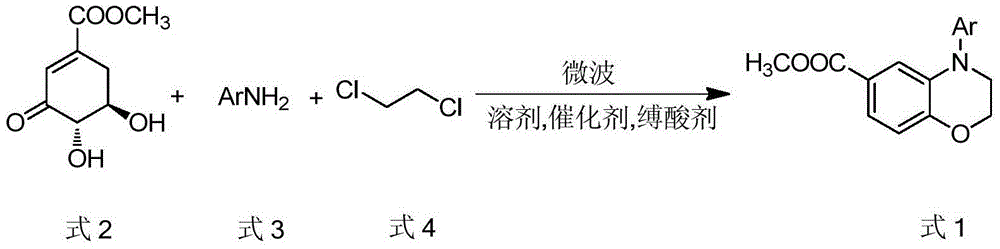
Preparation method for 4-aryl-6-methoxycarbonyl benzoxazine compound
A technology of methoxycarbonylbenzene and compounds, which is applied in the field of preparation of 4-aryl-6-methoxycarbonylbenzoxazine compounds, and can solve the problems such as many synthesis steps, long reaction time and poor selectivity of o-aminophenol , to achieve the effect of short reaction time, high yield and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Example 1: Preparation of 4-phenyl-6-methoxycarbonyl-3,4-dihydro-2H-1,4-benzoxazine
[0020] Methyl 3-dehydroshikimate (0.37g, 2.0mmol), aniline (0.19g, 2.0mmol), 1,2-dichloroethane (1ml), p-toluenesulfonic acid (0.02g, 0.10mmol), Dimethylsulfoxide (5ml) was added to the microwave reaction vial. The above system was reacted in a microwave reactor at 120°C (160W) for 5min, followed by TLC. After the aniline reaction was complete, cesium carbonate (1.95g, 6mmol) was added to the above system, and reacted at 180°C (800W) for 5min, TLC Follow up until the response is complete. After cooling the reaction solution, pour it into a beaker filled with 20ml of saturated saline, stir, and extract the product with ethyl acetate (3×20ml), combine the organic layers, dry, remove the solvent by rotary evaporation, column separation, ethyl acetate-petroleum Eluted with ether, crystallized to obtain 0.48 g of white solid 4-phenyl-6-methoxycarbonyl-3,4-dihydro-2H-1,4-benzoxazine, yield...
Embodiment 2
[0021] Example 2: Preparation of 4-(4-methoxyphenyl)-6-methoxycarbonyl-3,4-dihydro-2H-1,4-benzoxazine
[0022] Methyl 3-dehydroshikimate (0.41g, 2.2mmol), 4-methoxyaniline (0.25g, 2.0mmol), formic acid (3.74μl, 0.10mmol), 1,2-dichloroethane (0.5 ml), dimethylsulfoxide (5ml) were added to the reaction flask. The above system was reacted in a microwave reactor at 130°C (240W) for 5min, followed by TLC. After the reaction of p-methoxyaniline was complete, cesium carbonate (1.95g, 6mmol) was added, and reacted at 180°C (800W) for 5min, TLC Follow up, after the response is complete. After cooling the reaction solution, pour it into a beaker filled with 20ml of saturated saline, stir, and extract the product with ethyl acetate (3×20ml), combine the organic layers, dry, remove the solvent by rotary evaporation, column separation, ethyl acetate-petroleum Eluted with ether, crystallized to give light yellow crystal 4-(4-methoxyphenyl)-6-methoxycarbonyl-3,4-dihydro-2H-1,4-benzoxazine ...
Embodiment 3
[0023] Example 3: Preparation of 4-(4-methylphenyl)-6-methoxycarbonyl-3,4-dihydro-2H-1,4-benzoxazine
[0024]Methyl 3-dehydroshikimate (0.46g, 2.4mmol), p-toluidine (0.21g, 2.0mmol), 1,2-dichloroethane (1ml), acetic acid (5.72μl, 0.10mmol), di Methyl sulfoxide (1.5ml) was added to the reaction flask, and the above system was reacted in a microwave reactor at 130°C (240W) for 5min, followed by TLC. After the reaction of p-toluidine was complete, cesium carbonate (1.95g, 6mmol) was added, React at 180°C (640W) for 5 minutes, followed by TLC, after the reaction is complete. After cooling the reaction solution, pour it into a beaker filled with 20ml of saturated saline, stir, and extract the product with ethyl acetate (3×20ml), combine the organic layers, dry, remove the solvent by rotary evaporation, column separation, ethyl acetate-petroleum Eluted with ether, crystallized to give 0.51 g of white solid 4-(4-methylphenyl)-6-methoxycarbonyl-3,4-dihydro-2H-1,4-benzoxazine, yield: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com