precipitated, which was filtered off with suction. The solid was recrystallized from dichloromethane-petroleum ether to obtain white crystals 3-anilino-4-hydroxyl Methyl benzoate 1.14 g, yield: 94%. m.p.160~162℃. 1 H NMR (DMSO-d 6 , 400MHz) δ: 10.48 (s, 1H, 4-OH), 7.74 (d, J=2.0Hz, 1H, 2-ArH), 7.40 (dd, J 1 =8.0Hz, J 2 =2.0Hz, 1H, 6-ArH), 7.37 (s, 1H, NH), 7.22 (t, J=7.6Hz, 2H, 3', 5'-ArH), 7.04 (d, J=7.6Hz, 2H) , 2′, 6′-ArH), 6.91 (d, J=8.0Hz, 1H, 5...
Embodiment 2
[0022] Example 2: Methyl 3-(4'-methylanilino)-4-hydroxybenzoate
[0023] Methyl 3-dehydroshikimate (0.93g, 5.0mmol), p-toluidine (0.54g, 5.0mmol), p-toluenesulfonic acid (0.05g, 0.25mmol) and 5ml of ethylene glycol were added to the microwave reaction flask at one time middle. The reaction system was reacted in a microwave reactor at 140 °C for 8 min. The reaction was monitored by TLC. After the reaction was completed, after cooling, the reaction solution was poured into 80 ml of saturated brine, stirred continuously, a solid was precipitated, filtered with suction, and the solid was recrystallized from ethyl acetate-petroleum ether to obtain 1.03 g of a white solid. Yield: 80%. m.p.152~153℃. 1 H NMR (DMSO-d 6 , 400M Hz) δ: 10.45 (s, 1H, 4-OH), 7.66 (d, J=2.0 Hz, 1H, 2-ArH), 7.34 (dd, J 1 =8.4Hz, J 2 = 2.0Hz, 1H, 6-ArH), 7.18 (s, 1H, NH), 7.05 (d, J=8.4Hz, 2H, 2', 6'-ArH), 6.98 (d, J=8.4Hz, 2H) , 3′, 5′-ArH), 6.88 (d, J=8.4Hz, 1H, 5-ArH), 3.73 (s, 3H, OCH 3 ), 2.22(s, ...
Embodiment 3
[0024] Example 3: Methyl 3-(4'-Methoxyanilino)-4-hydroxybenzoate
[0025] Methyl 3-dehydroshikimate (1.11 g, 6.0 mmol), p-methoxyaniline (0.62 g, 5.0 mmol), p-toluenesulfonic acid (0.05 g, 0.25 mmol), and 5 ml of diethylene glycol were added at one time in a microwave reaction vial. The reaction system was reacted in a microwave reactor at 170 °C for 5 min. The reaction was monitored by TLC. After the reaction was completed, after cooling, the reaction solution was poured into 80 ml of saturated brine, stirred continuously, a solid was precipitated, suction filtered, and dried. : 87%. m.p.153~154℃. 1 H NMR (DMSO-d 6 , 400MHz) δ: 10.42 (s, 1H, 4-OH), 7.51 (d, J=2.0Hz, 1H, 2-ArH), 7.28 (dd, J 1 =8.0Hz, J 2 =2.0Hz, 1H, 6-ArH), 7.06(d, J=6.8Hz, 2H, 3', 5'-ArH), 6.88(d, J=8.0Hz, 1H, 5-ArH), 6.85(d , J=6.8Hz, 2H, 2', 6'-ArH), 3.72(s, 3H, COOCH 3 ), 3.71(s, 3H, OCH 3 ); MS(EI): m / z=273 [M] + , 258[M-CH 3 ] + , 170, 156, 141, 129.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com