Method for preparing fluralana intermediate, prepared intermediate and application of fluralana intermediate
A technology of Frellaner and intermediates, which is applied in the field of organic synthesis, and can solve problems such as difficulty in purchasing zinc dimethoxide, low yield, and difficulty in obtaining products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
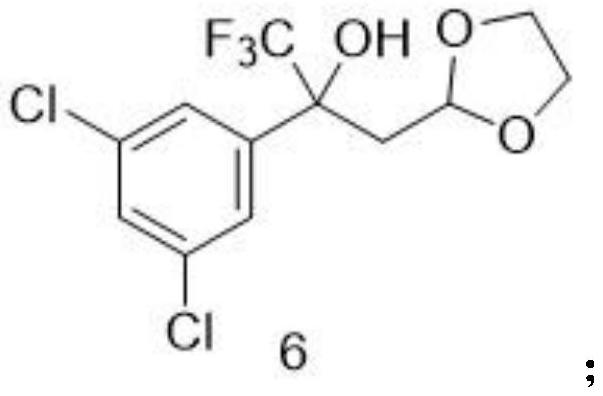
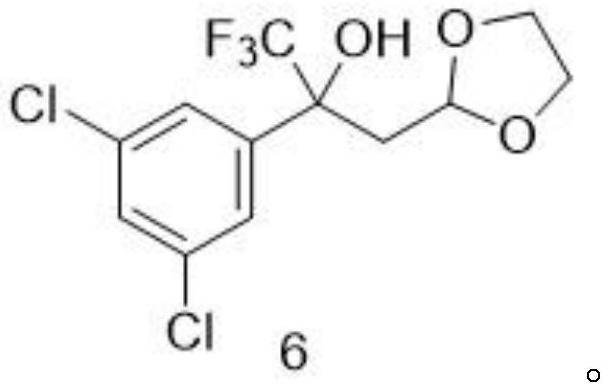
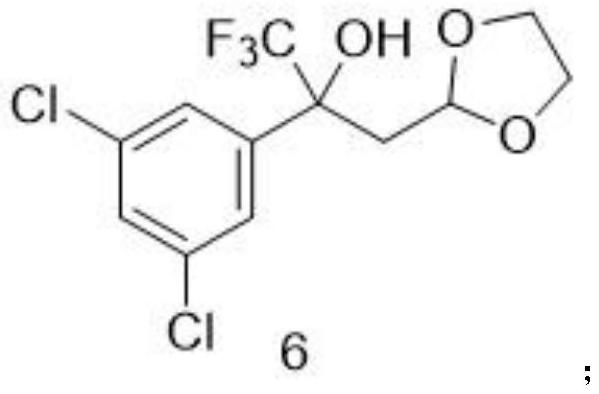
[0058] Embodiment 1: the synthesis of step (1) intermediate 6
[0059] 1-(3,5-dichlorophenyl)-2,2,2-trifluoroethanone (50 g, 206.65 mmol) in tetrahydrofuran (206.65 mL) was placed in a reaction vessel with a thermometer, and (1 , 3-dioxolan-2-ylmethyl)magnesium bromide in tetrahydrofuran (0.5M) (1.03L, 516.63mmol), the dropwise addition was completed, and the reaction system was warmed up to 60°C and stirred until the reaction was completed as monitored by TLC. After ice-water bath to 0°C, add saturated ammonium chloride aqueous solution (800mL) to quench, add ethyl acetate (800mL) to extract, and the organic layer is washed with water (150mL*2 times) and saturated sodium chloride aqueous solution (150mL*2 times) Then dry with anhydrous sodium sulfate. After suction filtration, the filtrate was evaporated to dryness and the residue was purified by silica gel column chromatography to obtain white solid intermediate 6 (51.10 g, 75.0%). 1 H NMR (300MHz, DMSO-d) δ7.58(s,1H),7.34...
Embodiment 2
[0060] Embodiment 2: the synthesis of step (2) intermediate 3*
[0061] Tetrahydrofuran (1.03L) of intermediate 6 (51.10g, 154.33mmol) was placed in a reaction vessel with a thermometer, and aqueous hydrochloric acid (6N) (514.3mL, 3.09mol) was added dropwise. After the addition was complete, the temperature of the reaction system was raised to Stir at 60°C until the reaction is complete as monitored by TLC, yielding intermediate 3*. The reaction solution was quenched by adding saturated aqueous sodium carbonate solution (800mL) at 0°C, extracted with ethyl acetate (800mL), and the organic layer was washed with water (150mL*2 times) and saturated aqueous sodium chloride solution (150mL*2 times). Dry over anhydrous sodium sulfate. Suction filtration, the filtrate was evaporated to dryness, and the residue 3* was directly thrown into the next step.
Embodiment 3
[0062] Embodiment 3: step (3) intermediate 4 * Synthesis
[0063] The tetrahydrofuran solution (771.50mL) of Grignard reagent (46.54g, 185.20mmol) was placed in a reaction vessel with a thermometer, and the temperature of the reaction solution was lowered to -30°C by using a low-temperature reaction tank, and the residue (154.33mmol ) in tetrahydrofuran (771.50 mL), after the dropwise addition was completed, keep stirring at -30° C. until the reaction was completed as monitored by TLC. Add saturated aqueous ammonium chloride solution (600mL) to the reaction solution at -30°C to quench, add ethyl acetate (700mL) to extract, and wash the organic layer with water (150mL*2 times) and saturated aqueous sodium chloride solution (150mL*2 times) Then dry with anhydrous sodium sulfate. Suction filtration, after the filtrate was evaporated to dryness, the residue DCM (2.50 L) was dissolved, and Dess-Martin oxidant (78.55 g, 185.20 mmol) was added at room temperature and stirred until ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com