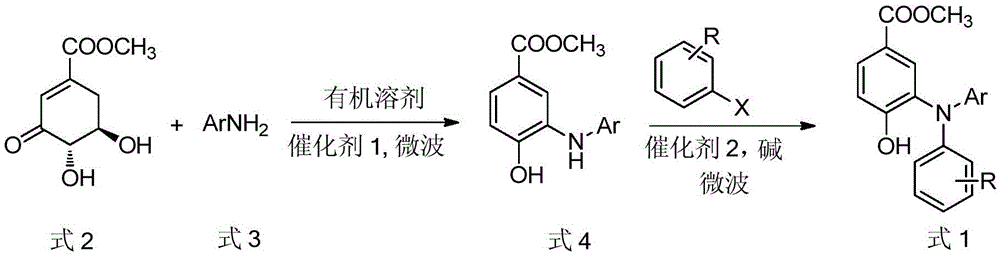
A kind of preparation method of hydroxyl and methoxycarbonyl substituted triarylamine compounds
A technology of methoxycarbonyl and triarylamine, which is applied in the field of preparation of triarylamine compounds, can solve problems such as harsh reaction conditions, difficulties, and narrow substrate adaptability, and achieves short reaction time, simple operation and convenient post-processing. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: Preparation of N-phenyl-N-(2-carboxyphenyl)-2-hydroxyl-5-methoxycarbonylaniline
[0025] Methyl 3-dehydroshikimate (0.41g, 2.2mmol), aniline (0.19g, 2.0mmol), p-toluenesulfonic acid (19.0mg, 0.1mmol), and 5ml N,N-dimethylformamide were added to the microwave at one time in the reaction vial. The reaction system was reacted at 130° C. for 8 min in a microwave reactor. TLC monitors the reaction. After the reaction is completed, potassium carbonate (0.83g, 6mmol), cuprous oxide (0.27g, 2mmol), o-iodobenzoic acid (0.50g, 2mmol) are added to the above system, and the system is placed in a microwave reactor for 130 Reaction at ℃ for 5min. The reaction was monitored by TLC. After the reaction was completed, it was cooled and filtered with suction. The filtrate was poured into 100ml of ice water and acidified with dilute hydrochloric acid. A large amount of off-white solids were precipitated, filtered with suction, dried, and recrystallized with ethyl acetate-pe...
Embodiment 2
[0027] Embodiment 2: Preparation of N-(4-methylphenyl)-N-(2-carboxyphenyl)-2-hydroxyl-5-methoxycarbonylaniline
[0028] Methyl 3-dehydroshikimate (0.37g, 2.0mmol), p-methylaniline (0.22g, 2.0mmol), formic acid (4.6mg, 0.1mmol), and 5ml N,N-dimethylformamide were added to the microwave at one time in the reaction vial. The reaction system was reacted at 130° C. for 6 min in a microwave reactor. The reaction was monitored by TLC. After the reaction was completed, potassium carbonate (0.83g, 6mmol), cuprous oxide (0.27g, 2mmol), and o-iodobenzoic acid (0.50g, 2mmol) were added to the above system in a microwave reactor at 130°C. React for 5 minutes. The reaction was monitored by TLC. After the reaction was completed, it was cooled and filtered with suction. The filtrate was poured into 100ml of ice water and acidified with dilute hydrochloric acid. A large amount of off-white solid precipitated, filtered with suction, dried, and recrystallized with ethyl acetate-ethanol to obta...
Embodiment 3
[0030] Example 3: Preparation of N-(4-bromophenyl)-N-(2-carboxyphenyl)-2-hydroxyl-5-methoxycarbonylaniline:
[0031]Methyl 3-dehydroshikimate (0.45g, 2.5mmol), p-bromoaniline (0.35g, 2.0mmol), p-toluenesulfonic acid (28.5mg, 0.15mmol), 5ml N,N-dimethylacetamide in one go Add to microwave reaction vial. The reaction system was reacted at 130° C. for 8 min in a microwave reactor. The reaction was monitored by TLC. After the reaction was completed, potassium carbonate (0.83g, 6mmol), cuprous chloride (0.20g, 2mmol), and o-iodobenzoic acid (0.50g, 2mmol) were added to the above system at 110°C in a microwave reactor. Under reaction 8min. The reaction was monitored by TLC, after the reaction was completed, suction filtered after cooling, the filtrate was poured into 100ml of ice water, acidified with dilute hydrochloric acid, a large amount of off-white solid was precipitated, filtered with suction, dried, and recrystallized with ethanol-water to obtain white crystal N-(4 -Bromo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com